Keywords
Acid etching, contact angle, roughness, sandblasting, surface morphology, titanium alloy
Surface modifications of titanium alloys used in dental implants can significantly change the implants’ micro and nano topography and composition. These alterations can lead to substantial improvements in mechanical properties, hydrophilicity, osseointegration, and antibacterial performance, as well as positively influencing osteoblast activity and bone formation processes. This paper investigates the effect of the combination of acid etching on titanium alloys’ surface morphology, roughness, and hydrophilicity.
A titanium alloy (Ti6Al4V) disc with a diameter of 12 mm and thickness of 2 mm was sandblasted using aluminum oxide Al2O3 by particle sizes 100 μm, 250 μm, and 500 μm and followed by acid etching. Acid etching was carried out on these samples using different combinations of acid, i.e., hydrofluoric acid (HF), hydrochloric acid (HCL), and sulfuric acid (H2SO4), with the same temperature and time exposure. All titanium surfaces were characterized using a scanning electron microscope (SEM) and contact profilometer.
This study showed that the type of acid combination influenced the surface morphology and roughness profile. After sandblast and acid etching, the titanium alloy has roughness between 1.369 μm – 4.304 μm. There is no significant difference in surface roughness between using a combination of HF, HCL, H2SO4 and only the combination of HCL and H2SO4.
Titanium alloy has achieved the desired morphology and roughness after sandblasting with Al2O3 250 μm and acid-etching with HCL and H2SO4. Acid etching with a combination of HCL and H2SO4 at 120 degrees can produce micro-topography in a shorter duration.
Acid etching, contact angle, roughness, sandblasting, surface morphology, titanium alloy
Surface modification of titanium significantly enhances its topography and composition for dental implants, optimizing roughness, wettability, and antibacterial properties to promote osteoblast attachment and facilitate osseointegration. The method of implant surface modification can be categorized into two groups: subtraction and addition. Subtraction techniques, such as sandblasting, acid etching, sandblasting acid-etched (SLA), anodizing, and laser radiation, effectively modify the titanium surface’s roughness, hardness, and oxidation of Ti. In contrast, addition techniques involve coating the titanium surface with various substances, significantly altering its composition and enhancing biological properties through plasma spraying and sol-gel.1,2 Acid-etching is often performed using hydrofluoric (HF), nitric (HNO3), sulfuric (H2SO4), hydrochloric (HCL) acids, and combinations thereof. The surface features after acid etching may vary depending on the time, type, and concentration of the acid.1 Regarding implant surface roughness, previous research concludes that osteoblast activity increases with a moderately micro-roughness profile with a Ra value range from 1 to 2 μm. The implant surface topography can be classified as smooth (Ra < 0.5 μm), minimally rough (Ra 0.5–1.0 μm), moderately rough (Ra 1.0–2.0 μm), and highly rough (Ra > 2.0 μm).3 The microtopographic features of the implant surface, such as peaks and valleys, are an essential factor in the biological response and the configuration of the bone-implant interface. However, optimal roughness and superficial morphology are still controversial and must be more clearly defined. Therefore, the present study aimed to investigate the effect of the combination of acid etching on titanium alloys’ surface morphology, roughness, and hydrophilicity. SEM analysis has shown that the combination of sandblasting followed by acid etching produces a honeycomb-like pattern.
Titanium alloy (Ti6Al4V) discs with a diameter of 12 mm and thickness of 2 mm (n=12) were machined using a 5-axis milling machine, as shown in Figure 1. Ti-disc was blasted with large grit aluminum oxide Al2O3 by particle size 100 μm, 250 μm, and 500 μm for 30 seconds from a distance of 10 mm, with a pressure of 5 bar. These machine Ti-discs were cleaned in an ultrasonic cleaner using acetone, ethanol, and distilled water for 5 minutes each to remove machining chips, oil, and any organic contamination. After cleaning, samples were dried in a vacuum oven 60-80°C. Acid etching was carried out on these samples in hydrofluoric acid (HF) 48%, hydrochloric acid (HCL) 37%, and sulfuric acid (H2SO4) 97%. The etching process in this research was carried out in two methods, which included immersion of titanium in HF at room temperature for 30 seconds followed by immersion in a mixture of HCL and H2SO4 in a ratio of 2:1 at 120 degrees for 5 minutes and the other method was immersion of titanium in a mixture of HCL, H2SO4, H2O in a ratio of 2:1:1 at 120 degrees for 5 minutes. After the completion of the etching process, the specimens were subjected to ultrasonic cleaning using distilled water and then dried.
Evaluation of implant surface morphology using Scanning Electron Microscope (SEM) (Thermoscientific Quanta 650) with 1000× and 3000× magnification. The surface roughness of the titanium sample was also measured with a contact profilometer (MarSurf PS 10, Mahr GmbH) to obtain the Ra value. Surface wettability was tested through contact angle measurement using the sessile drop method. Analysis and measurement of contact angle were done with ImageJ software.
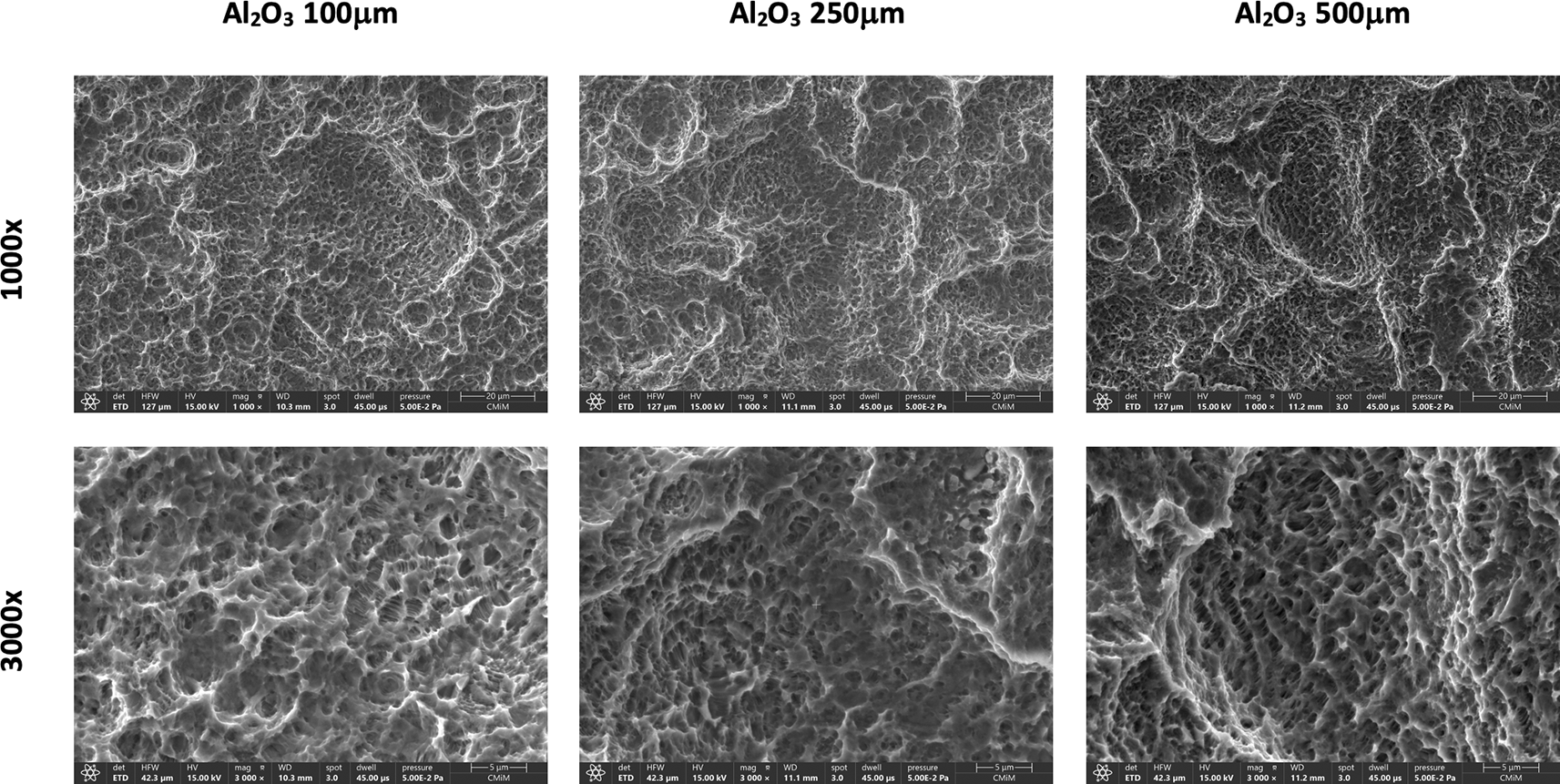
The acid type significantly affects the titanium alloy’s morphology (Ti6Al4V) surface. Figure 1 presents the Ti-disc specimen and SEM images after sandblasting with Al2O3 particles. After sandblasting, SEM results show a heterogeneous and irregular titanium surface with varying degrees of roughness, depending on the size of the Al2O3 particles used. SEM results show that the sandblasting process on the surface of titanium alloy using Al2O3 particles produces a unique and irregular surface topography with peaks and valleys of varying sizes ranging from shallow and small depressions to more extensive and more profound depressions. The difference in Al2O3 particle size also affects the surface morphology. SEM results show that titanium sandblasted using Al2O3 500 μm produces a more extensive and deeper valley shape when compared to Al2O3 100 μm.
The results of etching specimens using HF at room temperature for 30 seconds and continued with a mixture of HCL and H2SO4 (2:1) at 120 degrees for 5 minutes are shown in Figure 2. Based on the SEM results, the titanium surface topography still shows an irregular surface with varying width and depth, but the etching process creates micro-roughness on the surface after sandblasting. The etching results with this method produce a topographic structure of the titanium surface that shows an irregular surface, more of a rounded porous structure, the peak area is still clearly visible, and the surface texture is less homogeneous. The white structure resulting from the reaction of titanium with H2SO4 and HCL appears to produce deposits and may be attached to the titanium surface.

The results of etching with HCL, H2SO4, and H2O (2:1:1) at 120 degrees for 5 minutes are shown in Figure 3. Based on the SEM results, the peak area is reduced and becomes flatter, the sandblast character is less visible, and the resulting surface texture is more homogeneous than the previous method. The combination of sandblasting followed by acid etching with this method results in a honeycomb-like pattern on the titanium surface. In addition, SEM analysis also shows the surface characteristics of titanium with a more even texture and a smaller cavity size than the previous method.
Based on Table 1, the average roughness of titanium alloy after etching using a combination of HF, HCL, and H2SO4 is higher when compared to using only HCL and H2SO4. Titanium alloy specimen has roughness between 1.369 μm – 4.304 μm depending on the size of Al2O3. There is no significant difference in surface roughness between using a combination of HF, HCL, H2SO4 and only the combination of HCL and H2SO4 without HF.
| Al2O3 particle size | Ra Value (Mean ± SD) | p-value | |
|---|---|---|---|
| HF, HCL + H2SO4 | HCL + H2SO4 + H2O | ||
| 100 μm | 1.482 ± 0.025 | 1.369 ± 0.114 | 0.226 |
| 250 μm | 2.224 ± 0.050 | 2.016 ± 0.150 | 0.127 |
| 500 μm | 4.312 ± 0.263 | 4.304 ± 0.225 | 0.970 |
Titanium surface modification not only shows an increase in surface roughness but also undergoes chemical modification that will affect the wettability and interaction with biological fluids. The hydrophilicity of the titanium alloy surface after sandblasting and acid etching was determined by measuring the contact angle using the sessile drop method. The results of contact angle measurement are shown in Figure 4. In general, all titanium specimens show contact angle values < 90°, so it can be concluded that the surface is hydrophilic.
The present study’s finding has proved that acid etching is a relatively dominant factor in deciding the surface texture and roughness. The SEM figure shows that the different acids used to modify the implant surfaces lead to different surface morphology. In this study, the etching of titanium alloy (Ti6Al4V) was optimized to obtain micro- and nano-topography and to improve hydrophilicity and surface roughness. The etching process in this study uses three different acids: hydrofluoric acid (HF) 48%, hydrochloric acid (HCL) 37%, and sulfuric acid (H2SO4) 97%. Etching on titanium surfaces significantly affects titanium’s surface morphology and mechanical properties. Each acid contributes uniquely during the etching process to improve surface roughness. HF is known to have aggressive etching ability even at low concentrations. In addition, HF and HCL also contribute effectively to removing the passive oxide layer on the titanium surface, while H2SO4 increases the overall surface roughness due to its strong corrosive properties. Combining these acids will produce a more significant etching effect than using each acid individually.4
Liang et al.5 showed that HF can create micro- and nanostructured surfaces on titanium surfaces, significantly improving osteogenic activity and cell attachment. HF effectively removes the passive oxide layer because it naturally reacts with titanium in the TiO2 layer to promote better cell adhesion. In addition, the concentration and duration of HF etching also play an essential role in determining the surface characteristics of titanium. Zahran et al.6 found that etching time significantly affects the topography and chemical composition of the titanium surface. In that study, it was observed that fluoride ions began to appear on the surface after the titanium was etched for two minutes using HF.
HCL and H2SO4 are the most widely used acid types for etching titanium. The etching process is done by mixing HCL and H2SO4 to accelerate the etching process. In addition, no reaction occurs due to the mixing of the two acids. Previous research showed that the etching process of titanium using HCL and H2SO4 at room temperature takes a very long time to obtain micro-textures on the titanium surface because both acids have a low etching rate at room temperature. Nadar et al.7 found that although the sandblasting process increased surface roughness, etching performed afterward at high temperatures could produce smoother titanium alloy surfaces. Another study by Hung et al.4 also showed that higher etching temperatures resulted in lower surface roughness for titanium alloy (Ti6Al4V) when etched using HCL. This phenomenon is related to increased activation energy at higher temperatures, which results in more uniform etching and reduces the formation of sharp microstructures that affect roughness. Although higher temperatures can reduce roughness, it is crucial to consider the balance between achieving the desired surface characteristics and maintaining the roughness required for biological interactions.
In this study, titanium alloy etching with HF, HCL, and H2SO4 showed an irregular surface, and the surface texture is less homogeneous than the acid etching method without HF. These results align with a previous study by Madaan et al., which found that using HF has disadvantages, including the potential to create a non-uniform surface profile. Prolonged etching time can also lead to the dissolution of the titanium oxide layer, which can potentially cause excessive roughness and impair mechanical properties, including reduced ductility and increased brittleness of the titanium alloy. This is particularly relevant in the development of dental implants, where the mechanical stability of the implant is crucial.8 Etching using HF can also contribute to the release of fluoride ions into the surrounding environment, which can potentially cause cytotoxicity and have negative impacts on cell function and viability. Due to these factors, the use of HF for etching titanium alloys requires careful consideration and strict safety protocols, so additional etching methods are needed to achieve optimal results for titanium surface modification.8,9 In this study, an HF solution with a concentration of 48% was used. The etching rate of titanium in 48% HF is very high, which can be advantageous for achieving precise desired surface characteristics within a short time.
In this study, titanium alloy etching with HF, HCL, and H2SO4 showed a higher roughness value with a 1.482 μm – 4.312 μm range. However, there is no significant difference in surface roughness between using a combination of HF, HCL, H2SO4 and the combination of HCL and H2SO4. Based on previous research, the average roughness of commercial titanium implants was about 1-2 μm.10 Therefore, in this study, the Ti-disc specimen, after being sandblasted using 250 μm Al2O3 followed by etched with a combination of HCL and H2SO4 had a surface roughness close to the roughness of commercial implants and had the desired surface morphology. The effect of sandblasting and acid etching on surface roughness has been highlighted in various studies.11 Acid etching, in conjunction with sandblasting, refines the surface topography further. This technique introduces micro-roughness on the already roughened surface, creating a more complex topography. The roughness of titanium surfaces and irregular surface features created by these methods can significantly improve the mechanical interlocking between the implant and surrounding bone tissue and improve biological responses from osteoblasts.12,13
All titanium specimens show contact angle values < 90°, so it can be concluded that the surface is hydrophilic. The contact angle of dental implants is an important factor affecting their wettability, which can affect osseointegration and overall implant success. Various surface treatments can significantly change the contact angle of titanium implants, thereby improving their biological performance. This study’s results align with research conducted by Yang and Huang, which showed that the titanium surface after sandblasting and acid etching indicated a moderate level of wettability. Although titanium surfaces are not entirely hydrophilic, they have better wettability than untreated ones, which usually have a higher contact angle.14 This relationship is crucial for the design of titanium implants to improve osseointegration and overall biocompatibility.
From the result of this study, it was suggested that sandblast followed by acid etching method was an effective way to roughen titanium alloy surface for dental implant application. Although acid etching creates a rough surface, the etching process must proceed with a control condition. A combination of HCL and H2SO4 seems to be an effective solution for etching in getting the desirable roughness and surface morphology in terms of the micro pit presence and homogenous texture, which may lead to good attachment of cells and might increase bone-to-implant contact. However, further study must evaluate the in vitro and in vivo bone response to these surfaces.
Figshare: SEM Images of Titanium Alloy Disc after Sandblasting and Acid Etching, https://doi.org/10.6084/m9.figshare.27987959.15
This project contains the following underlying data:
Figshare: Roughness Measurement of Titanium Alloy Disc after Sandblasting and Acid Etching, https://doi.org/10.6084/m9.figshare.27987935.16
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Biomaterials, surface modification of metal implants, material engineering
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Abdulla MA, Hasan RH, Al-Hyani OH: Impact of Er,Cr:YSGG Laser, Sandblast, and Acid Etching Surface Modification on Surface Topography of Biodental Titanium Implants.J Lasers Med Sci. 2023; 14: e38 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Dental Implants, Prosthodontics
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 17 Feb 25 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)