Keywords
Staphylococcus hyicus, Zoonoses, Livestock exposure, Chronic osteomyelitis, Matrix-Assisted Laser Desorption-Ionization, Polymerase chain reaction, Methicillin resistance, Antimicrobial resistance
This article is included in the Pathogens gateway.
Staphylococcus hyicus is a zoonotic pathogen primarily associated with animal infections. Human infections are exceedingly rare, with only six cases documented in the literature. The pathogen’s role in chronic osteomyelitis of long bones has not been previously reported, presenting unique diagnostic and therapeutic challenges.
We report the first case of chronic osteomyelitis of long bones caused by methicillin-resistant S. hyicus in a 34-year-old male farmer with a history of recurrent osteomyelitis following trauma and livestock exposure. The patient presented with purulent discharge from two fistulae on the medial aspect of the right thigh. Imaging studies revealed bony sequestration, periosteal reaction, and multiple fistulous tracts consistent with Cierny-Mader stage III chronic osteomyelitis. Microbiological identification of coagulase-negative methicillin-resistant S. hyicus was achieved via culture and phenotypic analysis of aspirates from the fistulae and intraoperative samples, with confirmation via matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS). A multidisciplinary treatment strategy was implemented, combining extensive surgical debridement and dead space management with a 24-week tailored antibiotic regimen. The regimen included intravenous teicoplanin and ciprofloxacin for 14 days, followed by oral ciprofloxacin and cotrimoxazole. The patient achieved sustained remission with no recurrence at 2.5 years of follow-up.
This case underscores the zoonotic potential of S. hyicus and highlights its capacity to cause severe, persistent infections in humans, particularly in individuals with occupational or environmental exposure to livestock. Advanced diagnostic techniques such as MALDI-TOF MS are essential for accurate identification, mitigating the risk of misdiagnosis due to phenotypic similarities with other staphylococcal species. Clinicians should consider S. hyicus in the differential diagnosis of chronic osteomyelitis in patients with relevant exposure histories to enable timely and targeted therapeutic interventions.
Staphylococcus hyicus, Zoonoses, Livestock exposure, Chronic osteomyelitis, Matrix-Assisted Laser Desorption-Ionization, Polymerase chain reaction, Methicillin resistance, Antimicrobial resistance
Staphylococcus hyicus is primarily recognized as a pathogenic bacterium in animals, particularly swine, where it is a causative agent of exudative epidermitis.1 However, its involvement in human infections remains exceedingly rare and is poorly documented in medical literature.2–7 Evidence suggests that while human infections caused by S. hyicus are infrequent, they can result in severe and destructive conditions, especially in individuals with close occupational or environmental exposure to animals.2 The limited number of cases and the variable phenotypic properties of S. hyicus can lead to underdiagnosis, suggesting that the true prevalence may be higher than reported and highlighting the need for clinical vigilance.3,4 Chronic osteomyelitis of long bones is a persistent and challenging infection characterized by significant morbidity and a high rate of recurrence, requiring a multidisciplinary and systematic approach to both diagnosis and management. We report the first documented case of chronic osteomyelitis of long bones caused by S. hyicus.
A 34-year-old male farmer residing in a rural area was admitted in July 2022 for evaluation and management of two fistulae discharging purulent material located on the medial aspect of the right thigh.
His medical history was significant for an episode of acute distal femoral osteomyelitis at the age of 9, following trauma sustained on a farm with exposure to livestock. Initial management consisted of surgical debridement and unspecified antibiotic therapy. The condition progressed to chronic osteomyelitis, characterized by six septic relapses between 1998 and 2020, each managed surgically with empiric antibiotic therapy, though no pathogen was identified.
In June 2022, the patient experienced persistent purulent discharge from two fistulae at the site of a previous surgical scar. Fourteen days prior to admission, he sought outpatient care and was prescribed amoxicillin-clavulanic acid (1 g three times daily) without clinical improvement.
Upon admission, he was afebrile and in good general condition. Physical examination revealed two draining fistulae with frank purulent discharge overlying the operative scar on the medial aspect of the right thigh. No additional abnormalities were identified.
Laboratory investigations showed a white blood cell count of 9,000/mm3, hemoglobin level of 13.2 g/dL, platelet count of 195,000/mm3, and a C-reactive protein (CRP) level of 10 mg/L (reference range <6 mg/L).
Standard radiographic imaging of the right femur revealed hypertrophy with heterogeneous remodeling of the bone, accompanied by periosteal reaction, distal metaphyseal osteolysis, and the presence of bony sequestrum. Computed tomography of the right thigh demonstrated sclerotic remodeling of the distal femur, a central bony sequestrum, periosteal reaction, and a 50 × 30 mm soft tissue abscess associated with a fistulous tract. Magnetic resonance imaging confirmed chronic distal femoral osteomyelitis with three fistulous tracts extending over 197 mm, a bony sequestrum measuring 58 × 8 mm, and a soft tissue abscess measuring 60 × 20 mm ( Figures 1 and 2).

Coronal (A) and axial (B) T2-weighted MRI of the femur and knee joints demonstrate an extensive inflammatory process in the distal right femur. Findings include thickening and diffuse edema of muscles and soft tissues (yellow arrows), a soft tissue collection (black arrow), fistulous tracts (orange arrows), diffuse cortical thickening (blue arrows), and a large intramedullary collection with a necrotic center (green arrows) containing a bony sequestrum (red arrows).
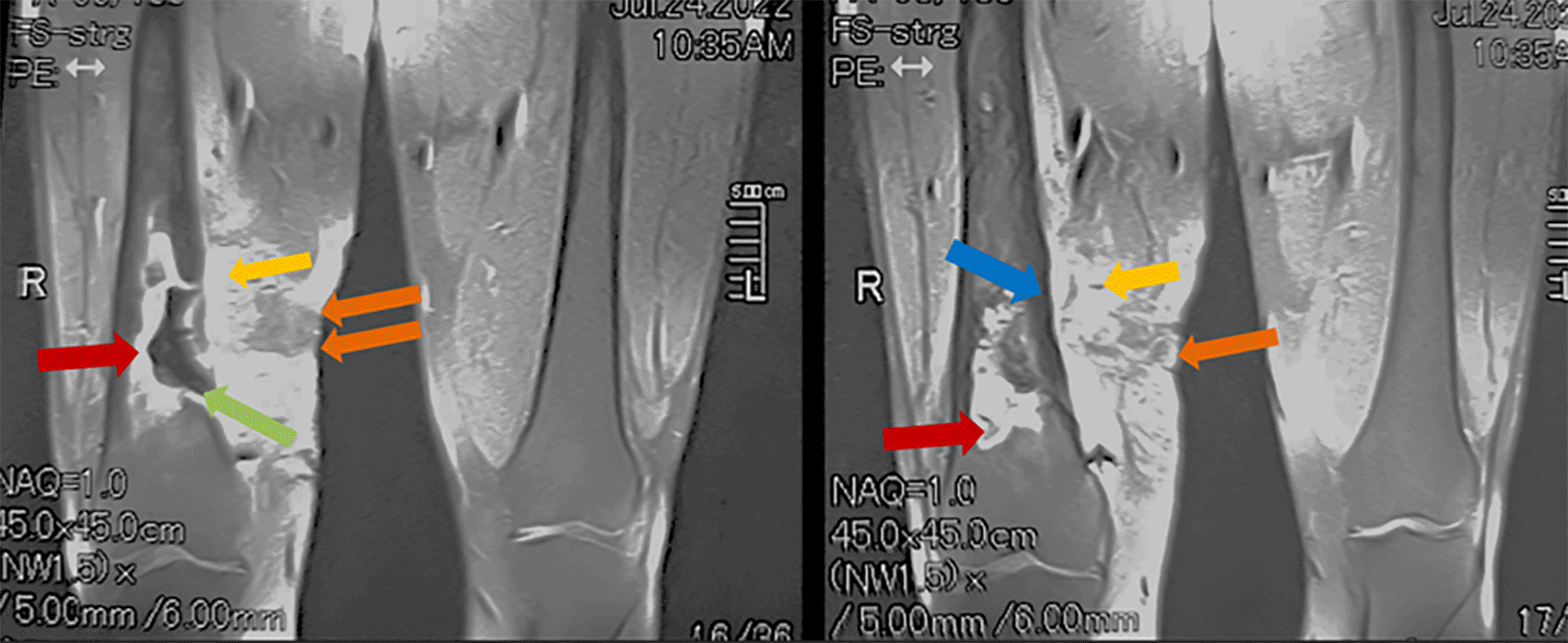
Findings reveal a significant inflammatory process in the distal right femur characterized by heterogeneous enhancement. Notable features include thickening and diffuse edema of the muscles and soft tissues (yellow arrows), the presence of fistulous tracts (orange arrows), diffuse cortical thickening (blue arrow), and a large intramedullary collection with a necrotic center (green arrow) and peripheral enhancement (red arrows).
A diagnosis of stage III A chronic osteomyelitis was established according to the Cierny-Mader classification, complicated by a soft tissue abscess.
A 14-day therapeutic window was implemented during which two aspirates were collected from the fistulae. Following a 24-hour incubation period under conditions of 37°C and a 5% CO2-enriched atmosphere on blood agar, both specimens yielded growth of small white non-hemolytic colonies. Preliminary biochemical analysis revealed that the isolates were catalase-positive but tube coagulase-negative. Microbiological identification and antimicrobial susceptibility testing were performed using standardized phenotypic methods with the MicroScan WalkAway 40 Plus system (Beckman Coulter, Brea, CA, USA), confirming the presence of coagulase-negative methicillin-resistant S. hyicus. To ensure diagnostic accuracy, species-level identification was further corroborated through proteomic analysis utilizing Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS, Bruker Daltonics, Bremen, Germany).
Upon identification of the isolated microorganism, the patient was re-interviewed and reported close contact with pigs.
The therapeutic approach comprised an integrated medical and surgical strategy. Surgical management entailed reopening the previous incision, resection of the fistulous tracts, excision of necrotic and infected tissues, diaphyseal trepanation, extensive irrigation and debridement, and the utilization of gentamicin-loaded ceramic carriers for effective dead space management.
Intraoperative microbiological cultures, including three bone and two pus samples, confirmed the presence of coagulase-negative methicillin-resistant S. hyicus.
The pathology results of the bone biopsy showed chronic plasma cell-dominant infiltrates, accompanied by fibrosis and sequestration of necrotic bone.
Postoperatively, the patient received intravenous teicoplanin (loading dose and maintenance regimen) and ciprofloxacin for 14 days, followed by oral ciprofloxacin (750 mg twice daily) and cotrimoxazole (960 mg three times daily) for a total antibiotic therapy duration of 24 weeks.
The patient demonstrated a favorable clinical outcome, with sustained remission and no recurrence of septic relapses after 2.5 years of follow-up.
S. hyicus is predominantly recognized as a zoonotic pathogen. Its primary clinical manifestation is exudative epidermitis in swine.1,8 Additionally, S. hyicus has been implicated in a variety of other infections across species, including subclinical mastitis in cows and sows, metritis in sows, arthritis in horses and chickens, ophthalmic infections in poultry, and, on rare occasions, osteomyelitis.3,9–12
Human infections typically arise from repeated contact with farm animals or occupational exposure to livestock, such as in farmers and veterinarians.3,4 Its rarity in human pathology necessitates a heightened index of suspicion to ensure accurate diagnosis.2 To date, only six cases of human infection caused by S. hyicus have been documented in the literature, with detailed clinical descriptions available for four.2–7
This case of chronic osteomyelitis of long bones caused by methicillin-resistant S. hyicus represents a novel occurrence and underscores several critical points for clinical practice and research.
Consistent with prior reports, our case underscores the ability of S. hyicus to cause severe infections in humans, highlighting recurring challenges in timely and accurate diagnosis. Kirk et al. described the first reported case of S. hyicus induced infective endocarditis in a sheep-shearer, notable for its destructive progression requiring mitral annular reconstruction.2 Foissac et al. reported an immunocompetent male with livestock exposure who developed spondylodiscitis and bacteremia due to S. hyicus.3 Another case involved a farmer with bacteremia in which S. hyicus was initially misidentified as Staphylococcus aureus, underscoring the diagnostic challenges posed by this pathogen.4 Furthermore, a wound infection attributed to S. hyicus was reported following a donkey bite.7
S. hyicus is a Gram-positive, aerobic, cluster-forming cocci that is non-hemolytic and typically appears white when cultured. It is a coagulase-variable species, including both coagulase-positive and coagulase-negative isolates. This bacterium expresses several virulence factors, including coagulase, lipase, and a homolog of the immunoglobulin G-binding protein (staphylococcal protein A).2,8 Additionally, it produces exfoliative toxins (ExhA, ExhB, ExhC, ExhD) responsible for cleaving desmoglein-1, leading to epidermal cell adhesion loss.2,13 However, human desmoglein-1 is resistant to these toxins.4
Diagnostic challenges are a recurring theme in S. hyicus infections, necessitating a multidisciplinary approach to avoid potential diagnostic pitfalls. Effective collaboration between clinicians and microbiologists is paramount in ensuring accurate diagnosis and management.4 Clinicians should meticulously gather the patient’s medical history, with particular attention to any documented exposure to animals, as highlighted in our case. Simultaneously, it is crucial to recognize the inherent limitations of traditional phenotypic diagnostic methods. The overlapping features of colony morphology and coagulase activity, combined with the limited resolution of biochemical characterization methods in differentiating closely related species or subspecies, often result in the misidentification of S. hyicus as Staphylococcus aureus or coagulase-negative staphylococci.2,4,14,15 This highlights the necessity of employing advanced complementary diagnostic techniques to achieve accurate identification, including molecular methods such as 16S rRNA gene sequencing and polymerase chain reaction (PCR), as well as MALDI-TOF MS.3,4,8,14 Specifically, PCR targeting genes such as sodA serves as a reliable approach for distinguishing toxigenic strains.8
S. hyicus exhibits significant antimicrobial resistance, with multidrug resistance observed in many strains. This resistance complicates treatment strategies further reinforcing the importance of early and accurate microbiological diagnosis.8,13
Comparatively to previous reports, this case adds a new dimension to the clinical understanding of this pathogen. Unlike infections described in earlier studies, chronic osteomyelitis of long bones represents a persistent and complex manifestation requiring multidisciplinary management. It is characterized by a persistent infection of the bone and marrow leading to bone destruction (sequestra) and new bone formation (involucra).16,17 It often arises from poorly managed acute osteomyelitis, open fractures, or implant-related infections.17–19 In adults, trauma involving open fractures is a primary etiological factor, while hematogenous spread is more prevalent in patients with diabetes or immunosuppression.17,18 The tibia and femur are the most frequently affected bones, with males and individuals aged 35–50 years exhibiting higher prevalence rates.19
Clinically, it manifests as persistent pain, swelling, and purulent discharge from sinus tracts.16,19 It can be associated with systemic symptoms like fever, especially in cases with acute exacerbations.20 If untreated, it can lead to significant disability and chronic pain.16,19 Symptoms are often nonspecific, necessitating a multimodal diagnostic approach.16,19
Plain radiographs (X-rays) are the first-line imaging modality for chronic osteomyelitis, though they lack sensitivity for early-stage disease.21 In contrast, magnetic resonance imaging (MRI), with its superior soft tissue contrast and heightened sensitivity, is the preferred modality for early detection and comprehensive assessment of osteomyelitis, enabling detailed visualization of bone marrow edema, abscesses, soft tissue involvement, bony sequestrum, sinus tracts, and both intramedullary and extracompartmental disease.21
The identification of the causative microorganism is fundamental to the effective management of chronic osteomyelitis. Cultures obtained from bone debridement during surgery remain the most reliable method for organism identification, whereas sinus discharge cultures, especially for anaerobic bacteria, are less dependable.19 Chronic osteomyelitis is predominantly caused by pyogenic bacterial infections, with S. aureus being the primary pathogen.22 However, Gram-negative bacilli such as Pseudomonas aeruginosa, and enterococci also play significant roles depending on geographic and clinical factors. Anaerobic bacteria are particularly relevant in prolonged infections.19 Many cases involve polymicrobial infections, complicating both diagnosis and treatment, particularly in post-traumatic or post-surgical settings.19 The emergence of multi-drug-resistant organisms further challenges the management of chronic osteomyelitis.23 Persistent infections are frequently due to the same bacterial species, with S. aureus, coagulase-negative staphylococci, and P. aeruginosa being notable for their persistence.24 The recurrent relapses observed in the present case may be attributed to staphylococcal mechanisms of persistence within bone tissue, including biofilm formation, intracellular persistence, antimicrobial resistance, immune evasion, and adaptation to host environment.25,26 These pathogenic mechanisms are likely relevant to S. hyicus, potentially accounting for the chronicity and recurrence of infection in this patient.
Chronic osteomyelitis treatment requires a multidisciplinary approach, including surgical debridement and antimicrobial therapy. Antibiotic management traditionally involves 6 to 12 weeks of therapy, with an initial intravenous phase of 2 to 6 weeks.16,27 Recent evidence, however, supports shorter regimens of 4 to 6 weeks, showing comparable efficacy to longer courses.16,27 Oral antibiotics, when pathogen susceptibility is confirmed, are increasingly favored for their comparable effectiveness and advantages in cost, convenience, and reduced complications.28 Therapy often targets S. aureus, the predominant pathogen, with combination regimens such as rifampin being explored, though evidence of superiority remains limited.16 The emergence of methicillin-resistant strains, as observed in the present case, poses significant therapeutic challenges. In this instance, a tailored regimen achieved sustained remission, underscoring the significance of personalized treatment strategies.
Surgical debridement remains essential, facilitating the removal of necrotic tissue and reducing bacterial load. Recent studies challenge the necessity of wide tumor-like resections, advocating for adequate debridement combined with local antibiotic delivery as an effective alternative.29 For complex cases, such as Cierny-Mader type IV osteomyelitis, bone and soft tissue reconstructive techniques are often necessary.29
Future research should focus on epidemiology, virulence mechanisms, and resistance patterns of S. hyicus to better inform clinical practice. Furthermore, collaborative studies exploring zoonotic pathogen surveillance and antimicrobial stewardship in agricultural settings are essential to address the broader implications of this emerging public health concern.
This case represents the first documented instance of chronic osteomyelitis of long bones caused by methicillin-resistant S. hyicus in a human, expanding the known clinical spectrum of this zoonotic pathogen. The diagnostic complexity of S. hyicus, stemming from its phenotypic similarity to other staphylococcal species, underscores the critical role of integrative molecular and proteomic methodologies in the accurate identification of suspected zoonotic infections. Furthermore, this case highlights the zoonotic potential of S. hyicus, emphasizing the need for increased vigilance among clinicians, particularly in patients with a history of livestock exposure. Incorporating S. hyicus into the differential diagnosis of chronic osteomyelitis could facilitate prompt targeted therapeutic strategies and ultimately improve patient outcomes.
HE: Conceptualization, Investigation, Methodology, Writing – Original Draft, Writing – Review & Editing. SK: Investigation, Methodology, Writing – Original Draft. SB: Methodology, Writing – Original Draft. SS: Investigation, Methodology. AT: Investigation, Methodology. AB: Investigation. AZ: Investigation. SD: Writing – Review & Editing. SBB: Writing – Review & Editing. IK: Writing – Review & Editing.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Microbiology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 18 Feb 25 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)