Keywords
Multidrug-resistant pathogens; Antibiotic resistance; Citrullus colocynthis; Antibacterial activity; Fatty acid metabolism; GC-MS/MS; Climate change adaptation.
The rise of multidrug-resistant pathogens and emerging new microbes due to climate change highlight the urgent need for alternative antimicrobials. Plants, as they adapt to environmental shifts, produce diverse metabolites with potent antibacterial properties, offering a sustainable source to combat antibiotic resistance and emerging microbial threats. In this context, Citrullus colocynthis fruits, known for their antimicrobial activity and adaptation to the hot Gulf region, were screened to identify accessions with strong antibacterial activity and distinct metabolic profiles.
The rinds and pulps of three C. colocynthis accessions were screened for their effectiveness against Staphylococcus aureus and Escherichia coli. Minimum inhibitory concentration (MIC50) tests determined activity levels, and GC-MS/MS metabolic profiling analyzed the chemical composition of rind and pulp extracts. Enrichment and network analyses were performed to identify metabolic pathways and potential bacterial targets.
Rind extracts demonstrated stronger antibacterial activity than pulp, with accession S2 showing the highest activity against S. aureus (MIC50 = 15.74 μg/ml), outperforming other accessions. Metabolic profiling revealed distinct metabolite clusters between rind and pulp, with the rind containing unique compounds like butyric acid, α-linolenic acid, and β-sitosterol. Enrichment analysis indicated that unsaturated fatty acid biosynthesis and other fatty acid metabolism pathways were enriched in the accession S2 rind, supporting its antibacterial potency. Network analysis pinpointed bacterial fatty acid synthase enzymes (FabZ, FabI, and FabH) as potential S. aureus targets of C. colocynthis rind fatty acids.
The distinct metabolic profiles and strong antibacterial activity of C. colocynthis rind, especially in accession S2, underscore its potential as a sustainable source for plant-based nutraceuticals. Its unique adaptability and antimicrobial properties present a promising strategy for combating multidrug-resistant pathogens.
Multidrug-resistant pathogens; Antibiotic resistance; Citrullus colocynthis; Antibacterial activity; Fatty acid metabolism; GC-MS/MS; Climate change adaptation.
Antimicrobial resistance (AMR) has become a critical global health threat, necessitating urgent prioritization of research and intervention efforts.1 The 2024 WHO Bacterial Priority Pathogens List (BPPL) ranks antibiotic-resistant pathogens into high-priority pathogens, like methicillin-resistant Staphylococcus aureus (MRSA) and multidrug-resistant Neisseria gonorrhoeae, present major challenges in healthcare and community settings.1 In the Gulf Cooperation Council (GCC) region, MRSA, extended-spectrum beta-lactamase (ESBL)-resistant Enterobacteriaceae, and vancomycin-resistant Enterococcus faecium are especially concerning.2 In Bahrain, ESBL-resistant Enterobacteriaceae ranks highest, followed by MRSA, reflecting the region's high prevalence of multidrug-resistant pathogens.2 The United Arab Emirates (UAE) faces a significant burden from priority pathogen infections and a worrisome rise in antibiotic resistance.3 The top five reported pathogens in the UAE include Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Candida spp., and Pseudomonas aeruginosa.3 Among these, S. aureus and E. coli present major public health threats, causing infections ranging from skin conditions to severe bloodstream infections.4 The increasing prevalence of antibiotic-resistant strains, such as MRSA, is particularly alarming due to the challenges they pose in treatment and the severe health risks they carry.5 Moreover, the UAE's hot climate contributes to the spread of microbial diseases, with high humidity and temperatures promoting bacterial growth and transmission.6 Recent research shows that rising local temperatures are linked to increased antibiotic resistance in pathogens, with a 10°C rise correlating with 4.2% and 3.6% increases in resistance for E. coli and S. aureus, respectively.6 This underscores the need for novel approaches to mitigate AMR in regions like the UAE.
The escalating global antimicrobial resistance (AMR) poses a dire threat to public health, diminishing the effectiveness of traditional antibiotics and leaving infections increasingly untreatable.1 This alarming trend demands urgent innovation, particularly in exploring novel plant-based antimicrobials as sustainable and effective alternatives.7 Given the alarming rise in AMR, Citrullus colocynthis stands out as a native Gulf plant with promising potential due to its unique secondary metabolites, which have demonstrated antimicrobial properties against resistant pathogens like S. aureus.8,9 Harnessing bioactive compounds from plants reduces dependence on synthetic drugs, slowing resistance development. With lower toxicity and eco-friendly production, plant-based solutions offer a vital strategy to combat antimicrobial resistance (AMR) and protect future generations from this growing threat.7,10
Climatic changes, particularly shifts in temperature, significantly impact the production of plant metabolites, including those with antimicrobial properties.11,12 Plants adapt to these environmental stressors by altering their metabolic pathways, often increasing the production of secondary metabolites that serve protective functions.13 For example, elevated temperatures and CO2 levels have been shown to enhance the accumulation of bioactive compounds such as phenylpropanoids, alkaloids, and terpenoids, which are known for their antimicrobial properties.14,15 These compounds not only help plants survive under harsh conditions but also exhibit strong antimicrobial activities, making them a potential source for novel treatments against resistant pathogens.16 Additionally, climate-induced stress enhances the plant’s ability to produce these antimicrobial metabolites, highlighting the interconnection between environmental changes and plant resilience.17 This adaptive response positions plants as a sustainable source of bioactive compounds, offering valuable solutions in addressing the rising issue of antimicrobial resistance.
Citrullus colocynthis, a native plant of the Gulf region, produces a wide range of secondary metabolites, including phenolics, flavonoids, and essential oils, which exhibit potent antibacterial, antifungal, and antioxidant activities.18,19 However, its reported antimicrobial activity varies across studies, depending on various factors such as environmental conditions and genetic variability.20,21 The bioactive compounds of C. colocynthis are known to be influenced by environmental factors such as temperature, the specific fruit part, and the particular plant accession being studied. Previous studies on C. colocynthis have shown promising antimicrobial properties but have not systematically examined the interplay between genetic variability, environmental conditions, and specific fruit parts.22,23 This study seeks to bridge this gap by identifying accessions with superior antimicrobial profiles.
Our earlier investigations demonstrated that plants growing in the extreme desert conditions of the UAE exhibited significant variations in their total phenolic content and antioxidant activities depending on the fruit part analyzed, whether rind, pulp, or seed. Notably, the rinds and pulps of C. colocynthis fruits contained higher phenolic content than seeds, especially in fruits harvested during the summer. Furthermore, the antioxidant and phenolic content were higher in some accessions compared to others, highlighting the genetic influence on metabolite production in this species.24 This variability underscores the necessity of further investigating the antimicrobial properties of the rinds and pulps of fruits collected in summer from different accessions. The higher phenolic content and unique metabolites found in these fruit parts, particularly under harsh environmental conditions of summer, highlight C. colocynthis as a promising source of antimicrobial agents.18 We hypothesize that the unique metabolic adaptations of C. colocynthis accessions to the UAE's extreme climate not only enhance their antimicrobial activity but also provide a blueprint for developing plant-based solutions to AMR. By identifying the specific metabolites and their pathways, this study aims to open new avenues for sustainable pharmaceutical applications. Therefore, the present study aimed at examining the variation in antimicrobial activities and metabolic profiles of the rind and pulp from different C. colocynthis accessions grew during the summer. By focusing on S. aureus and E. colimodel pathogens representing Gram-positive and Gram-negative bacteria, respectively we aim to evaluate the antimicrobial spectrum of C. colocynthis metabolites robustly. This approach is expected to identify accessions with superior antimicrobial activity, which can then be optimized for further applications.
Fresh Citrullus colocynthis fruits were collected from three accessions (numbers 6, 10, and 13 reported in Al-Nablsi et al.16 from the desert of Al Faya, Sharjah Emirate, UAE (25.051065° N 55.795627° E). Our previous study revealed significant genetic, morphological, and seed dormancy variations among these accessions.24 Accession 13 displayed larger fruit size, heavier seeds, and lower seed dormancy, resulting in faster germination, particularly under higher temperatures. In contrast, accessions 6 and 10 exhibited smaller fruits, lighter seeds, and higher seed dormancy.24 In this study, the same three accessions are referred to as S1, S2, and S3 for accessions 6, 10, and 13, respectively. Three distinct individuals representing each accession were collected from a single population in September 2019 ( Figure 1). The collected fruits were washed and separated into pulp, rind, and seeds. Each part was dried separately in the shade at room temperature for two weeks, ground into a fine powder using a laboratory blender, and stored at room temperature prior to antibacterial analysis.
Metabolic extraction was performed according to the previously reported method,24 breiefly100 mg of air-dried rinds and pulps samples from three accessions of C. colocynthis fruits were extracted by soaking in 1 ml of absolute methanol overnight, followed by water bath sonication at RT for 2 h. The extracts were filtered using Whatman No.1 filter paper (Whatman International Ltd., England), dried under a vacuum, dissolved in DMSO, and stored in the fridge until used for biological screening.
To identify the antibacterial activity of C. colocynthis fruit extracts, a spotting assay was conducted to screen the antibacterial activity of C. colocynthis fruit extracts of the pulp and rind of the three accessions S1, S2, and S3. The extract solutions at 10 mg/ml were employed. Each extract was tested on both Gram-positive S. aureus (ATCC 29213) and Gram-negative E. coli (ATCC 25922). A standard inoculum of the testing bacteria was prepared in 0.9% saline solution from a 24 h agar plate using the direct colony suspension method. The inoculum optical density was adjusted using dens check device to give a 1 × 108 CFU/ml concentration equivalent to 0.5 McFarland standard solution. The standard inoculum was further diluted to give a final bacterial concentration of 5 × 105 CFU/ml, following the Clinical and Laboratory Standards Institute (CLSI) guidelines.25 Nearly 5 μl of each prepared extract was spotted on the streaked agar plate, in addition to positive and negative controls: Colistin (0.7 μg/ml), Vancomycin (3 μg/ml), and DMSO (100%), respectively. The plates were incubated at 37oC, and the results were observed and recorded after 24 h.
The broth microdilution method using a 96-well microplate was performed to measure the minimal inhibitory concentration (MIC) that inhibits visible bacterial growth. Around 100 μl of bacterial cultures in Muller-Hinton Broth (MHB) was added to each well of a 96-well plate. Then 100 μl of each prepared plant extract were added to each well at different concentrations (0, 50, 100, 200, 300 μg/ml). DMSO and Vancomycin were employed as negative and positive controls, respectively. The plate was sealed by parafilm to avoid the evaporation of the solution, and incubated overnight at 37 °C. The bacterial growth was measured using a microplate reader (Epoch TM 2 Microplate spectrophotometer, BioTek Instruments, Inc., Winooski, VT USA) at OD570. Excel and GraphPad prism software (La Jolla, CA, USA, version 9, https://www.graphpad.com) was used to analyze the data obtained from the microplate reader.
The dried extract was treated with 20 μL of 20 mg/ml methoxyamine in pyridine and 50 μL of hexane. The mixture was vortexed and incubated in a water bath at 37 °C for 1.5 h.26,27 Following this, 90 μL of N-trimethylsilyl- N-methyl trifluoroacetamide and trimethylchlorosilane (MSTFA + 1% TMS) were added, and the samples were vortexed for 30 sec and further incubated in a water bath at 37 °C for 1 h. The resulting solution was filtered using 0.45 μm syringe filters (nylon syringe filter, Membrane Solutions, Auburn, WA, USA) and subjected to GC-MS/MS analysis using multiple reaction monitoring (MRM).28,29 The samples were incubated at 330 minutes and then analysed using GC-MS, following a method previously described.30 GC-MS analysis was conducted using a Shimadzu QP2010 GC-2010 coupled with a GC-MS QP-2010 Ultra and equipped with an auto-sampler (AOC-20i + s). A Restek Rtx-5 ms column was used, with helium as the carrier gas. The analysis temperature was initially set at 35°C for 2 min, followed by an increase of 10°C/min until reaching 250°C. Subsequently, the temperature was increased by 20°C/min until reaching 320°C and held for 23 min. The injection volume and temperature were 1 μl and 250°C, respectively, using splitless-injection mode. The mass spectrometer operated in electron compact mode with an electron energy of 70 eV. The ion source and interface temperatures were set at 240°C and 250°C, respectively. The MS mode was set to scan mode 35–450 m/z with a scan speed of 1428. Spectral data were interpreted using the NIST 14 Mass Spectral Library.30
The antimicrobial metabolites were imported into MetaboAnalyst (v.5.0), available a (https://www.metaboanalyst.ca/home.xhtml) for further analysis. Hierarchical cluster analysis (HCA), partial least squares-discriminant analysis (PLS-DA), and metabolite set enrichment analysis were conducted.31
The STRING (Search Tool for the Retrieval of Interacting Genes/Proteins) database (version 10.0) at http://string-db.org// was used to identify protein interaction involved in unique metabolic pathways in S. aureus. The search parameters were adjusted according to interaction types and confidence thresholds. The results comprising protein interaction networks were obtained and examined to find protein clusters associated with metabolic pathways. Additional research, including validation and functional enrichment analysis, verified the proteins involved in S. aureus metabolic activities.
The data were collected and analyzed using GraphPad Prism 9.0 (GraphPad Software, La Jolla, CA, USA, https://www.graphpad.com)). Statistical significance was measured using one-way ANOVA for measuring MIC of the plant extracts. Every test was conducted at least in three independent replicas, and the values were expressed as the mean ± standard error (SE) of the mean. The significance was adjusted at P < 0.05.
The antibacterial activity of different parts (rind and pulp) of C. colocynthis fruits collected in summer was assessed using spotting assay against the gram-positive S. aureus and gram-negative E. coli. Interestingly, rinds and pulp extracts showed positive effects against S. aureus ( Figure 2A), but not against E. coli ( Figure 2B). The best activity was mainly for the rind extract of samples 1, 3, 5, 7, 9, 11, 13, 15, and 17, while only sample 12 of the pulp from accession S2 showed inhibition against S. aureus. Selected samples that showed an inhibitory zone were selected for further microdilution assay to measure their MIC50 values. MIC50 value of accession S2 was 15.74 μg/ml, followed by the pulp of accession S2 with a MIC50 value of 165.6 μg/ml. On the other hand, other accessions showed lower activity with higher MIC50 values of 259 μg/ml for accession S3 and 462.8 μg/ml for accession 1 ( Table 1, Figure 3).
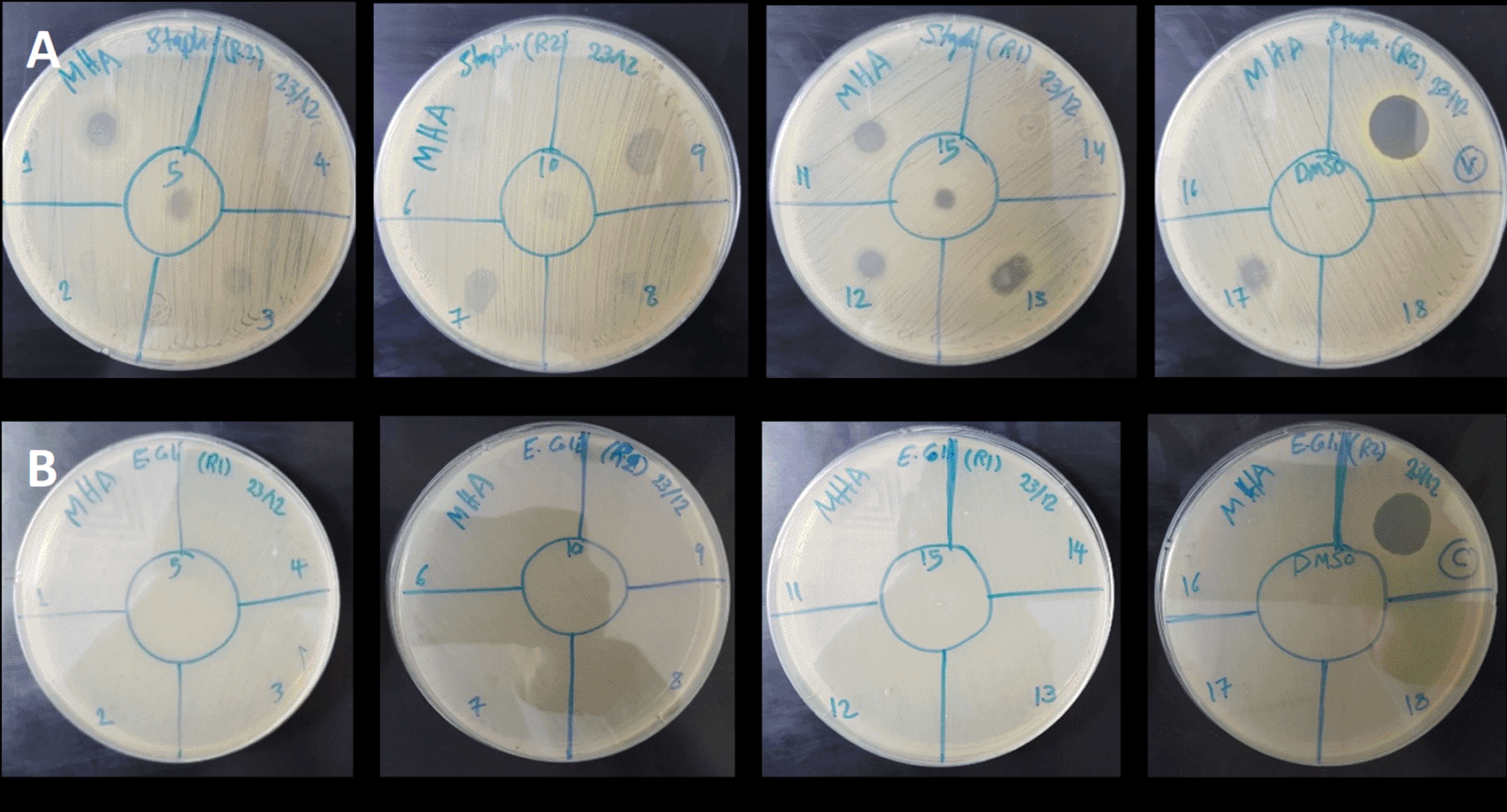
(A) S. aureus. (B) E . coli. Vancomycin and Colistin at 3 μg/ ml and 0.7 μg/ml, respectively and DMSO were employed as controls. Where sample codes are the following, 1: (S1/rind/R1), 2: (S1/pulp/R1), 3: (S1/rind/R2), 4: (S1/pulp/R2), 5: (S1/rind/R3), 6: (S1/Pulp/R3), 7: (S2/rind/R1), 8: (S2/pulp/R1), 9: (S2/rind/R2), 10: (S2/pulp/R2), 11: (S2/rind/R3), 12: (S2/pulp/R3), 13: (S3/rind/R1), 14: (S3/pulp/R1), 15: (S3/rind/R2), 16:(S3/pulp/R2), 17: (S3/rind/R3), and 18: (S3/pulp/R3).
| C. colocynthis accession/fruit parts | MIC50 |
|---|---|
| (S1/rind) | 462.8 ±0.06 |
| (S2/rind) | 15.74 ± 0.34 |
| (S2/pulp) | 165.6 ± 0.76 |
| (S3/rind) | 259.0 ± 0.85 |
Multivariate analyses, including hierarchical cluster analysis (HCA), were performed to assess the metabolic profile in different accessions and parts of C. colocynthis fruits. This analysis revealed two distinct clusters, one representing the rind and the other for the pulp ( Figure 4A). Additionally, partial least squares discriminant analysis (PLS-DA) was conducted to investigate the natural variations in metabolic traits among different accessions of C. colocynthis fruit rind and pulp as indicated in Figure 4B. The analysis identified distinct metabolic signatures, with the rind extract containing predominantly unique antimicrobial metabolites. These metabolites include 1-monolinolein, α-linolenic acid, dodecanoic acid, mandelic acid, dodecane acid, butanoic acid, β-tocopherol, 1-heptacosanol, 9,12-octadecadienoic acid, D-mannopyranose, β-sitosterol, D-glucitol, and palmitic acid. Conversely, only three unique metabolites were found exclusively in the pulp, namely D-(-)-tagatofuranose, D-fructose, and 1,3,5-cycloheptatriene ( Table 2).
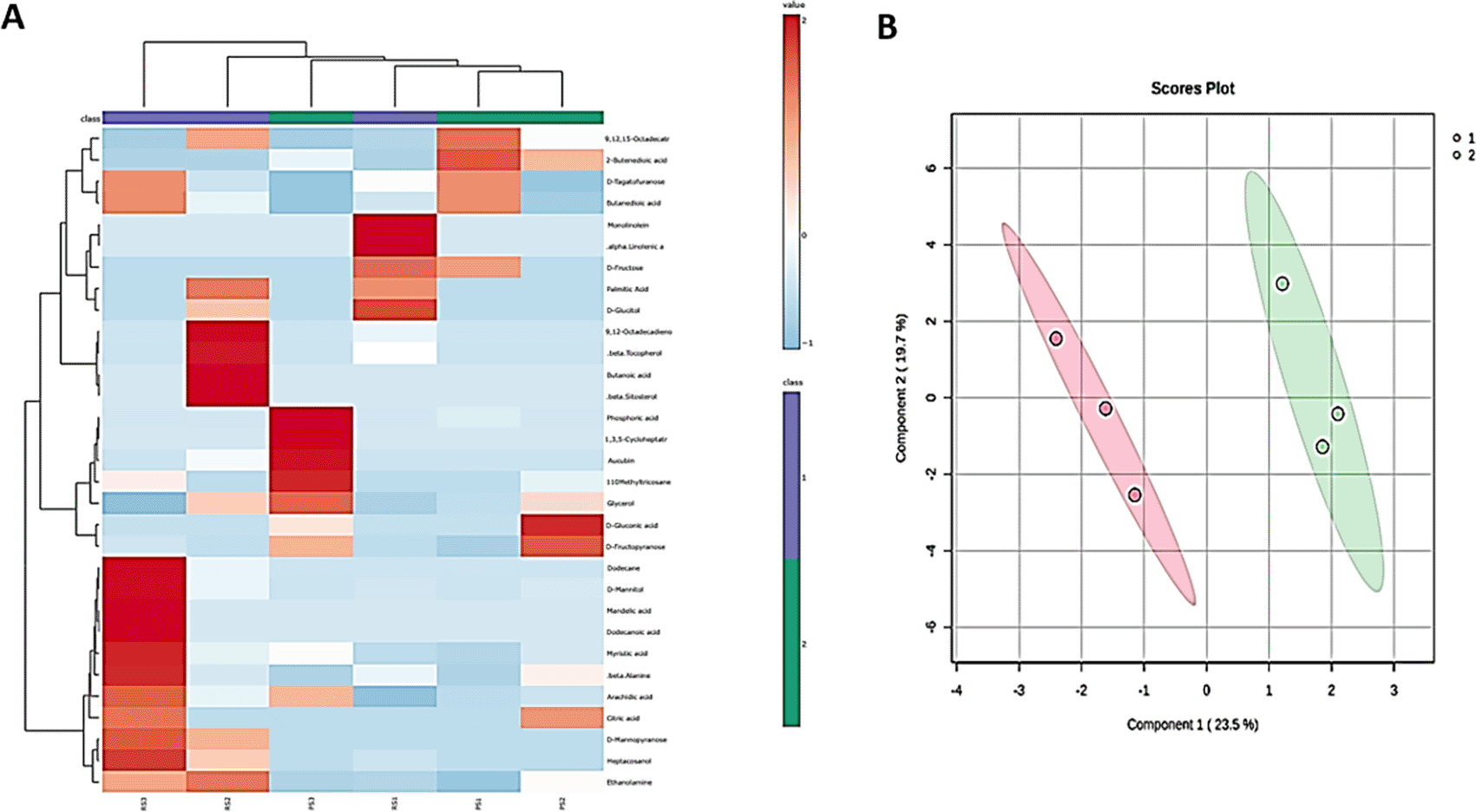
(A) Heat map analysis was conducted on the metabolic profiles of C. colocynthis fruit rind and pulp using GC-MS/MS analysis. Three rind accessions (RS1, RS2, RS3) and three pulp accessions (PS1, PS2, PS3) were included. The color scale ranging from red to blue indicated differences in metabolite expression, with red indicating high expression and blue indicating low expression. Mean percentage area values were calculated for each group. Euclidean distance was used to measure similarity. (B) PLS-DA was used to analyze all C. colocynthis fruit rind and pulp metabolites. 1 represents rind, while 2 represents pulp.
Means (% ± SD) were detected with the same letter with a metabolite do not differ significantly at P ≤ 0.05.
The rind of C. colocynthis showed a higher metabolic spectrum compared to the pulp. The rind of accession S2 displayed the highest number of antimicrobial metabolites compared to accessions S1 and S3, with 21, 15, and 18 metabolites, respectively. For the pulp, the numbers of compounds recorded in accessions S2, S1, and S3 were 14, 11, and 10, respectively ( Figure 5).
Heat map analysis indicated that the rind of accession S2 demonstrated a unique metabolic, represented by distinct metabolite clustering from the other accessions ( Figure 6). This unique metabolite profile can be represented by butanoic acid, β-sitosterol, and butenedioic acid. VIP metric was also used to determine the most important variables among the shared metabolites in the PLS-DA model, particularly in comparison to the other accessions. Accession S2 showed palmitic acid, ethanolamine, 9,12-octadecadienoic acid, and 9,12,15-octadecatrienoic acid as the highly expressed metabolites compared to other accessions ( Figure 7). These metabolites are of particular interest due to their potential antimicrobial activity.32
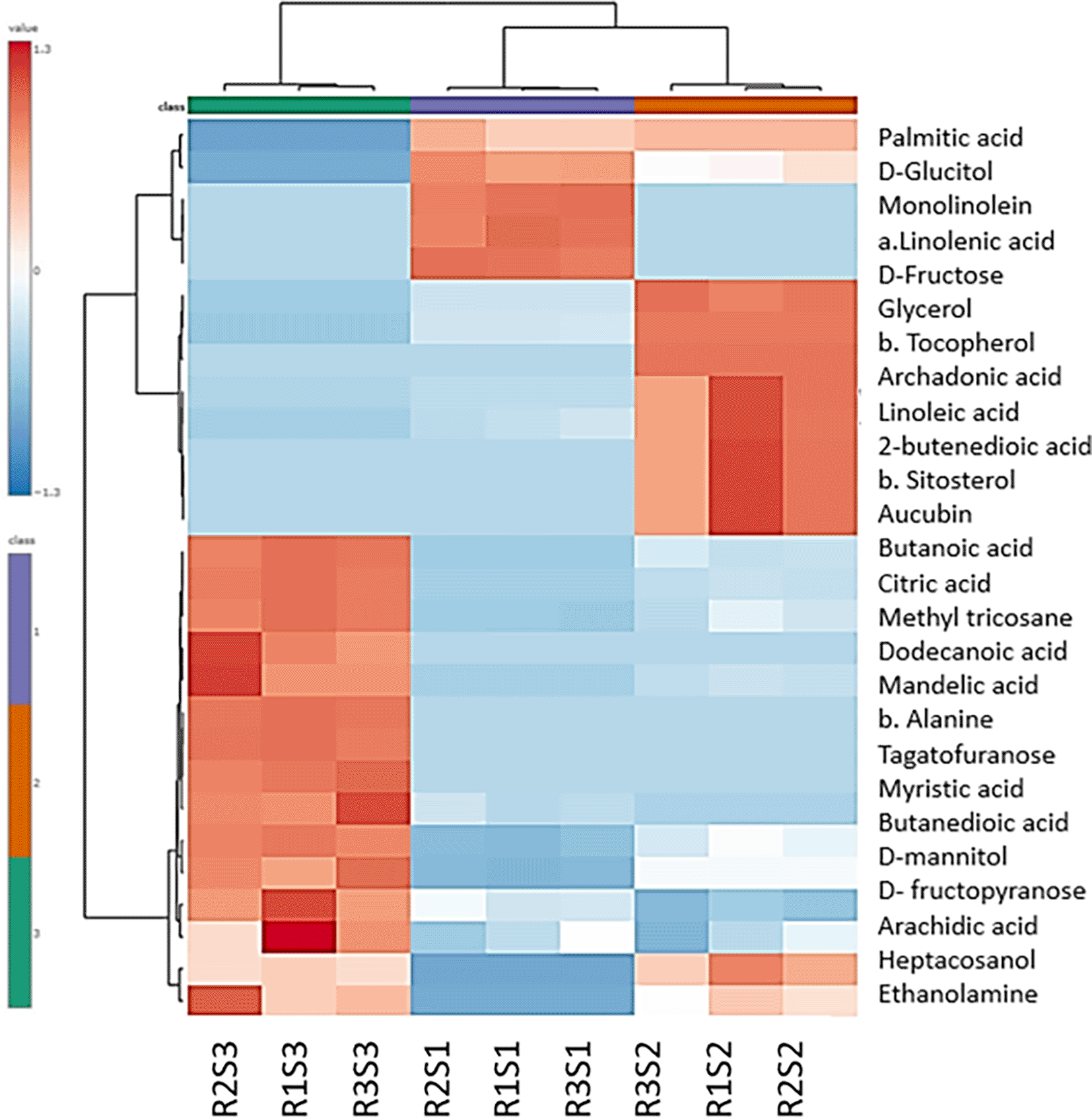
The color scale ranging from red to blue indicated differences in metabolite expression, with red indicating high expression and blue indicating low expression.
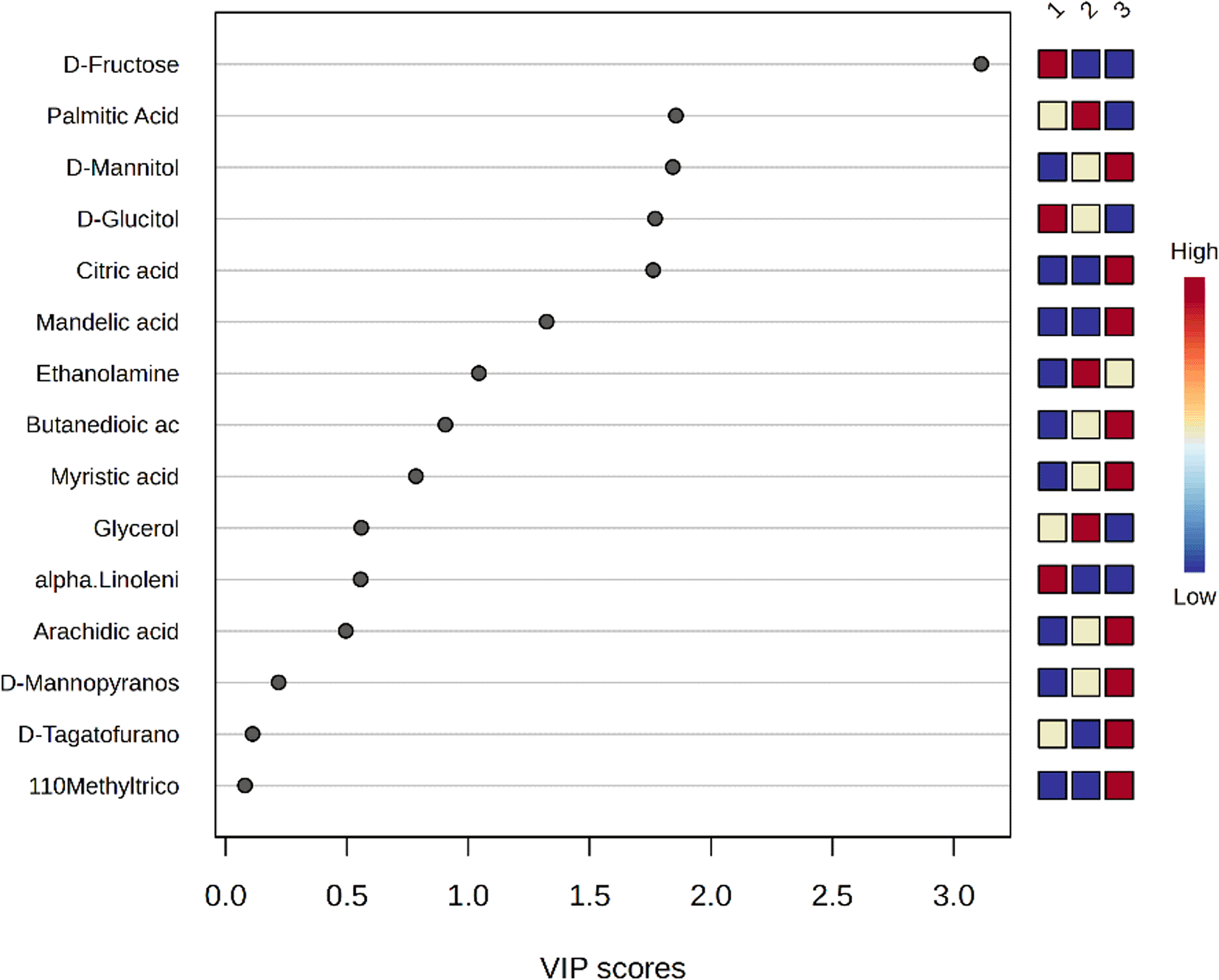
It wascalculated as the weighted sum of absolute regression coefficients. Where Sample 1; RS1, 2; RS2, 3; RS3 and the color-coded boxes on the right side indicate the relative concentrations of each metabolite in the studied groups, with red indicating high expression and blue indicating low expression.
The top enriched pathways of the identified unique metabolites are unsaturated fatty acid biosynthesis, fatty acid biosynthesis, linolenic acid metabolism and α-linolenic acid metabolism, elongation of fatty acid, and butyrate metabolism ( Figure 8). Fatty acid was reported to exhibit antimicrobial activity by targeting essential enzymes in bacterial survival.33
STRING, a website employed to identify molecular targets influenced by enriched pathways in different organisms,34 was employed here to identify S. aureus targets affected by C. colocynthis enriched pathways. The network analysis involved constructing a protein-protein interaction (PPI) network to reveal both known and predicted targets, highlighting crucial interactions with S. aureus proteins. The results pinpointed three bacterial enzymes, FabZ, FabI, and FabH, as probable targets for intervention ( Figure 9). These three enzymes are essential to the synthesis of fatty acids, a critical step in the metabolism of bacteria.35 Moreover, the bacterial FAS differs from mammalian FAS analogues providing a safety and selectivity profile.
The present study indicated that the antibacterial activity of C. colocynthis differed between different accessions, the fruit parts, and the tested microorganism. Several studies have shown variations in the antimicrobial activity of C. colocynthis extracts among different populations.21 It has been shown that the ethanol extract of C. colocynthis fruit from a Pakistan population exhibited inhibitory activity against Bacillus subtilis, but not against the Gram-negative bacteria E. coli and P. aeruginosa.19 It is reported that the ethanolic extract of C. colocynthis fruit extract from an Iranian population showed an inhibitory effect comparable to that of the antibiotic novobiocin.36 Additionally, in a Tunisian population, the aqueous fruit extracts demonstrated strong antimicrobial activity against Candida albicans, Candida glabrata, Escherichia coli, and Pseudomonas aeruginosa.37 This is consistent with prior research highlighting the variable antimicrobial efficacy of C. colocynthis extracts against diverse microorganisms.29,30 These findings underscore the influence of genetic and environmental factors on the bioactive potential of C. colocynthis, supporting the need for studies like this one that systematically examine accessions adapted to extreme environments like the UAE.
This study also showed greater antibacterial activity for the fruit rinds rather than the pulps. The rinds of the three accessions showed significant growth inhibition against S. aureus, while only the pulp of one accession, S2, showed such inhibition. Similarly, the methanolic extract of dried fruit pulp of C. colocynthis was assessed and showed no effects against thirty bacterial isolates (10 Gram-positive and 20 Gram-negative) and five fungal species.38 On the other hand, antimicrobial activities of fruit rind, pulp, and seeds against S. aureus were reported,39 with greater activity in the pulp. This robust antibacterial activity suggests that C. colocynthis could be a valuable source of natural antimicrobial compounds that may eventually replace synthetic derivatives.30 The metabolic distinctions between rinds and pulps may reflect tissue-specific roles in plant defense mechanisms, offering insights into the evolutionary adaptation of C. colocynthis to its harsh environment. Future studies should explore the biochemical pathways that underlie this tissue-specific bioactivity.
The fruits collected in the summer exerted effects on the Gram-positive S. aureus but not on the Gram-negative E. coli. In consistence, Arora et al., 1999, showed that the antibacterial activity of C. colocynthis fruit extracts exhibited more pronounced growth inhibition against S. aureus and minimum effect on E. coli.40,41 Similarly, it has been reported that ethanolic extracts of C. colocynthis fruits were more effective against S. aureus than E. coli.42 The same authors also observed that the inhibitory effect of ethanolic extract was higher than water extract, and the ethyl acetate extract showed greater antibacterial activity against S. aureus than E. coli.43 This differential efficacy highlights the potential specificity of C. colocynthis metabolites toward Gram-positive bacterial cell wall structures. This specificity could serve as a focus for developing targeted antibacterial therapies.
The highest antibacterial activity of the rind of accession S2 can be attributed to the existence of unique metabolites, in addition to the predominance of other metabolites reported with antimicrobial activity ( Table 2). These metabolites include butyric acid, α-linolenic acid and other unsaturated fatty acids such as 9,12-octadecadienoic acid and 9,12,15-octadecatrienoic acid, which could potentially serve as biomarkers for strong antibacterial activity. Butyric acid, a short-chain fatty acid, has been shown to significantly suppress the growth of S. aureus in vivo and in vitro.44 The butyric acid derivative has also shown the ability to stimulate the production of pro-inflammatory interleukin (IL)-6 and drastically diminish S. aureus colonization.45 This emphasizes the potential of butyrate derivatives as anti-inflammatory agents with bactericidal action against S. aureus.45 Additionally, recent research underscores the influence of α-linolenic acid metabolite on fatty acid (FA) biosynthesis in S. aureus, indicating that it primarily targets the FA biosynthesis pathway, particularly the FabI enzyme in S. aureus.46
These unsaturated fatty acid metabolites have been reported for their antimicrobial effects.47,48 9,12-Octadecadienoic acid has demonstrated significant antimicrobial activity against multidrug-resistant S. aureus (MRSA) with potent anti-inflammatory properties.47,49 Similarly, 9,12,15-octadecatrienoic acid is known for its antimicrobial and anti-inflammatory activity.50 Additionally, they are linked to the enzymes involved in fatty acid synthesis (FAS)51 and act as regulators or substrates of these enzymes; hence, they can affect the rate and composition of fatty acid synthesis within the bacterial cells.52 Consequently, these metabolites could serve as valuable biomarkers for identifying accessions with strong antibacterial activity, offering potential for pharmaceutical applications, particularly in topical treatments and managing chronic infections caused by Gram-positive bacteria.
Pathway analysis indicated the enrichment of unsaturated fatty acid biosynthesis and other fatty acid metabolism in the fruit rind. This suggests that fatty acid biosynthesis pathways may play a crucial role in the antimicrobial activity of C. colocynthis, possibly by targeting essential bacterial enzymes such as FabZ, FabI, and FabH.53 The identification of these enzymes as potential targets against S. aureus provides a molecular basis for developing novel antimicrobials.54 Targeting bacterial fatty acid synthesis pathways is effective in inhibiting bacterial growth.55 Interestingly, the bacterial fatty acid biosynthesis pathway differs from its mammalian counterpart, making it a selective and safe target for therapeutic intervention.56,57 This property enhances the potential clinical applicability of the metabolites identified in this study.
This study revealed that some individuals of the C. colocynthis population adapted to the hot climate of the UAE and developed a promising selective antibacterial activity by expressing unique metabolite profile. Improving Citrullus plants' adaptability to hot temperatures and antibacterial capabilities could have significant consequences in the pharmaceutical industry and nutraceuticals. Comprehending the genetic and external factors that impact the production of antimicrobial secondary metabolites is essential; direct breeding and selection initiatives towards cultivating genotypes with enhanced antibacterial capabilities could be the strategy of choice.58 Techniques like metabolic engineering and genetic engineering can also be used to manipulate key pathways involved in the synthesis of antimicrobial metabolites like fatty acids.59 Moreover, the potential synergy between these plant metabolites and conventional antibiotics warrants exploration, as such combinations may help overcome existing drug resistance mechanisms.
Investigating the role of the plant microbiome in boosting antimicrobial activity may provide new approaches to boosting Citrullus plants' antibacterial capabilities.60 A comprehensive strategy combining genetic, environmental, and cultural strategies is required to enhance the antibacterial capabilities of Citrullus plants and accomplish their full potential, while adapting to the hot climate. Additionally, studies on the stability and bioavailability of these metabolites under various conditions could pave the way for their practical application in drug formulation. These developments may eventually result in novel plant-based therapies for combating drug-resistant pathogens.
This study highlights the potential of C. colocynthis as a source of antibacterial agents, particularly the rind. The unique antibacterial activity and metabolic profile of accession S2 suggest that it can serve as a promising candidate for further investigation and optimization to develop a plant variety that can tolerate the hot environment while exerting valuable pharmaceutical potential. The findings underscore the importance of environmental and genetic factors in shaping the bioactive potential of C. colocynthis metabolites.
Conceptualization, A.E., S.S.; methodology, S.A., S. H.; software, R.H.; validation, A. E, S.S.; formal analysis, S. A., R. H, and S.H.; resources, F.L.R.; data curation, S. A, A.E., S.S., R.H.; writing original draft preparation, S.A; writing—review and editing, A.E. S.S., R.H; supervision, A.E., S.S.; project administration, A.E., S.S., and F.L.R.; funding acquisition F.L.R.
Figshare: Prioritizing Citrullus colocynthis accessions and fruit parts with promising antibacterial activity and adaption to the hot climate of the UAE for the future pharmaceutical industry, DOI: https://doi.org/10.6084/m9.figshare.25125647.v2
The project contains the following underlying data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The project includes the file GC-MS_Data.xlsx, which contains processed GC-MS, Number of metabolites, MIC analysis data, number of metabolites, including peak intensities and identified metabolites.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Extractions and relevant biological activities
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Valorization of Food and dairy by- products
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 10 Mar 25 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)