Keywords
nanoplasctic, toxicology, diseases, food and feed, ingestion
The extensive use of plastic without an effective management system is linked to significant environmental pollution issues. The fragmentation of various types of plastic waste leads to the formation of microplastics (MPs) and nanoplastics (NPs). NPs, measuring less than 0.1 μm pose a latent danger to the human food chain caused by the ability to traverse biological membranes than MPs, potentially leading to various chronic diseases. The widespread distribution of NPs across diverse environmental matrices and their subsequent infiltration into food and feed chains precipitates various emerging health concerns. NPs contaminate food production systems and leach from plastic packaging, infiltrating organisms at various trophic levels. Seafood, processed foods, and drinking water serve as vectors for absorption and accumulation in human tissues. The pervasive contamination pathway poses substantial risks to human health through multiple exposure routes, primarily ingestion. It can lead to cytotoxicity, inflammation, genotoxicity, and apoptosis. This review summarizes the implications of NPs exposure that triggers various diseases such as inflammatory bowel disease (IBD), kidney dysfunction, liver disease, heart problems, brain disorders, reproductive issues, and cancer. Currently, no established method exists to treat NPs that humans may have already ingested. Hence, it is urgent to mitigate the harmful effects of NPs through the development and implementation of innovative, efficient, and sustainable environmental decontamination strategies. This discussion highlights several advanced remediation techniques that can effectively reduce the toxicity of NPs in environmental systems, thus mitigating their associated risks.
nanoplasctic, toxicology, diseases, food and feed, ingestion
Plastic is widely recognized as an integral part of human life, offering flexibility, durability, and high efficiency. The prolonged usage continues to rise annually, with production reaching 368 million tons worldwide in 2019 and expected to reach 100-250 million tons by 2025.1,2 This poses a serious threat concerning microplastics (MPs) degradation into ultra-small plastic particles. Within the total amount of plastic produced, only about 6-26% is recycled, 71-74% is lost to the environment, and about 10% of the total production is released as waste into the ocean.3,4 The enormous burden of plastic waste potentially causes environmental pollution, which deserves global attention due to the possible significant implications on various aspects, particularly human health.
Current plastic waste management systems such as landfilling and incineration are inadequate to handle the high amount of plastic waste produced annually. The COVID-19 pandemic also contributed to the accumulation of medical plastic waste released into the ocean, reaching 25.9 million tons, with 12.3 million tons fragmented into nanoparticles.5 Plastic landfilling potentially causes leaching through groundwater, leading to river pollution before it flows into the ocean.6 Plastic contaminants released into the sea interact with chemical, physical, and biological agents, degrading from micro (< 5mm) to nano (< 100 μm).7
Nanoplastics (NPs) pose a greater risk and danger than micro-sized plastic (MPs). NPs can accumulate in various food sources such as fish, shellfish, sea salt, and drinking water. Both processed food and animal feed are susceptible to contamination by these particles, transmitted to humans through the food chain.8 Hence, the complexity of the transmission process poses a dual threat, affecting the environment and human health.
Ingestion is the main pathway of NPs transmission, resulting in various diseases through the absorption and depletion of harmful substances in human body tissues.9 Exposure to NPs in the ingestion system culminates in oxidative stress and DNA damage, dysbiosis, systemic penetration, and distribution, leading to a variety of chronic diseases.10 However, cytotoxicity from NPs exposure in humans via ingestion pathways at the subcellular or molecular level has not been widely investigated.
This review assessed NPs contamination found in food and feed products to complement the limited information about the implications on human health. The objective was to comprehensively outline the process of NPs formation and distribution in the environment, the transmission in food and feed through the food chain, toxicity levels, and impact on human health. Recognizing the effects of NPs exposure on human health and treatment to reduce plastic pollution is pivotal in addressing this problem. In addition, using technology to detect NPs precisely is an essential step to protect the environment and human health. This review offers insights into the merging of advanced technology with interdisciplinary sciences.
Plastic is an extensively used material due to its flexibility, durability, and cost-effectiveness; however, its large-scale application causes environmental pollution. Pollution caused by plastic can be classified based on size into macroscopic plastic (>2.5 cm), MPs (micrometer-scale plastic), and NPs (nanometer-scale plastic). According to the European Commission, MPs have a size range of 100 nm–5 mm, while NPs range from 1–100 nm.11
MNPs originate from two main sources, namely primary and secondary.12 Primary MNPs are generated by releasing nanosized manufactured plastic polymer particles such as cosmetics, synthetic paints, coatings, and personal care products into the environment.13 Meanwhile, secondary MNPs pollution are formed by the incomplete degradation of macro-sized plastic polymers due to interactions with chemical, physical, and biological agents. For instance, motor vehicle tire abrasion, textile wastewater, and the use of plastics in the fisheries and agro-industry sectors.14 Macroscopic plastic waste is the primary source of secondary MNPs due to the variety of plastic types used globally with various applications.15 The different types of commonly used plastics and their polymer structures are shown in Table 1. Each MNPs is associated with a polymer hazard index (PHI) value, indicating the level of polymer risk to human health. The value differs depending on the type of MNPs and the sample of organisms analyzed.16–18
| Types of Plastics | Code | Polymer Structure | Uses | Reference |
|---|---|---|---|---|
| Polyethylene Terephthalate | 
| 
| Plastic containers, plastic bottles | 15 |
| High-Density Polyethylene | 
| 
| Plastic packaging for medical use, food packaging | 19 |
| Polyvinyl Chloride | 
| 
| Food packaging, electronics, coating | 20,21 |
| Low-Density Polyethylene | 
| 
| Packaging, plastic bag, coating, bubble wrap | 22 |
| Polypropylene | 
| 
| Plastic cup, packaging | 23,24 |
| Polystyrene | 
| 
| Styrofoam, disposable cup, food container | 25 |
| Others | 
| Example: polyurethane 
| All plastic polymers other than the previous 6 categories, example: Polyurethane | 25 |
The formation of NPs in the environment involves a complex series of processes ( Figure 1). The initial stage of secondary NPs formation is physical degradation, leading to the fragmentation of plastic into smaller sizes in the form of flakes, discs, rectangles, and cylinders.26 Plastic particles interact with environmental factors, such as UV light, leading to further degradation into nanometer-scale particles. Sorasan et al. (2021) disclosed NPs forming from LDPE-MPs exposed to UV light.27 Wang et al. (2023) also investigated the effect of UV light exposure on the degradation of PVC, PS, PP, and PE-NPs, triggering alterations in the physicochemical structure leading to NPs formation.28

The interaction between plastics and biotic factors also leads to the formation of secondary NPs via biodegradation. Microorganisms facilitate this process through several stages, such as colonization, biodeterioration, biofragmentation, assimilation, and mineralization.29 The colonization of MPs by degrading microorganisms will form a biofilm, which then undergoes biodeterioration and biofragmentation to form a nanometer-scale particle. On the condition wherein biodegradation continues to the mineralization stage, the NPs are depolymerized by microorganisms to form molecules that can be assimilated into cells. However, NPs remain unchanged in the void of the mineralization stage. Hence, this indicates the presence of NPs in nature is due to biodegradation by microorganisms that have not attained the mineralization stage ( Figure 1).30
The accumulation of NPs in the terrestrial environment, mainly soil, is caused by various factors, including human activities, such as agriculture. Li et al. (2022) reported that applying plastic mulch in cultivated land over 32 years has detected MPs content in the soil extending down to the subsoil.31 Wahl et al. (2021) also reported the occurrence of PE, PS, and PVC NPs in agricultural fields in Central France.32 NPs contamination in the soil may also occur due to irrigation using NPs-contaminated wastewater and agrochemicals encapsulated with plastic polymers.33 Compared to NPs, more studies reported the discovery of MPs in terrestrial environments, specifically agricultural fields. This is due to the limited availability of analytical methods to detect and count plastic particles smaller than < 5-10 μm in the soil.34 Existing methods for analyzing and detecting NPs still focus primarily on the aquatic environment.35
NPs in soil can be found in free form or bound to soil particles, including organic matter, iron oxide, and others.36 NPs bound to soil particles may experience a desorption process, leading to detachment from the bonds and forming a free state. Free-form NPs in the soil, specifically groundwater, are more mobile and have a higher bioavailability to undergo biological and non-biological transport.37 Biological transport occurs in the presence of organisms such as food crops that interact with NPs.38 NPs contamination in the terrestrial environment has the potential to be absorbed by food crops and enter the bodies of other organisms through the food chain.39
The aquatic environment is one of the sites where secondary NPs accumulate because aquatic environments such as rivers, lakes, and even oceans are involved in transforming large plastic polymers into MPs and NPs.40 Marine ecosystems with high salinity levels and microbial diversity support the formation of secondary NPs.11 The accumulation of NPs is also caused by the erosion of fishing equipment and the painting of cruise ships.41 Moon et al. (2024) reported the discovery of nanofiber, PET, nylon, and PS NPs in various marine waters areas, such as Shenzhen in China, Jeju in South Korea, Los Angeles and Corpus Christi in the United States, as well as the Gulf in Mexico.42 Various types of NPs have also been reported in rivers,43 lakes,44 and sea ice in the Antarctic Sea.45
NPs contamination in various aquatic environments marks the initial stage of NPs internalization into the body tissues of aquatic organisms,46 including organisms that are subsequently used as food and feed. This occurs as NPs in the aquatic environment undergo biological transport resulting from interactions and the ability to form aggregates with aquatic organisms, such as algae. Long et al. (2017) reported that NPs aggregates with algae cells are often mistaken as food by aquatic organisms, such as plankton, shellfish, crustaceans, and fish.47,48 Thus, algae serve as one of the NPs carriers in the aquatic environment.49
NPs infiltrate the atmospheric environment through wind currents sourced from household furniture, buildings, industrial emissions, motor vehicle tire friction, and agricultural products to pollute the air.50,51 Sheng et al. (2023) observed the presence of PS-type NPs in indoor and outdoor air environments in Eastern China. However, studies on the detection of NPs in the air are still limited.52
NPs pollution in the air can be transported to both aquatic and terrestrial environments via dry and wet deposition processes.53 Dry deposition is the process of exposure between objects and organisms in the atmosphere through precipitation due to gravity and adsorption supported by the presence of SO2 and NOx gases as acid formers.54 This process is affected by the size of NPs in the air, as smaller particles tend to remain suspended for extended periods and transported to distant regions before finally being deposited on the surface of terrestrial and aquatic areas due to gravitational forces.55 The presence of NPs in terrestrial environments due to dry deposition was identified by Materić et al. (2021) in the snow surface of the Alpine mountains.56 Besides that, wet deposition occurs due to NPs transportation along with precipitation to the surface of the terrestrial and aquatic regions.57
A progressive increase in global plastic waste consequently increases NPs pollution in various ecosystems. The NPs will subsequently penetrate the food chain and influence all organisms involved. The properties of NPs with a high volume-to-surface ratio and colloidal nature facilitate the mobilization of NPs through materials greater than MPs.58 NPs high mobility supports the transport and integration of NPs into the bodies of animals and plants.59 Several investigations have shown that NPs can invade the food chain from below the peak of trophic levels. The process occurred via contamination during food production and leaching from plastic packaging of food and beverages.60,61
Plants absorb NPs in the soil through root uptake, which consists of several stages, i.e. endocytosis, transport in plasmodesmata, and absorption through lateral root fissures along with water and nutrient flow until finally accumulating in leaves or other parts of the plant.62–64 Accumulation of NPs in plants is determined by on species, sizes and NPs charges. The rate of absorption is inversely proportional to the size of the material. Organs in plants will more readily absorb smaller NPs than larger ones. Moreover, particle charges also affect the location of the material accumulation. NPs with positive charges tend to accumulate on the root surface, while negatively charged ones accumulate in the protoplasm and the roots. Neutral-charged particles showed increased absorption and accumulation rates compared to negatively charged ones.65
Accumulations of NPs in plants, especially in food and feed crops, will affect the organisms that consume the plants. Rats, birds, chickens, snails, worms, and ruminants are directly affected by consuming plants contaminated with NPs ( Figure 2).66–68 In addition, NPs can infiltrate the bodies of terrestrial animals and even humans through contaminated water,69 feeds from fish farms and marine aquaculture,70 other food products like shellfish,71,72 and salt.60,73 Seafood such as shellfish absorbs many NPs and induces bioaccumulation in their digestive tract.74 As a source of protein for humans, shellfish have the potential to be a vector of NPs with varying levels.75 Other marine species, such as shrimp, squid, fish, crabs, and sea urchins, can also serve as human NPs vectors.70,76,77 In addition, NPs levels are generally higher in viscera than in the flesh despite the lack of focused research.78 Further, the presence of NPs in the feed was also reported. NPs contamination in feed predominantly originates from processed feed products for cultivated animals. Commercial fish feed has become a product with the highest risk due to using leftover products from fishermen and industry as raw materials.79 NPs may contaminate feed through mechanical contamination, cross-contamination, and packaging and storage contamination. 80 Table 2 shows the various NPs detected in food and feed. The number of NPs listed may be lower than the actual quantity present in nature due to the limitations of current technology in identifying sizes. The existence of degradation factors resulting from the interaction of various environmental parameters can hinder the identification process. PE, PS, and PP are the most common types of NPs found in various materials ( Table 2), as these NPs represent the most widely utilized polymers for producing various materials. These three NPs types also have a relatively similar density to water, 0.88–1.50 g/cm3, compared to PA, ACRY, and PVS.87 Those characteristics facilitate the movement of PE, PS, and PP in various environmental areas in the form of suspension instead of deposition.88 Additionally, PE, PS, and PP detection methods are well-established compared to other types of NPs, making their presence more straightforward to detect.
| Food product | NPs Type | NPs Amount | Sources |
|---|---|---|---|
| Animal | |||
| Mugil cephalus (Ebro River, Spain) | PE PP | 0.8-80 μg/g 6-9 μg/g | 81 |
| Liza sp. (Ebro River, Spain) | PE PP | 20-300 μg/g 8 μg/g | 81 |
| Dicentrarchus labrax (Ebro River, Spain) | PE | 8-200 μg/g | 81 |
| Cyprinus carpio (Ebro River, Spain) | PE | 0-80 μg/g | 81 |
| Pseudorasbora parva (Ebro River, Spain) | PE | 0 μg/g | 81 |
| Silurus glanis (Ebro River, Spain) | PE | 80 μg/g | 81 |
| Squalius latetanus (Ebro River, Spain) | PE PP | 500-5,000 μg/g 5 μg/g | 81 |
| Rutilus rutilus (Ebro River, Spain) | PE PP | 300-5,000 μg/g 8 μg/g | 81 |
| Alburnus alburnus (Ebro River, Spain) | PE PP | 0-500 μg/g 8μg/g | 81 |
| Barbus graellsii (Ebro River, Spain) | PE | 6,000 μg/g | 81 |
| Ictalurus punctatus (Ebro River, Spain) | PE | 40-500 μg/g | 81 |
| Carassius auratus (Ebro River, Spain) | PE PS | 30-100 μg/g 1 μg/g | 81 |
| Mussels (China) | Cellophane PET PES | 0.9-4.6 particles NPs/gram wet weight (mixed with various types) | 71 |
| Mussels (Apulian, Italy) | PE (37%) PP (27%) PVC PS | 187±27 ng/mg | 82 |
| Atlantic bluefin tuna (Thunnus thynnus) specimens from Mediterranean Sea (Sardinia, Italy) | phthalate di-2-ethylhexyl phthalate (DEHP) mono-2-ethylhexyl phthalate (MEHP) | 9.14± 3.27 ng/g 2.13±1.52 ng/g | 83 |
| Zebra snail (Neritina sp.) (Indonesia) | PS | 0.785±0.028 μg/g | 84 |
| Misgurnus anguillicaudatus (Hongze Lake, Suqian City, Jiangsu Province) | PS | 0.262 μg/g | 84 |
| Portunus gladiator (purchased from fish markets located in Gezhou, Raoping County, Chaozhou City, Guangdong Province) | PS | 0.093 μg/g | 84 |
| Plants | |||
| Cowpea | PET PVC PE | 414.3–1430.1 mg kg− 1 DW N.D.–703.1 mg kg− 1 DW 124.8− 462.9 mg kg− 1 DW | 85 |
| Flowering Cabbage | PA66 PVC PE | N. D.− 141.6 mg kg− 1 DW 215.1 to 954.3 mg kg− 1 DW 138.7− 345.8 mg kg− 1 DW | 85 |
| Rutabagas | PVC PE | 54.6− 279.5 mg kg− 1 DW 101.3 to 135.1 mg kg− 1 | 85 |
| Chieh-Qua | PA66 PVC PE | N. D.− 91.2 mg kg− 1, DW 35.4− 367.9 mg kg− 1 DW 110.8− 267.8 mg kg− 1 DW | 85 |
| Another Food Product | |||
| Huai Salt (China) | Combination of PE, PS, PP, PMMA, PVA, and PVC | 234,000±56,000 NP/200 g salt | 86 |
| Salt from Mediterranean Sea | Combination of PE, PS, PP, PMMA, PVA, and PVC | 692,000±94,000 NP/200 g salt | 86 |
| Salt from Australia and Antartica | Combination of PE, PS, PP, PMMA, PVA, and PVC | 130,000±42,000 NP/200 g salt | 86 |
| Salt from North Atlantic Ocean | Combination of PE, PS, PP, PMMA, PVA, and PVC | 54,000±31,000 NP/200 g salt | 86 |
| Salt from Seto, Japan | Combination of PE, PS, PP, PMMA, PVA, and PVC | 69,000±12,000 NP/200 g salt | 86 |
| Salt from Sinan Sea | Combination of PE, PS, PP, PMMA, PVA, and PVC | 251,000±32,000 NP/200 g salt | 86 |
Aside from being contained in food and feed, NPs can also occur as surface contamination. This phenomenon occurs due to the release of NPs from plastic products that directly contact food and beverages, such as food containers, plastic bottles, tea bags, and cutting boards. Table 3 shows several studies on NPs leaching from plastic products that encounter food products.
| Food package | NPs Type | NPs Amount | Sources |
|---|---|---|---|
| Tea from Tea Bag | PET and Nylon | 7 million particles/mm2 | 89 |
| Baby Food Container | PP | After microwave = 169 million NPs/cm2 High temperature = 38.6 million NPs/cm2 Room temperature = 47.9 million NPs/cm2 Refrigeration = 11.5 million NPs/cm2 | 90 |
| Kitchen Sealant | Silicone | 2–10 debris/10 μm | 91 |
| Blender | Acrylonitrile Butadiene Styrene (ABS) and PS | 0.78 × 109 | 92 |
| Dish Sponge | Nylon | ~1.2 million NPs/0.1 mm | 93 |
| Cutting Board | PP | ~3,000 NPs/mm2/cut | 94 |
| Ziplock | PE | ∼50 particles/mm | 95 |
| Cookware | PTFE | 2,300,000 NPs (each crack) 5–18.7/μm2 | 96 |
| Food container | PS | 4.0 ± 0.5 ng·g−1 | 97 |
| Food container | PP | 7.0 ± 0.8 ng·g−1 | 97 |
| Cup | PE | 29.0 ± 1.0 ng·g−1 | 97 |
| Disposable Paper Cup | LDPE | 1.9 × 107 | 98 |
| Disposable Paper Cup | PLA | 2.5 × 106 | 98 |
| Tea bag | PP | 302 ± 21 ng·g−1 | 97 |
| Tea bag | PA | 1960 ± 87 ng·g−1 | 97 |
The table above indicates that every use of plastic has the potential to release NPs, starting from simple activities such as opening the lid of a plastic bottle or pouring hot water into a plastic cup.97 This demonstrates the ease with which NPs can penetrate the human body. Hence, the continual utilization of plastic containers will facilitate NPs' transfer to food and beverages. The exposure route is described comprehensively in the subsequent section.
NPs can penetrate the human body through three primary pathways: ingestion, inhalation, and dermal contact. The ingestion pathway is via consuming contaminated food and beverages, while inhalation involves exposure to air containing NPs particles. In the digestive systems, NPs are endocytosed by intestinal epithelial cells and subsequently enter the lymphatic and circulatory systems. This process contrasts with inhalation, in which particles trapped in the respiratory tract are subjected to clearance via the mucociliary mechanism.99 Dermal exposure, the least significant route compared to ingestion and inhalation, occurs through contact with dust, clothing, and personal care products contaminated by NPs, primarily via wounds, sweat glands, or hair follicles.
Ingestion is recognized as the primary route of NPs accumulation in the human body through the food chain.74 Although direct studies on this mechanism are limited, evidence from previous research supports this theory, as NPs have been detected in human fecal samples, indicating their presence in consumed food and water.61 For instance, polyethylene (PE) particles ingested by Corbicula fluminea mussels are excreted through feces, serving as a food source for benthic detritivores such as crustaceans. These crustaceans are consumed by larger predators, which humans ultimately ingest. This process, termed biomagnification, refers to the progressive increase in pollutant concentration as it moves up the trophic levels of the food chain.100,101 As humans occupy the highest trophic level, the concentration of NPs accumulated in their bodies is significantly elevated.101
NPs are suspected of permeating gastrointestinal organs through endocytosis via the lymphatic tissue, subsequently infiltrating microfold (M) cells.102 This process results in systemic exposure, which presents a significant problem to humans. Its severity is greatly influenced by the NPs' rate of absorption, which relies on their size and chemical structure.103
As a crucial channel in the digestive system, the lumen is acknowledged for its role in detecting NPs absorption rate. Several molecules are present within this channel, such as proteins, lipids, carbohydrates, nucleic acids, ions, and water interacting with NPs. Molecular bonds enable NPs-protein interaction, leading to the coating of NPs by a group of proteins, identified as corona.104 Corona formation also promotes aggregation and increases cell absorption.105,106 The composition of these corona proteins differs based on the type of NPs, which affects the properties and toxicity levels. Elevated concentrations of NPs are also known to accelerate the formation of coronal proteins, thereby enhancing the ability of these particles to translocate into cells.107
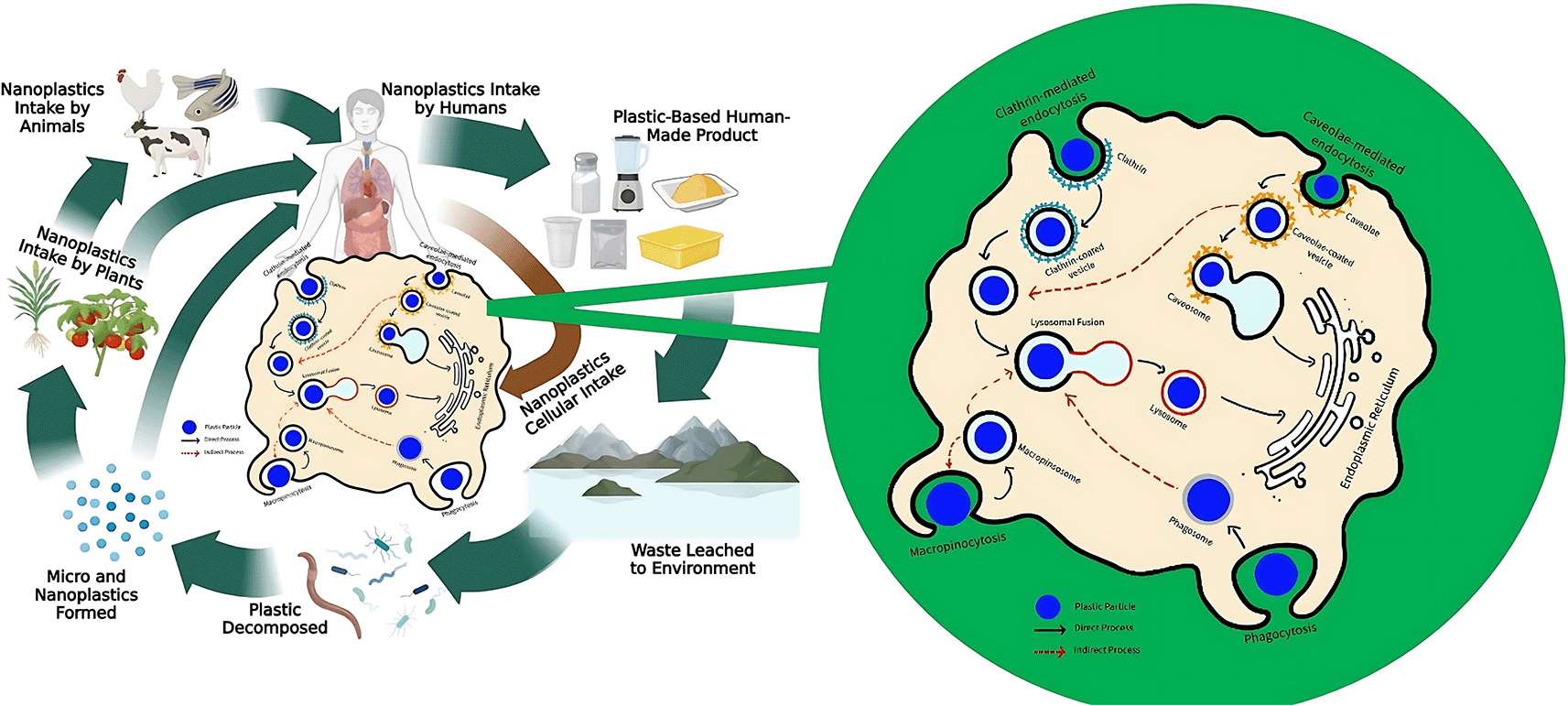
The penetration of NPs into the plasma membrane will be followed by their binding to receptors on the cell surface, which leads to damage to the phospholipid bilayer and disrupts the normal function of the cell. NPs can intervene in the integrity of the cell membranes and internalize through endocytosis mechanisms, such as phagocytosis, macropinocytosis, clathrin, and caveolae-mediated endocytosis105,108,109 ( Figure 2). Phagocytosis means immune cells eliminate nanoparticle (NP) contaminants from the digestive tract. Phagocyte cells recognize and bind NPs through surface receptors and then envelop the particles by forming phagosomes. The process involves the contraction of the actin-myosin system to ensure that the particles are properly trapped. Phagosomes interconnect with lysosomes to form a fusion called phagolysosome, containing proteases, lipases, and nuclease enzymes that break down NPs into smaller components.110
Macropinocytosis is the process of internalizing molecules into cells by bending the plasma membrane, forming large vesicles called macropinosomes.110 While NPs contamination occurs along with the permeation of fluid from the external environment, macropinosomes envelope both the contaminant and the extracellular fluid. Once formed, macropinosomes combine with lysosomes containing hydrolytic enzymes to digest NPs into smaller components. Macropinocytosis tends to facilitate the uptake of larger NPs (0.5–1 μm) into the cell, such as 500 nm polystyrene particles (PS500).111
Cells may also take up NPs via clathrin and caveolae-mediated endocytosis. Clathrin-mediated endocytosis (CME) occurs when NPs bind to cell surface receptors, then signals the formation of clathrin-coated pits (CCPs), which are the invagination of the plasma membrane coated by clathrin proteins. The particles afterward penetrate the cell through the CCP and are carried by clathrin-coated vesicles (CCVs). Aside from CME, NPs can also be taken up by cells via caveolae-mediated endocytosis through a process similar to CME.112 Clathrin- and caveolae-mediated endocytosis can facilitate the permeation of 50 nm NPs, such as PS50 particles.111 This declares that endocytosis plays a vital role in the internalization of NPs into cells.
In addition to endocytosis, NPs can infiltrate cells through transcytosis, interaction with transporters, or passive diffusion through lipid membranes, particularly in intestinal epithelial cells.108 The particle size dramatically influences the internalization. Smaller-sized particles have difficulty interacting with protein receptors on the membrane surface, whereas bigger-sized ones encounter challenges penetrating the cell membrane, thus limiting the internalization process.113 Although NPs can pass through paracellular pathways via tight junctions, the limitation of pore size (0.3–1 nm) makes these pathways less effective. Therefore, NPs often penetrate cells with permeation enhancers such as EDTA, chitosan, and sodium caprate, which enlarge the pores of tight junctions by up to 20 nm.114
Various vesicles encapsulating NPs throughout the translocation process produce hydrogen peroxide, which facilitates their degradation. The remaining digestive products are removed from the cells through exocytosis.
NPs penetrate cells via various endocytosis mechanisms and exert harmful effects by producing reactive oxygen species (ROS). Elevated ROS levels in intestinal epithelial cells result in cytotoxicity, which triggers inflammation, apoptosis, necrosis, and gastrointestinal damage, including intestinal perforation.115 NPs-induced cytotoxicity, driven by excessive ROS production in the early stages of intestinal injury, leads to inflammation and apoptosis depending on the severity.116
Structural properties and interactions with biological cell membranes trigger the formation of ROS influenced by NPs. The interaction between NPs and membrane lipids induces peroxidation, generating ROS as a byproduct. This increase in ROS levels leads to changes in cell integrity and cellular damage that triggers oxidative stress. Excessive ROS levels exceeding the antioxidant system's capacity can activate signaling pathways like NF-κB and MAPK, which mediate inflammation and apoptosis. The inflammatory response is initiated when TLR-4, a transmembrane pattern recognition receptor (PRR), activates the innate immune system by triggering NF-κB signaling and promoting inflammatory cytokine production.117 ROS overproduction activates pathways that contribute to chronic inflammation, oxidative stress in the gut, intestinal microbiota dysbiosis, and metabolic diseases.118,119
The accumulation of NPs in the intestine disrupts macrophage function by impairing their ability to recognize and bind foreign particles. NPs promote the activation of helper T cells (Th1), producing pro-inflammatory cytokines such as TNF-α and IFN-γ. An increase in these cytokines aggravates inflammation and affects phagocytosis function. Jiang et al. (2024) reported that PS-NPs above the threshold (50 μg/mL) would independently affect the polarization imbalance of macrophages leading to pro-inflammatory features.113 Li et al. (2024) reported that chronic exposure to PS-NPs for 32 weeks in a rat model led to intestinal inflammation, characterized by villi erosion and reduced crypt numbers.116 The study also reported an increase in the expression of the NF-κB target gene that plays a role in the inflammatory process. NF-κB was shown to induce pro-inflammatory genes, polarize M1, and increase the production of IL-6, TNF-α, and IL-1β.116 Another study conducted by Forte et al. (2016) observed the upregulation of pro-inflammatory cytokine genes IL6, IL8, and IL-1β in human gastric adenocarcinoma cells following PS-NPs exposure.120 High-dose NPs exposure in animal models significantly increased the levels of the pro-inflammatory molecule NF-κB and inflammatory interleukins. In contrast, the anti-inflammatory molecules such as Nrf2 would decrease. In addition, increased NF-κB synthesis correlated with elevated expression of most immune-related genes, suggesting that the NF-κB pathway is mechanically implicated in this event.121
NPs are also known to induce cell death through autophagy pathways. Autophagy is a process of cell degradation through lysosomal control to eliminate components that cause cell damage. NPs localization inside and outside cells enhances lysosome production, as detected by fluorescence staining.101 Furthermore, NPs induce autophagy and apoptosis simultaneously by depolarizing the mitochondrial membrane potential and forming autophagosomes.
Apoptosis is the programmed death of cells to help the body eliminate damaged and irreparable cells. As previously explained, NPs can infiltrate cells up to lysosomes through endocytosis. NPs entry into lysosomes is thought to activate apoptosis pathways, including p53, PI3K–Akt, and Bcl-2/Bax. These findings are supported by Li et al. (2024), which indicate that chronic exposure to high doses of PS-NPs (10 mg/L) for 28 days in shrimp Litopenaeus vannamei led to increased cell apoptosis in intestinal tissue.122 Umamaheswari et al. (2021) also conducted a study on Danio rerio with 10 mg/L of PS-NPs measuring 0.10-0.12 mm for 35 days.123 They reported a significant increase in TNF-α, p53, casp3b, gadd45ba, and ptsg2a gene expression as evidenced by histological observations where cytoplasmic degradation, necrosis, lamellar fusion, and epithelial aneurysms occur.123 The study also showed an increase in the production of ROS, resulting in 4-hydroxynonenal and malondialdehyde, which promote DNA addition and apoptosis. Overproduction of ROS can interfere with mitochondrial redox homeostasis and contribute to further cellular damage.124 These results confirm that oxidative stress triggered by ROS is the primary mechanism of NPs toxicity.109,125 Regular exposure also triggers uncontrolled intracellular calcium release, affecting cellular signaling pathways and accelerating apoptosis.
NPs induce genotoxicity through multiple mechanisms, including direct interaction with DNA strands and indirect effects mediated by ROS production. Studies by Yang et al. (2024) and Roursgaard et al. (2022) demonstrated that exposing Caco-2 cells, an epithelial cell line derived from colon cancer, to PET-NPs for 3 hours resulted in DNA strand breaks without a corresponding increase in ROS levels.126,127 These findings suggest that genotoxicity may not solely result from oxidative stress but could involve direct interactions between NPs and DNA or the release of chemicals, such as bisphenol A (BPA), from NPs, leading to DNA damage.
Genotoxicity can also occur indirectly due to elevated ROS levels. In addition to damaging cellular proteins and causing inflammation and apoptosis, ROS accumulation harms DNA by inducing strand breaks or base deletions, which may result in mutations, genomic instability, and potentially carcinogenesis.127,128 An in vitro study reported that exposure of carboxylated polystyrene sphere NPs to small intestinal epithelium models led to a significant increase in intracellular ROS levels within 6 hours. This ROS accumulation was accompanied by observable DNA damage after 48 hours, indicating that ROS production and accumulation play a critical role in NPs-induced genotoxicity.129 The genotoxicity of NPs can also trigger the occurrence of senescence, necrosis, and apoptosis, which contribute to tissue damage and organ dysfunction.
The gut microbiome is a highly complex ecosystem comprising diverse microorganisms in the human intestine.130 The general composition of the gut microbiome in humans includes six bacterial phyla: Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Fusobacteria, and Verrucomicrobia. In addition to bacteria, the gut microbiome contains several fungal genera, such as Candida, Saccharomyces, Malassezia, and Cladosporium. Other components include viruses, bacteriophages, and archaea.131 A balanced gut microbiome is essential for nutrient absorption, immune system regulation, detoxification, and protection against pathogens.130
The accumulation of NPs in the intestine has been implicated in gut microbiome dysbiosis, potentially contributing to gastrointestinal and extraintestinal disorders and increasing the risk of chronic diseases.132 Zhang et al. (2023) reported that exposure to PS-NPs in mice altered the gut microbiome's composition, reducing functional microbiota and inducing dysbiosis.133
Various hypotheses have been proposed to explain how NPs contribute to gut microbiome dysbiosis. One widely accepted hypothesis suggests that the presence and accumulation of NPs directly influence intestinal microbiota's growth and metabolic activity.134 Another hypothesis posits that pathogens introduced via NPs alter the microbiota's composition and function, as demonstrated in the zebrafish model.135 Furthermore, NPs are often associated with endocrine-disrupting chemicals (EDCs), such as diethyl-hexyl phthalate (DEHP) and bisphenol A (BPA). These additives have been shown to alter gut microbiota composition in zebrafish and rat models.136,137
Dysbiosis of the intestinal microbiota due to NPs exposure at high chronicity leads to IBD caused by the activation of the immune response.138 Antigens from dysbiotic microbes activate M1 macrophages, thereby triggering an immune response in the lamina propria.139 These macrophages produce pro-inflammatory cytokines such as IL-1β, IL-6, TNF-α, and IFN. Consequently, inflammation and continuous cell damage occur due to the thickening of lamina propria as a marker of IBD.138
The potential of IBD occurrence due to NPs contamination via food ingestion was validated in the study conducted by Ma et al. (2023).140 The study discovered that NPs exposure for 28 days increased inflammation and intestinal ulcers in mice induced with sodium dextran sulfate (DSS).140 In a subsequent study, Zhang et al. (2023) revealed that exposure to PS-NPs in mice caused intestinal barrier dysfunction, leading to inflammation.133 Wounds and intestinal inflammation were identified as early markers of IBD in the reported study, although further research is needed to confirm these findings.
NPs penetrate biological membranes and access the circulatory system owing to a small size (<10 μm), large surface area ratio, and negatively charged surfaces, leading to high reactivity. Thus, NPs can interact with the endothelial cells of blood vessels, triggering various toxic mechanisms that primarily affect the cardiovascular system.141 in vitro research on cardiac organoids determines that exposure to NPs could trigger oxidative stress, inflammatory responses, apoptosis, and collagen accumulation, resulting in heart failure. These particles effectively induced IL-6 and TNF-α expression, which worsened the inflammatory response and limited the heart’s ability to function normally. The results suggest NPs may contribute to myocardial fibrosis, structural changes, and heart dysfunction.142 Another study reported that exposure to NPs in zebrafish caused significant alterations in the development of the cardiovascular system, such as pericardial edema, abnormal heart chamber morphology, changes in heart rate, and impaired blood circulation.143 Furthermore, the exposure of PS-NPs in zebrafish larvae reduced the heart rate dose-dependently by 5–10%.144
As previously mentioned, NPs can induce inflammation by activating the NF-κB pathway and the NLRP3 inflammasome, disrupting the gills' structural integrity and leading to apoptosis.145 Meanwhile, in carp larvae (Cyprinus carpio), combinations of MNPs at low concentrations (10 μg/L) caused tachycardia.146 Disclosure to NPs at the prenatal level can have a transgenerational impact on cardiovascular function.
Studies in mice showed that exposure to PS-NPs affected the expression of genes associated with apoptosis, such as cleaved caspase-3 and fibrosis genes (Zfp36, Tfdp2, Map 3k6, Igfbp3, Mkrn1, and Sik1).147 These alterations impair endothelial function, interfere with nitric oxide (NO) signaling, and trigger peroxynitrite formation. Peroxynitrite compound worsens tissue damage and affects the integrity of blood vessels.148 NPs can also affect the expression of cardiac development-related genes, such as myh6, NKX2.5, and Tnnt2a, leading to dysplasia and abnormalities in cardiac morphology.143 Furthermore, there was a decrease in the expression of VEGF and VEGFR genes, which led to impaired formation of new blood vessels. These findings show that NPs can affect the cardiovascular system at both the molecular and organ levels.
The impact of NPs on the human cardiovascular system is evidenced by the appearance of arterial plaques, which are associated with an increased risk of myocardial infarction, stroke, and premature death within 34 months of exposure.149 The formation of arterial plaques was attributed to high expression of IL-18, IL-1β, IL-6, and TNF-α, along with increased infiltration of CD68+ macrophages and CD3+ lymphocytes, leading to chronic inflammation. In addition, activation of NLRP3 inflammasomes by NPs exacerbated vascular tissue damage, increasing the risk of atherosclerosis and hypertension.150 It was concluded that alterations in the cardiovascular system due to exposure to NPs could worsen over time, characterized by progressive damage to vascular tissue and the heart. Chronic inflammation and further hemodynamic damage potentially increase the risk of arrhythmias, thromboembolism, and cardiovascular death.
The distribution of NPs in the liver may impair nutrient absorption, leading to metabolic diseases such as liver injury and dysfunction.151–153 A study by Zhang et al. (2024) reported that long-term exposure to PS-NPs (100 nm) in mice caused histological damage such as vacuole degeneration and vascular disorganization in the liver.154 This mechanism involves an increase in proinflammatory signals (IL-6, IL-1β, TNF-α) and a decrease in anti-inflammatory proteins (IL-10). Exposure also increases oxidative stress through elevated ROS, decreasing the activity of antioxidant enzymes such as CAT, SOD, GSH-Px, and TAOC while increasing MDA levels. Furthermore, changes in the profile of 276 lipid metabolites were observed, leading to liver metabolic diseases due to PS-NPs exposure.154
Another study using zebrafish exposed to NPs (PS, PP, and PE) for 30 days showed varying levels of liver damage. PS-NPs caused the most severe damage, namely cytoplasmic vacuolation, eosinophilia, nuclear pyknosis, and narrowing of the bile ducts that triggered cholestasis. This mechanism is attributed to activating the P38-MAPK signaling pathway, which elevates the expression levels of pro-inflammatory cytokines such as IL-1β and TNF-α, worsening inflammation and liver damage. PP-NPs inflict moderate liver damage, including mild vacuolation and minor pyknosis, triggered by the inflammatory response involving NLRP6 and the overexpression of NOD1, NLRP3, and IL-6. Meanwhile, PE-NPs exhibited the lowest toxicity, inducing negligible liver damage owing to the activation of the JNK-MAPK pathway, which triggered slight cellular stress without significant injury.155
Liver inflammation, changes in lipid metabolite profiles, and structural damage from exposure to NPs can progressively lead to liver dysfunction. Dysfunction inhibits the ability of the organ to detoxify, metabolize nutrients, and regulate the body's homeostasis. In the long term, liver damage caused by NPs exposure leads to severe conditions such as liver fibrosis, cirrhosis, and even hepatocellular carcinoma.156,157 An imbalance in lipid metabolism also risks worsening systemic metabolic diseases, such as obesity and type 2 diabetes.158 Therefore, the cumulative effects of liver damage due to NPs have a local impact and affect the body's overall health.
The kidneys accumulate NPs due to their vital function of filtering blood, including blood contaminated with particles from ingestion.115,159,160 This accumulation interferes with the morphology and function of the kidneys, as evidenced by histopathological analysis showing that NPs exposure causes progressive damage. At low doses, exposure leads to glomerular capillary dilation, tubule damage, and vacuolization in the renal medulla. At high doses, the damage extends to interstitial hemorrhage, fibrosis, and loss of cellular architecture.161 NPs can also decrease the expression of tight junction proteins, such as claudin-2, which compromises epithelial barrier integrity and leads to a subsequent increase in tissue damage.114,115,159,160 Exposure to PS-NPs in the kidneys causes tubule atrophy, glomerulopathy, and inflammation.162 The combination of NPs particles and other contaminants, such as BPA and DEHP, which often leach from plastic drinking bottles, also contribute to kidney dysfunction. Chronic exposure to these additives worsens oxidative stress, inflammation, and endocrine diseases, resulting in renal tubule cell injury, fibrosis, and the formation of kidney stones.163 In addition, NPs exposure can disrupt primary metabolic functions in renal cells by decreasing glycolysis activity, which was indicated by reduced GAPDH expression, resulting in impaired energy production.160 Impaired metabolic function contributes to decreased cellular function. It increases susceptibility to chronic kidney disease, characterized by a weakened ability to filter blood, regulate fluids and electrolytes, and remove metabolic wastes such as urea and creatinine.160,163
Contamination of NPs from drinking water absorbed through the ingestion route may lead to brain dysfunction. Fluorescence analysis in a previous study revealed the distribution of 50 nm NPs from the cortex to the hypothalamus in a mouse midbrain model after three days of exposure. The small size of NPs facilitates entry across the blood-brain barrier (BBB) and subsequent accumulation in various parts of the brain, such as the olfactory lobe, cortex, cerebellum, hippocampus, and brainstem.164–166 Accumulated NPs reduce the integrity of the BBB by decreasing the expression of PECAM-1 protein and tight junction proteins (ZO-1 and occludin). These changes lead to structural damage, increasing permeability to foreign particles.164 Despite numerous toxicological tests that have been conducted, the impact of nanoparticles on the brain remains unclear due to variations in deposition behavior influenced by NPs material composition. Current reports assume that the size of NPs contributes to the accumulation in the brain.167 In addition, NPs concentration also exerts a significant influence.
Apart from affecting the brain, NPs impact the nervous system through inflammatory mechanisms, as previously explained. Fluorescence staining presented that exposure to PS-NPs in mice caused cytoplasmic damage to neurons in the Niessl body and hippocampus. Additionally, PS-NPs induced the degradation of HT-22 neurons, as evidenced by cell shrinkage.164 Xian et al. (2024) found that the effects of PS-NPs caused neuronal death, neurotoxicity, neuronophagy, vasodilation, loss of granule cells, and coagulative necrosis in the brains of female zebrafish.168 Schröter et al. (2024) reported that exposure to PS-NH2 in Caenorhabditis elegans reduced neuronal growth. Exposure to NPs may also increase the expression of amyloid precursor protein (APP), neuron-specific enolase (NSE), synaptophysin (SYN), and βIII tubulin, signaling axon damage and cellular stress responses.169 PS-NH2 exposure has also been reported to disrupt synaptic structure and function, as shown by increased expression of NLGN1 (neuroligin 1) and decreased expression of NLGN3 (neuroligin 3).169
Long-term exposure to PS-NPs leads to neurobehavioral changes, such as Parkinson's disease and Alzheimer's. In a study on zebrafish, Xian et al. (2024) found symptoms such as anxiety behaviors characterized by shoaling and decreased locomotor activity. Consequently, zebrafish became more passive, increasing their vulnerability to predators after NPs exposure.168 Liang et al. (2022) confirmed these results in mice exposed to PS-NPs, showing decreased activity, such as grasping strength and motor coordination, despite the absence of Parkinson's symptoms.170 Impaired spatial working memory and cognitive function were also observed through the radial arm maze and NORT in vivo models in mice.165 PS-NPs exposure disrupts the structure of β-amyloid (Aβ), a key factor in the pathogenesis of Alzheimer's disease. NPs accelerate Aβ nucleation and transform protein structures into oligomers, increasing the toxicity of Aβ oligomers to nerve cells.171 Aβ toxicity due to NPs can exacerbate oxidative stress, damage cell membranes, and cause Ca2+ ion dysfunction, all contributing to neuronal cell death.171 Therefore, long-term exposure can damage the structure and function of the brain and nervous system.
Exposure to NPs can significantly disrupt both male and female reproductive systems, with long-term effects potentially impairing overall organ function. In males, NPs can result in testicular damage, curtailed sperm quality, and lower testosterone levels, eventually leading to infertility. An in vivo study with a mouse model exposed to PS-NPs found an accumulation of compounds in the seminiferous tubules, a critical site for spermatogenesis.172–174 The study also showed that NPs could penetrate the blood-testis barrier (BTB), altering issue ultrastructure and decreasing junction proteins such as occludin, ZO-1, β-catenin, and N-cadherin.173–175 Additionally, ROS overproduction led to high expression of IL-6, CXCL10, IL-1β, MCP-1, TNF-α, and Bax, along with the interaction of NPs with TLR4, triggering an inflammatory response through the oxidative stress pathway.174,176 These alterations cause damage to the basal membrane structure, spermatogenic cells, and inflammatory infiltration of the seminiferous tubules.172,174
High doses of PS-NPs increase sperm morphological abnormalities, including the absence of acrosomes, cervical folding, acephaly, and lack of tail.177–179 This is accompanied by decreased sperm quality and count.172,174,177,180 In addition to affecting organ structure, NPs exposure disrupts hormonal regulation in men, notably causing a significant decrease in testosterone levels.172,180 Exposure to PS-NPs can inhibit key genes implicated in testosterone synthesis, such as StAR and CYP11A1.176 The reduction in testosterone inhibits sperm maturation and disrupts the spermatogenic environment.176 NPs exposure reduces the expression of LH, which stimulates Leydig cells to produce testosterone but increases FSH, which is crucial for sperm maturation.172,173 The activity of enzymes such as succinate and lactate dehydrogenase decreases, which also affects sperm development and motility.175 Thus, exposure to NPs has a complex impact on the male reproductive organ system.
In the reproductive system of female mammals, chronic exposure to NPs disrupts ovarian structure, promotes hormonal imbalances, and alters the reproductive process.175 NPs exposure can reduce ovarian size and weight and trigger ovarian fibrosis, characterized by increased expression of fibrosis markers, including fibronectin, collagen I, and collagen III.181 This fibrosis damages the structure of the ovarian stroma and increases apoptosis in ovarian cells. An increase influences the changes in ROS and MDA, which is inversely proportional to the activity of SOD, CAT, and GPx enzymes. Furthermore, the expression of Bcl-2, Bax, and Caspase-3 genes significantly accelerates tissue damage.69,175
NPs exposure worsens follicular atresia by reducing the number of ovarian follicles at various developmental stages and increasing granulosa cell apoptosis.69,182 The reduction of granulosa cells in the ovaries due to apoptosis decreases egg reserve, promotes corpus luteum atrophy, increases the number of atrial follicles, and interferes with ovarian function.175,182 Exposure to NPs is also related to complex hormonal diseases in the female reproductive system. Specifically, it lowers LH, AMH, and P4 levels, inversely proportional to the expression of hormones T, E2, and FSH. Consequently, this imbalance interferes with the estrus cycle.69,180–183 The long-term effects of NPs exposure exacerbate reproductive organ dysfunction, disrupt hormonal balance, and potentially lead to permanent infertility.
Prolonged chronic exposure to NPs has the potential to cause carcinogenesis. As reported by Barguilla et al. (2022), exposure to PS-NPs for 120 days in PTP cells caused down-regulation of stress-related genes such as Keap, Nrf2, Pgp, SOD1, and SOD2.184 These processes cause cells to become more susceptible to oxidative damage and stress. The development of oncogenic phenotypes was aggressive in PTP cells during migration and invasion, which exceeded that of the control group after exposure to PS-NPs. This raises the possibility that the exact mechanism of carcinogenesis can occur in other cells. Domenech et al. (2021) reported that exposure to PS-NPs in Caco-2 cells causes changes in the expression levels of HO-1 and SOD2, which are stress-related genes.185 These studies provide information and preliminary evidence regarding the potential for carcinogenesis due to NPs contamination. Generally, some types of cancer that have the potential to occur due to NPs exposure through ingestion routes are colorectal and pancreatic.185,186
Various routes of NPs contamination, notably ingestion and the impact on human health are known, but data on the accumulation level in food and feed ingredients is still limited. This is due to the varying PHI value in each NPs materials and polymers being different and the lack of a precise identification method. The development of bioengineering plays a vital role in the detection of NPs, including biosensors, by using biological elements, namely specific antibodies, DNA aptamer, microscopy, imaging technologies, and microfluidic-based detection systems. Specific detection methods can differentiate various types of pollutants to be degraded and can be combined with mechanical, chemical, and biological methods.187
Degradation is a promising treatment method to alleviate plastic pollution in the environment. In general, plastics can undergo a natural process of biodegradation aided by microorganisms. The stages include biofilm formation, biodeterioration, fragmentation, assimilation, and mineralization.188 However, biodegradation of NPs for plastics with complex structures, such as contaminated plastics that bind to other compounds, remains difficult. Therefore, optimization is needed on each variable.189
Mechanical methods, namely centrifugation and ultrafiltration, are considered more impactful than natural biodegradation and filtration. However, the filtration method is limited due to the potential clogging in the membrane pores.190 In addition, centrifugation is a dispensable membrane that separates the NPs. The degree of separation depends on the duration and speed used. For instance, centrifugation at 10,000 rpm can separate 90% of NPs contamination from the contaminated material.191 Meanwhile, the ultrafiltration method reportedly separated NPs contaminants up to 88.1% without coagulation. Combination with coagulation treatments will increase the efficiency of NPs separation up to 99%.192 Chemical methods such as flocculation with aluminum sulfate and salt at a certain concentration decrease the number of NPs in contaminated materials by around 77-87% from an initial number of NPs.193
Biological methods of NPs treatments can be carried out using bioreactors and bioremediation. The bioreactor method entails two main components, membranes and enzymes, to separate NPs from other substrates.190 Meanwhile, bioremediation can be achieved with plants and microorganisms. Remediation using plants (phytoremediation) consists of phytoaccumulation, phytostabilization, and phytofiltration. Phytoaccumulation is a method by which plants absorb and accumulate pollutants, thereby reducing the percentage of pollutants in the environment—phytostabilization attempts to process pollutants into immobile in contaminated areas.194 Meanwhile, phytofiltration is a vegetation that restrains the movement of pollutants by filtration.195
A remediation method for NPs using microorganisms has also been reported. Achromobacter xylosoxidans M9 can biodegrade PS-NPs by up to 92.3% and change the chemical composition. The PS-NPs themselves will experience a decrease in weight by up to 7% within 30 days. The results suggest that microorganisms such as bacteria have the potential for NPs bioremediation.196,197 Bioremediation offers the advantage due to its constant efficiency over time, unlike non-organic materials, which gradually lose their effectiveness. Biological agents are also harmless to the environment and easily degraded. Several methods for NPs remediation through biological and non-biological strategies are summarized in Table 4.
| Method | NPs Type | Removal efficiency | Sources |
|---|---|---|---|
| Non-biological remediation | |||
| TEMPO-mediated Seaweed Cellulose Nanofibers and Quaternized Seaweed Cellulose Nanofibers | Various NPs | 98.71% | 198 |
| Wet oxidation at 190-220°C | PE PVC Total of various NPs | 100% 98.0% 97.4% | 199 |
| Biochar-derived dissolved matter (BCDM) and Biochar-derived particulate matter (BCPM) | PVC | Increase of total chlorophyll content and biomass in lettuce | 200 |
| NdFeB magnet (iron oxide nanoparticles (IONPs) with hydrophobic coatings) | PS | 90% | 201 |
| 3D printed moving bed water filter(M-3DPWF) | Polycarbonate | Zeta potential is lower for the treated solution (∼−3.0 mV) than the initial solution (∼−6.5 mV) | 202 |
| Magnetic biochar (Fe3O4-biochar) | Carboxylate-modified polystyrene latex microspheres (0.02 micrometer) | 87% | 203 |
| Fe2O3-modified graphene oxide | PS | Mitigate nanoplastic-induced damage in wheat by regulating water relations, protecting photosynthesis reactions and providing efficient ROS scavenging with high antioxidant capacity. | 204 |
| Carbon (3D Graphene-like) double oxide (layered) | PS | pH 3–11 = ≥ 80% pH 1 and 13 = 60% | 205 |
| Functional mesoporous biochar (MBC) | PS | 1st cycle = 92.2% 5th cycle = 70.2% | 206 |
| Fly ash + Fe ions | PS | 1st cycle = 94.1% 4th+ cycle = 89.8% | 207 |
| MXene-derived γ-Fe2O3/Pt/TiO2 microrobots | Carboxylated PS (50 nm) | 97% | 208 |
| Synthesized Zn-Al layered double hydroxide | Nano-scale plastic debris (NPDs) | pH 4 = 100 % pH 9 = 37 % | 209 |
| CuNi carbon material (CuNi@C) | PS | 0.3 g/L= 99.18% 0.1 g/L= 32.72% 4th+ cycles= ~75% | 210 |
| Framework-based composite material ZIF-8@Aerogel | poly(1,1-difluoroethylene)(60–110 nm)
PS(90–140 nm) | 91.4% 85.8% | 211 |
| Cellulose/LDHs composite beads | PS | 68-90 % (Depends on the cycle of use) | 212 |
| Mg/Al flocculants | PS | 90.0% | 213 |
| PVDF membrane | PS | 88.6 % removal efficiency for 100 nm microplastics | 214 |
| Remediation by plants | |||
| Coffee ground | fluorescent-orange amine-modified PS beads (fluo-NP, 100 nm) | 74% | 215 |
| Brassinosteroid | PS | inhibit accumulation of PS-NPs by affecting aquaporin expression | 216 |
| Remediation by microorganism | |||
| Achromobacter xylosoxidans M9 from gut microbiome of Tenebrio molitor larvae | PS | 92.3% | 196 |
| Lactic acid bacteria from infant feces | PP PE PVC | 78.57% 71.59% 66.57% | 217 |
| Lipase Pseudomonas aeruginosa O6 | PS, PET, PE | 97% | 197 |
| Aspergillus versicolor | LDPE | 77% | 218 |
| polycaprolactone-bound diatomite | PS | 69.81 % (10 ppm) 73.28 % (100 ppm) | 219 |
Remediation efforts can mitigate the presence of NPs in the environment. However, once ingested, these methods are no longer practical. In general, the organism can excrete NPs by themselves. Even so, there is a limit to the excretory capacity of organisms. The process depends on each species' speed and excretion ability, component, and type of NPs.220 Evidence from shellfish showed that NPs were excreted from body tissues after two days. Still, when the organism remained in the contaminated environment, the absorption continued to repeat until accumulation occurred.220 Various remediation methods for environmental NPs contamination can be carried out to optimally reduce or eliminate the transmission risk to food and feed. Consequently, various health problems in humans due to the penetration of NPs into the food chain can also be minimized.
In conclusion, NPs contamination in aquatic, terrestrial, and atmospheric environments mainly infiltrates the human body through ingestion. Food chains from those environments will eventually reach humans as organisms in the highest trophic levels. The ingested NPs are translocated into the cell through various endocytosis mechanisms, such as phagocytosis, macropinocytosis, clathrin-mediated, and caveolae-mediated endocytosis. The presence of accumulated NPs in cells will trigger toxicity, genotoxicity, and gut microbiome dysbiosis. Dysfunction of various organs that might lead to cancer will happen if the NPs are not promptly treated. Hence, efforts are needed to develop methods for detecting NPs in the environment. Pathological detection is also vital for preventive efforts against the impacts of NPs on human health.
The authors thank Lembaga Pengelola Dana Pendidikan (LPDP) for facilitating the funding via the Ministry of Finance Republic of Indonesia.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the topic of the review discussed comprehensively in the context of the current literature?
Partly
Are all factual statements correct and adequately supported by citations?
Partly
Is the review written in accessible language?
Yes
Are the conclusions drawn appropriate in the context of the current research literature?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Environmental pollution, environmental monitoring and remediation, microfluidic sensors
Is the topic of the review discussed comprehensively in the context of the current literature?
Yes
Are all factual statements correct and adequately supported by citations?
Yes
Is the review written in accessible language?
Yes
Are the conclusions drawn appropriate in the context of the current research literature?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Distribution in the environment, food chain etc
Is the topic of the review discussed comprehensively in the context of the current literature?
Yes
Are all factual statements correct and adequately supported by citations?
Yes
Is the review written in accessible language?
Yes
Are the conclusions drawn appropriate in the context of the current research literature?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Microplastic pollution
Is the topic of the review discussed comprehensively in the context of the current literature?
Partly
Are all factual statements correct and adequately supported by citations?
Yes
Is the review written in accessible language?
Yes
Are the conclusions drawn appropriate in the context of the current research literature?
Partly
References
1. Mallek M, Barcelo D: Sustainable analytical approaches for microplastics in wastewater, sludge, and landfills: Challenges, fate, and green chemistry perspectives. Advances in Sample Preparation. 2025; 14. Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: environmental chemistry
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
|
Version 1 12 Mar 25 |
read | read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)