Keywords
post-hepatectomy liver failure, post-hepatectomy liver dysfunction, royal jelly, rat, hepatectomy
This article is included in the Agriculture, Food and Nutrition gateway.
Posthepatectomy liver dysfunction can lead to fatal outcomes, making it a highly undesirable complication. Numerous studies have attempted to mitigate liver dysfunction after hepatectomy. However, in the context of hepatectomy, Royal Jelly, previously known for its various benefits to liver health, has not been studied.
In this study, thirty-two male Wistar rats were randomly divided into two groups. Just before undergoing 80% hepatectomy, 16 rats received intravenous Royal Jelly (RJ group), while the remaining 16 rats were administered intravenous phosphate-buffered saline in a blinded manner (PBS group). Four rats died before the scheduled time for specimen collection, leaving 28 rats for analysis. Forty-eight hours after hepatectomy, liver function (bilirubin and prothrombin time) was evaluated in all rats. Additionally, hepatocyte proliferation was examined using the Ki67 marker, which was obtained from the remaining liver specimens. The difference in bilirubin levels and prothrombin time between the two groups was tested using the Mann-Whitney U test. The difference in Ki-67 percentage between the two groups was analyzed using the t-test.
The RJ group exhibited a lower median total bilirubin level compared to the PBS group (p<0.001). Additionally, the prothrombin time in the RJ group was lower than that of the PBS group (p<0.05). The Ki-67 percentage in the RJ group was also lower than in the PBS group (p<0.05). These findings suggest that the rats administered Royal Jelly demonstrated better liver function compared to the other group.
Administer intravenous Royal Jelly to rats undergoing 80% hepatectomy helps to protect liver function. This study indicates the potential for further research on Royal Jelly as a possible alternative for reducing liver dysfunction following surgery or other medical conditions.
post-hepatectomy liver failure, post-hepatectomy liver dysfunction, royal jelly, rat, hepatectomy
Post-hepatectomy liver dysfunction is a significant challenge for surgeons dealing with liver-related diseases. One study reported that the incidence of post-hepatectomy liver dysfunction, ranging from mild to severe, can reach 27%.1 Furthermore, post-hepatectomy liver dysfunction contributes to 20-40% of post-hepatectomy mortality cases.2
Several risk factors are associated with post-hepatectomy liver dysfunction, including patient comorbidities; suboptimal liver condition; surgical factors such as extensive resection, excessive bleeding, and prolonged Pringle maneuver; and postoperative complications such as bile leakage and sepsis. post-hepatectomy liver dysfunction is characterized by impaired liver synthesis and excretory function.3
Numerous studies have investigated post-hepatectomy liver dysfunction in animal models, particularly in rats. The major liver resection model in rats is widely used for studying post-hepatectomy liver dysfunction.4 Various experimental approaches, such as the administration of N-acetylcysteine,5 steroids,6 and stem cells,7 have been explored to reduce liver dysfunction after hepatectomy.
Royal Jelly, a secretion produced by bee salivary glands, contains proteins that are known to benefit liver health. Royal Jelly have demonstrated protective effects against hepatotoxic substances,8 and can enhance hepatocyte proliferation in vitro.9 To date, no studies have examined the effects of Royal Jelly on major liver resection models in rats.
In this study, we aimed to evaluate the effect of Royal Jelly on liver function and regeneration following hepatectomy in a rat model. The researchers used bilirubin and prothrombin time to evaluate liver function, whereas Ki67 expression was used to assess liver regeneration.
The experimental animals used in this study were healthy, 10-12 week-old male Wistar rats, weighing 150-200 grams. Sick appeared animals were excluded. During the study, rats were housed individually and provided free access to food and water.
Royal Jelly was prepared as described in a previous study.10 Briefly, Raw Royal Jelly was obtained from local beekeepers (Tawon Gung, Malang, Indonesia) and stored in a freezer at -18°C until preparation of the water-soluble Royal Jelly. One gram of Raw Royal Jelly was homogenized in 10 mL of Phosphate Buffered Saline (Gibco®, 70011-044) containing 1 M Ammonium Sulfate (Nacalai Tesque, 02619-15). The solution was then centrifuged at 15,000 g for 30 minutes at 4°C. The supernatant was collected and passed through a 0.22-micron syringe filter. The water-soluble Royal Jelly solution was stored in a refrigerator at 4°C until use.
The total sugar content, fatty acid content, total protein content, and amino acid composition of water-soluble Royal Jelly preparations were analyzed using various methods.
Total sugar content was analyzed using the High-Performance Liquid Chromatography (HPLC) method. First, a series of standard sugar mixtures consisting of glucose (Sigma-Aldrich, G7528), fructose (Sigma-Aldrich, F0127), sucrose (Sigma-Aldrich, A2186), lactose (Sigma-Aldrich, 61339), and maltose (Sigma-Aldrich, 63418) were prepared in a 10 mL volumetric flask. Then, 2 mL of water soluble Royal Jelly was added into a 25 mL volumetric flask. Distilled water was added up to half the flask’s volume. Subsequently, 1 mL each of Carrez solution I and II (Chemtest) was added, and the mixture was shaken. The solution underwent sonication for 15 minutes. Distilled water was added up to the calibration mark, followed by homogenization. The solution was filtered using a 0.45-micron syringe filter into a 2 mL vial and injected into the HPLC Refractive Index Detector system (Waters 2414).
Saturated and unsaturated fatty acid content was analyzed using the Gas Chromatography (GC) method. Water-soluble Royal Jelly equivalent to 50 mg of fat was weighed and placed into a 20 mL screw-top vial. MTBE (Merck, 101849) and transesterification solutions were added, and the vial was vortexed for 10 seconds. Afterward, the vial cap was removed, and hexane was added, followed by the neutralizing solution. The mixture was centrifuged at 1750 rpm for 5 minutes. The organic phase was collected into a 2 mL vial and injected into the GC Flame Ionization Detector system (PerkinElmer Claurus 690).
Total protein content was determined using the Kjeldahl method. A total of 0.51 g of water-soluble Royal Jelly was placed in a 100 ml Kjeldahl flask. Then, 2 g of Kjeldahl tablet (Supelco, 1.10958.0250) and 25 ml of concentrated H2SO4 (Supelco, 1.00731.2500) were added. The mixture was heated to boiling for 2 hours. After cooling, the mixture was diluted and transferred to a 100 ml volumetric flask. Subsequently, 5 ml of the solution was placed into a distillation apparatus, and 5 ml of 30% NaOH (Supelco, 1.06498.5000) was added. The distillation process was carried out for 10 minutes, using 10 ml of 2% boric acid solution (Supelco, 1.00165.1000) mixed with an indicator as the receiving solution. The condenser tip was rinsed with distilled water. A 0.01 N HCl solution (JT. Baker, 9535-69) was used as the titrant.
A general amino acid analysis was conducted utilizing the Ultra-High Performance Liquid Chromatography (UHPLC) technique. A standard amino acid solution (TCI, A0280), incorporating an internal reference compound, was prepared at a fixed concentration. Subsequently, 1 mL of the water-soluble Royal Jelly solution was transferred into a 20 mL headspace vial and subjected to hydrolysis using hydrochloric acid. The resulting hydrolysate was then poured into a 50 mL volumetric flask, diluted with distilled water to reach the calibration line, and thoroughly mixed. The prepared solution underwent filtration through a 0.2-micron syringe filter, and the filtrate was collected. A second internal standard was introduced before derivatization, after which the solution was injected into the UHPLC Photodiode Array system (Waters Acquity H-Class).
The amino acids cystine and methionine were analyzed using the Liquid Chromatography-Mass Spectrometry (LC-MS/MS) method. A standard series (TRC, C994500 and M260515) was prepared in 2 mL vials using filtered solvents. A total of 0.5 g of water-soluble Royal Jelly was placed into a 20 mL headspace vial, then stored in a cooling water bath before being frozen for 15 minutes. An oxidizing solution was added, followed by homogenization. The preparation was returned to the freezer. Subsequently, sodium bisulfite (Sigma-Aldrich, 243973) was added, and the mixture was left to stand for 3 hours. A hydrolyzing solution was then added, and the mixture was heated in an oven. After cooling, the preparation was diluted with distilled water. The pH was adjusted to 2.2 in a cooling water bath, and homogenization was carried out with distilled water. The solution was centrifuged and filtered using a 0.2-micron membrane filter before injection into the LC-MS/MS Mass Spectrometer system (Sciex Triple Quad 4500).
Tryptophan was analyzed using High-Performance Liquid Chromatography (HPLC). After the preparation of the standard series (TRC, T947210), 0.1 g of water-soluble Royal Jelly was added to a 20 mL headspace vial. A 4.2 M NaOH solution (Merck, 106498) was then added, and the mixture was hydrolyzed at 110°C for 20 hours. The solution was transferred to a 50 mL beaker, and citrate buffer (Merck, 100244) was added. The pH of the solution was adjusted to 4.25, followed by homogenization with distilled water. The solution was centrifuged, and the supernatant was filtered using a 0.45-micron syringe filter before injection into the HPLC Photodiode Array system (Waters Alliance e2695).
In this study, we conducted two phases: a feasibility study and a main study. A feasibility study was performed to determine the extent of liver resection that could be performed while keeping the rats alive. Based on the findings of the feasibility study, the main study was conducted.
In the feasibility study, 10 rats were divided into four groups. The first group of rats underwent laparotomy without liver resection (n=2). The second, third, and fourth groups comprised 70% (n=2), 80% (n=2), and 90% (n=4) of the rats, respectively. The methods for anesthesia, prophylactic antibiotic administration, and pain management were consistent with those described in the main study section. All rats were monitored for survival up to 48 hours post-surgery. If they survived, they were sacrificed after 48 h, and bilirubin and prothrombin times were measured.
For the main study, surgical procedures were performed in the operating room of Airlangga University Animal Hospital. Thirty-two rats were weighed before the liver resection. Anesthesia was induced using 5% inhaled sevoflurane (Sevoflurane, Yarindo Farmatama) and maintained at 3.5% throughout the procedure. Sixteen rats were randomly assigned to receive an intravenous injection of Royal Jelly at a dose of 70 mg/kg via the tail vein immediately before liver resection, with a second dose administered 24 h post-surgery (RJ group). Another 16 rats were randomly assigned to receive an intravenous injection of 0.1 ml phosphate-buffered saline (PBS group) via the tail vein at the same time intervals. The injection via the tail vein was chosen as it is one of the recommended methods.11 Royal Jelly and phosphate-buffered saline were administered in a blinded manner. Prior to surgery, all rats received the prophylactic antibiotic Cefazolin (Cefazolin, Mahakam Betafarma) at a dose of 100 mg/kg. All rats were administered Flunixin Meglumine (Fortis®, Dong Bang) intramuscularly at a dose of 2.5 mg/kg. After surgery, the rats were housed individually with access to standard food and 10% glucose water. All rats were maintained on a 12-hour light-dark cycle. Flunixin injections were repeated every 12 h, using the same dose and route of administration. Animal suffering was minimized through the administration of analgesics, and the experimental procedures were conducted by experienced veterinary hospital staff. All the rats were sacrificed 48 h after surgery. The euthanasia method employed involves administering an intramuscular injection of a mixture of Ketamine (Ket-A-100®, AgrovetMarket) and Xylazine (Xyla®, Interchemie) at an excessive dose (150–15 mg/kg). Subsequently, the previously made laparotomy incision is reopened and extended towards the thoracic cavity. Intracardiac blood samples were collected for bilirubin and prothrombin time analysis. The remaining liver tissue was harvested and fixed in 10% formalin for pathological examination. We report this experiment in accordance with the ARRIVE guidelines.12 This study has received approval from the Animal Care and Use Committee of the Faculty of Veterinary Medicine, Airlangga University, under license number “2. KEH.047.04.2024” on April 4, 2024.
Once anesthetized, the abdominal fur of the rats was shaved. Antisepsis was achieved by applying 10% povidone-iodine to the abdominal skin. Surgery was performed in a sterile environment. A 3.5 cm midline incision was made on the cranial abdomen, starting from the xiphoid process and extending caudally. Abdominal wall was retracted to provide visibility of the liver. The ligaments were detached, and resection was performed on the median lobe, left lateral lobe, and caudate lobe following ligation by piercing with 4-0 Silk sutures placed 3 mm from the vena cava. The superior and inferior right lobes were preserved. The surgical area was then irrigated with 3 ml of saline solution. The abdominal wall was closed using continuous 4-0 Polyglactin sutures, and the skin was sutured using interrupted 3-0 Silk ( Figure 1).
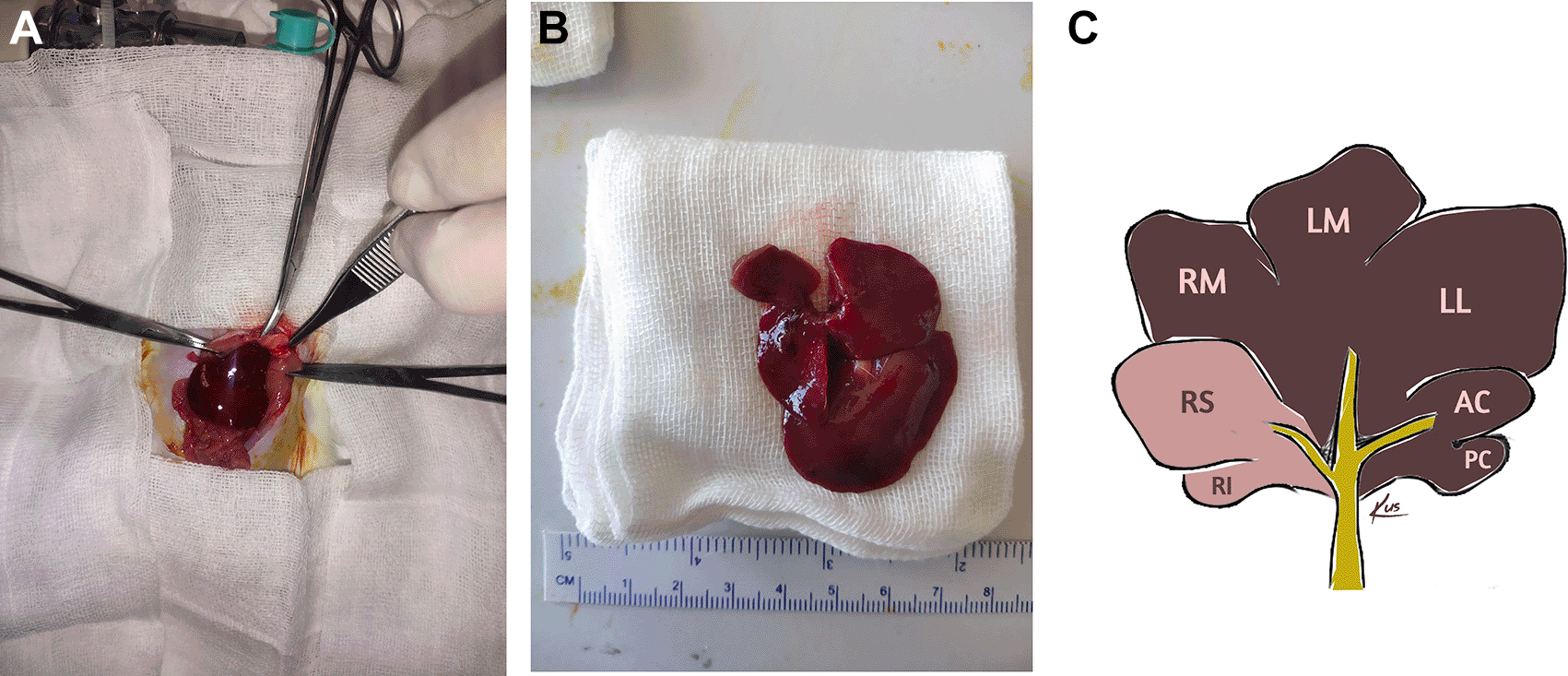
RM: Right Median Lobe; LM: Left Median Lobe; LL: Left Lateral Lobe; AC: Anterior Caudatus Lobe; PC: Posterior Caudatus Lobe; RS: Right Superior Lobe; RI: Right Inferior Lobe. The shaded area indicates the portion of the liver that was resected in this study.
Forty-eight hours post-surgery, the surviving rats were sacrificed and the superior right lobe of the liver was harvested and fixed in 10% neutral buffered formalin. Paraffin blocks were then prepared from the samples, sectioned at a thickness of 3-5 microns, and mounted onto special slides. Deparaffinization was performed using xylol, followed by rehydration in 96, 90, and 80% alcohol for 2 minutes each. The slides were rinsed with running and distilled water for 5 minutes. Next, the slides were placed in 3% H2O2 (Biogear, BGPB-0500) in methanol for 15 minutes at room temperature, rinsed with distilled water for 5 minutes, and heated in Target Retrieval Solution/Citrate Buffer (Biocare Medical, BRRR2004CMX) at pH 6 in a decloaking chamber for 20 minutes at 95°C. The slides were then washed with phosphate-buffered saline (Biogear, BGPBS-002) for 5 minutes. A background snipper was applied for 15 minutes, followed by application of the primary antibody. The study used rabbit monoclonal antibody, clone SP6, and isotype IgG against Ki67 (Biocare Medical, 901-325-060223) at a dilution of 1:50. The expression of Ki67 in hepatocytes was evaluated by identifying positively stained nuclei using an anti-Ki67 antibody. The Ki67 score was based on the percentage of positively stained cells (%) in hotspot areas, analyzed under a binocular light microscope (Olympus CX31) at 400x magnification, and documented using the Olympus DP2-BSW software (see Software Availability statement). Ki67 examination was conducted by an Anatomical Pathology specialist who was blinded to the treatments applied to the specimens under review.
The Mann-Whitney test was used to analyze bilirubin and prothrombin time values, while a t-test was used to analyze the Ki67 percentage. Statistical p-value of <0.05 was considered statistically significant. Statistical analysis was performed using SPSS software (IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY), and JASP software (JASP Team (2024) JASP (Version 0.18.3) [computer software]) (see Software Availability statement).
The water-soluble Royal Jelly used for intravenous preparations contained 1.04% total sugar. Several fatty acids were analyzed but not detected, including dodecanoic acid, decanoic acid, tetradecanoic acid, pentadecanoic acid, heptadecanoic acid, (Z,Z)-9,12-octadecadienoic acid, (Z,Z,Z)-9,12,15-octadecatrienoic acid, eicosanoic acid, docosahexaenoic acid, and tetracosanoic acid. The analysis detected the presence of saturated and unsaturated fatty acids, each at a concentration of 0.01%. Hexadecanoic acid and (9Z)-Octadec-9-enoic acid were detected at levels below 0.01%, while Omega-9 was detected at a concentration of 7.8 mg/100 g. The total protein content was 14.3%. Several amino acids were detected at varying levels. The most abundant amino acids were aspartic acid and glutamic acid. The least abundant amino acids were alanine, cystine, and methionine. The summary of the Royal Jelly component analysis are presented in Table 1. The chromatograms of the conducted analyses are presented in Figures 2-4.
| No | Parameter | Value | Unit | Method |
|---|---|---|---|---|
| 1 | Total Sugar | 1.04 | % | HPLC |
| 2 | Fructose | 0.39 | % | HPLC |
| 3 | Glucose | 0.35 | % | HPLC |
| 4 | Sucrose | 0.29 | % | HPLC |
| 5 | Hexadecanoic acid | 0.0084 | % | GC |
| 6 | (9Z)-Octadec-9-enoic acid | 0.0078 | % | GC |
| 7 | Saturated Fat | 0.01 | % | GC |
| 8 | Unsaturated Fat | 0.01 | % | GC |
| 9 | Monounsaturated Fat | 0.01 | % | GC |
| 10 | Omega-9 Fatty Acid | 7.8 | mg/100 g | GC |
| 11 | Total Protein | 14.3 | % | Kjeldahl |
| 12 | L-Alanine | 220.12 | mg/kg | UPLC-PDA |
| 13 | L-Arginine | 526.36 | mg/kg | UPLC-PDA |
| 14 | L-Aspartic Acid | 1018.55 | mg/kg | UPLC-PDA |
| 15 | Glycine | 320.48 | mg/kg | UPLC-PDA |
| 16 | L-Glutamic Acid | 654.73 | mg/kg | UPLC-PDA |
| 17 | L-Histidine | <295.11* | mg/kg | UPLC-PDA |
| 18 | L-Isoleucine | 387.02 | mg/kg | UPLC-PDA |
| 19 | L-Cystine | 164.55 | mg/kg | LC-MS/MS |
| 20 | L-Leucine | 595.2 | mg/kg | UPLC-PDA |
| 21 | L-Lysine | 546.53 | mg/kg | UPLC-PDA |
| 22 | L-Methionine | 109.09 | mg/kg | LC-MS/MS |
| 23 | L-Valine | 450.41 | mg/kg | UPLC-PDA |
| 24 | L-Phenylalanine | <476.07* | mg/kg | UPLC-PDA |
| 25 | L-Proline | 426.29 | mg/kg | UPLC-PDA |
| 26 | L-Serine | 538.1 | mg/kg | UPLC-PDA |
| 27 | L-Threonine | 379.87 | mg/kg | UPLC-PDA |
| 28 | L-Tyrosine | <608.01* | mg/kg | UPLC-PDA |
All rats that underwent 90% liver resection (4/4) died before the 48-hour mark, with the longest survival time in this group being 17 h. The other three groups survived for 48 hours post-surgery. From the feasibility study, we determined that 70% and 80% of liver resections were feasible. Liver dysfunction was more pronounced in rats that underwent 80% liver resection than in those that underwent 70% resection. Bilirubin and prothrombin time values are listed in Table 2. Based on the results of the feasibility study, we decided to perform 80% liver resection in the main study.
The rat body weight, surgery duration, and weight of the resected liver are presented in Figure 5. These parameters showed no significant differences (p>0.05). Four of the 4 rats out of 32 (two from the RJ group and two from the PBS group) died before the scheduled sacrifice time. Therefore, 28 rats were included in this analysis.
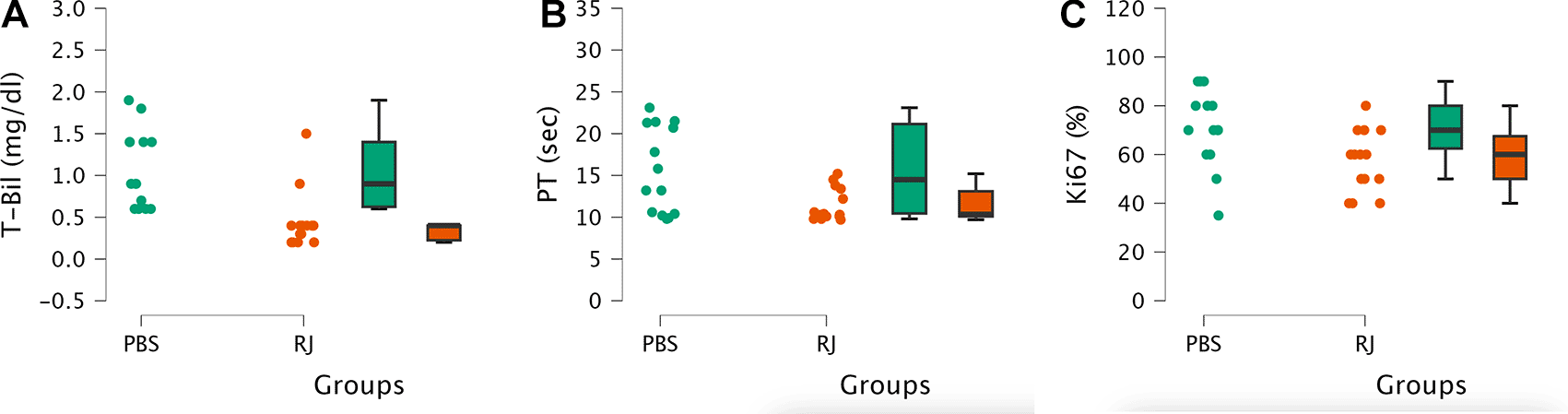
In both groups, some rats exhibited elevated total bilirubin levels and prothrombin time compared with rats without liver resection in the feasibility study ( Figure 6). The median total bilirubin level in the RJ group was significantly lower than that in the PBS group (0.4 [0.2-1.5] mg/dl versus 0.9 [0.6-1.9] mg/dl respectively, p<0.001). The median prothrombin time in the RJ group was lower than that in the PBS group (10.35 [9.7-15.2] sec versus 14.5 [9.8-23.1] respectively, p<0.05). In addition, the mean percentage of Ki67 in the RJ group was lower than that in the PBS group (57.14 ± 12.67% versus 71.07 ± 15.95% respectively, p<0.05). Several documented images of the Ki67 examination are shown in Figure 7.
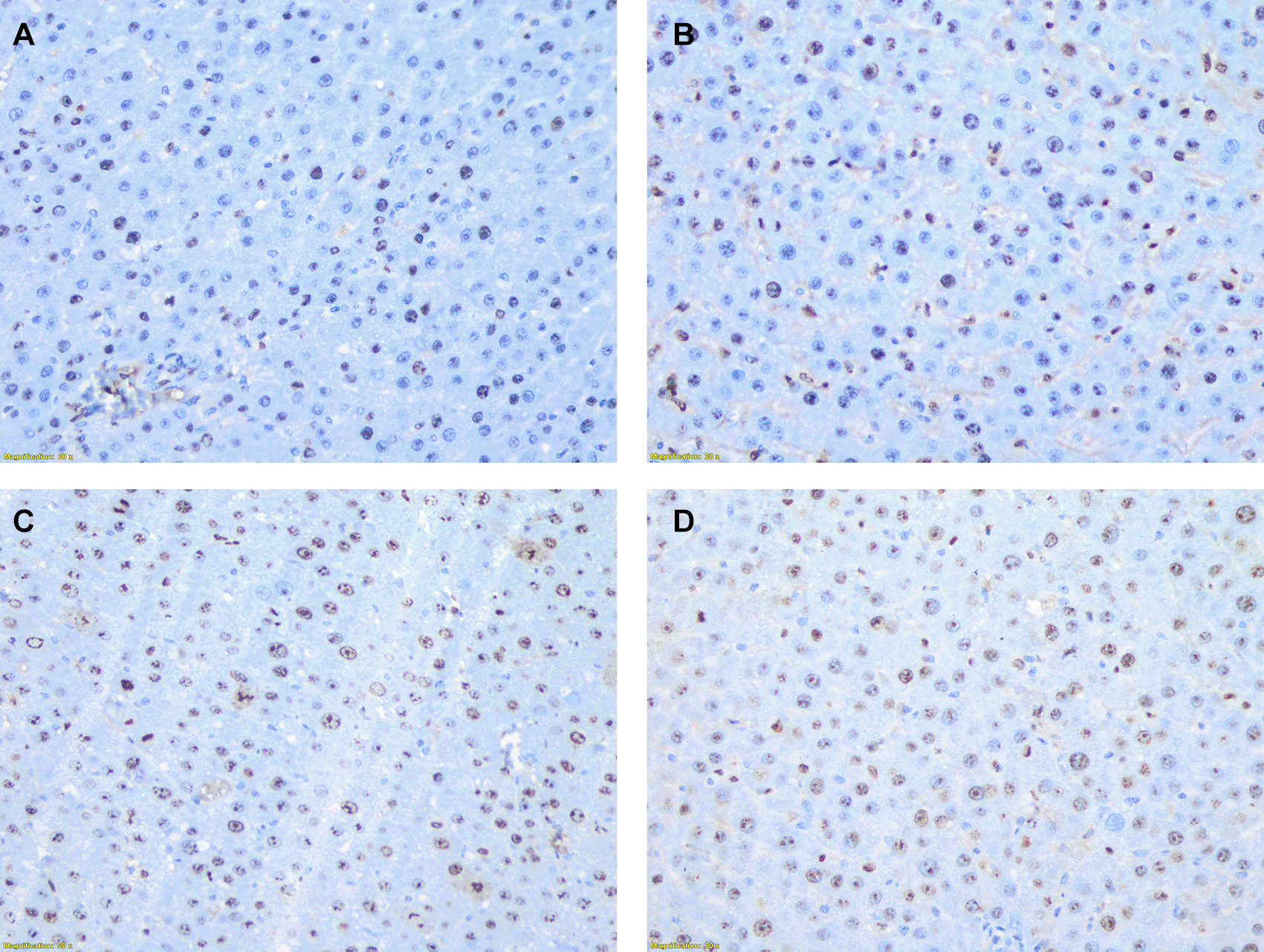
The image above illustrates the microscopic structure of liver tissue (400x magnification), showing the distribution of hepatocyte cells examined using Ki67 antibody staining. Hepatocyte cells are considered positive for Ki67 when their nuclei are stained with a brownish color. The staining is observed at varying intensities: weak intensity with light brown staining, moderate intensity with brown staining, and strong intensity with dark brown staining. In contrast, cells that are unstained or negative will have nuclei that remain blue. Image A-D show percentage of positively stained hepatocye with varying intensities. A: 35%; B: 50%; C: 80%, and D: 90%.
Post-hepatectomy liver dysfunction remains a significant concern for surgeons performing liver resections because it is associated with a high risk of mortality. Numerous studies have been conducted to prevent post-hepatectomy liver dysfunction, and this study aimed to contribute to the existing body of research on post-hepatectomy liver dysfunction prevention.
Rats were selected as experimental subjects because of their widespread use and the availability of extensive references on these techniques.4,13 Wistar rats were chosen for their accessibility, ease of handling, nonaggressive nature, and suitability for various types of research.14
In clinical practice, post-hepatectomy liver dysfunction is most commonly defined using criteria established by the International Study Group of Liver Surgery.3 However, no standardized criteria exist for assessing this condition in rats. In this study, we defined post-hepatectomy liver dysfunction as an increase in bilirubin levels (indicating impaired excretory function) and prolonged prothrombin time (reflecting reduced synthetic capacity) at the 48-hour assessment, relative to baseline values observed in non-hepatectomized rats from a preliminary analysis (feasibility study).
Before initiating this study, we conducted a literature review and found that the optimal liver resection volume in rats was 90%. Several authors have performed 90% liver resection, with varying outcomes. Some studies reported a 100% survival rate at 7 days post-resection,15,16 whereas others reported a 40% survival rate within 2 days.17 Based on these findings, a feasibility study was conducted, revealing that 90% liver resection was not feasible, as none of the 4 rats survived beyond 24 h. Rats that underwent 80% liver resection survived and exhibited the expected liver dysfunction. Therefore, 80% resection volume was selected for this study.
Bilirubin and prothrombin time were chosen as indicators of liver dysfunction because they are widely used.3 Ki67, a proliferation marker, was selected to investigate the relationship between the treatment effects and liver regeneration. Ki67 is a protein involved in the formation of the peri-chromosomal layer during mitosis and is expressed in all phases of cell proliferation, except G0.18 The 48-hour timeframe was selected based on previous studies, as it is optimal for capturing bilirubin elevation, liver synthetic dysfunction, and Ki67 proliferation markers.17,19,20
We focused on Royal Jelly as the primary intervention because of its well-known protective properties against liver damage8 and its demonstrated ability in in vitro studies to enhance hepatocyte proliferation.9 We hypothesized that Royal Jelly might have an effect in cases of major hepatectomy. To the best of our knowledge, no previous study has examined the effects of Royal Jelly on major hepatectomy.
Raw Royal Jelly was obtained from local Apis mellifera. For the preparation of the water-soluble Royal Jelly, we used raw Royal Jelly as the starting material. The raw Royal Jelly was superior because of its fresh storage from the beekeepers and preservation in frozen conditions, in contrast to commercially packaged Royal Jelly, which often contains additives and is stored at room temperature.21
Laboratory analysis using Kjeldahl method revealed a protein content of 14.3% in the water-soluble form for intravenous administration. Chromatographic analysis detected a variety of amino acids ( Table 1). The protein and amino acid content of water-soluble royal jelly does not differ significantly from that of raw royal jelly.22 The total sugar content was 1.04%, while the content of both saturated and unsaturated fatty acids was 0.01% each. The extraction process we employed appears to have minimized the sugar and fatty acid content of the raw Royal Jelly, which typically ranges from 2.4–6.4% for fatty acids23 and 7–18% for sugars.24 We suspect that the protein content in the water-soluble Royal Jelly preparation is responsible for the effects observed in our research.
Royal Jelly contains multiple proteins, collectively referred to as the Major Royal Jelly Proteins (MRJPs). Nine MRJPs with different benefits were identified. MRJPs play a critical role in its biological activities. MRJP 1 exhibits anti-inflammatory, antimicrobial, and cell proliferation effects; MRJP 2 has antioxidant and wound-healing properties; and MRJP 3 demonstrates immunomodulatory effects.25 In this study, we utilized the extraction technique described in previous research. This extraction process produced a water-soluble Royal Jelly preparation containing MRJP 1, MRJP 2, and MRJP 3 components.10 However, we did not isolate specific proteins to administer to rats undergoing hepatectomy. This decision was made because our primary objective was to determine whether the Royal Jelly extract could be safely administered intravenously and whether it could achieve the desired effects. The results of this study will serve as a foundation for future research to identify which specific protein isolates are responsible for the observed effects.
This extraction process produced a clear water-soluble Royal Jelly preparation, which, when combined with a syringe filter, was considered suitable for intravenous administration.26 This administration method was chosen because orally administered Royal Jelly proteins are broken down by digestive enzymes,27 with previous studies showing Royal Jelly protein inactivation by trypsin.9
The results of this study showed that rats treated with Royal Jelly had lower median bilirubin and prothrombin time levels, indicating a protective effect of Royal Jelly against liver dysfunction after hepatectomy. However, the RJ group also had a lower mean Ki67 percentage, indicating lower proliferation than the PBS group. Studies on the relationship between proliferation and liver function after hepatectomy have yielded mixed results. One study reported that higher proliferation was correlated with lower bilirubin levels, suggesting that high proliferation occurs alongside improved liver function.28 However, other studies have found that increased Ki67 proliferation is associated with elevated bilirubin levels, indicating poorer liver function.19,29 This study aligns with the findings of latter studies, suggesting that proliferating hepatocytes may be immature and have suboptimal function.30–32
Based on the liver function and proliferation results, we hypothesized two possible mechanisms for Royal Jelly in hepatectomized rats: Royal Jelly accelerates proliferation, leading to quicker cell maturation and improved function, or Royal Jelly exerts a protective effect on the remaining liver, reducing the need for a high proliferative response to maintain functional hepatocytes. The rationale behind the first hypothesis is based on previous studies, which demonstrated that the post-hepatectomy increase in Ki67 levels follows a parabolic curve, with a peak occurring around day 2 and declining from day 3 onward.19,20 The rationale for the second hypothesis was that Ki67 proliferation levels are closely and directly correlated with the degree of liver injury, as described in an earlier study.33 Further studies are required to confirm this hypothesis.
This is the first study to investigate the effects of Royal Jelly on liver surgery, yielding meaningful results. The limitations of the study include the single time point for specimen analysis and the limited examination of the mechanism of action of Royal Jelly. These findings raise questions for future research, such as the optimal dosage of Royal Jelly, comparisons with other nutrients, and the impact of laboratory and histopathological parameters in serial examinations at 24-hour intervals. In the future, if the results are promising, the specific Royal Jelly protein isolates responsible for these effects can be investigated.
This study demonstrates that intravenous administration of Royal Jelly in rats undergoing 80% liver resection successfully reduced liver dysfunction. The administration of Royal Jelly also decreased hepatocyte proliferation. These findings suggest that Royal Jelly warrants further detailed investigation for its potential benefits in the field of hepatology, both in surgical and medical contexts.
Zenodo: Royal Jelly Rat Hepatectomy. https://doi.org/10.5281/zenodo.14700286.34
The project contains the following underlying data: Supplementary File 1.xlsx.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Reporting guidelines
Zenodo: ARRIVE checklist for Royal Jelly Rat Hepatectomy. https://doi.org/10.5281/zenodo.14736694.35
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
Ki67 documentation used Olympus DP2-BSW software and is available to download at https://evidentscientific.com/en/downloads?product=DP2.
Statistical analysis may be performed using free software from JASP and is available to download at https://jasp-stats.org/.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)