Keywords
Frozen embryo transfer, Infertility, Assisted reproductive technologies, Isthmocele, Intra-cavitary fluid, Residual myometrial thickness.
Isthmocele, or uterine niche, is a defect in the anterior wall of the uterine isthmus, often following a cesarean section. It can lead to complications such as abnormal uterine bleeding, infertility, and pregnancy risks.
This case report describes a successful live pregnancy after isthmocele repair and embryo transfer. A 28-year-old woman (para 1, living 1) presented with prolonged periods, dysmenorrhea, and secondary infertility. Transvaginal ultrasound revealed a large isthmocele and intra-cavitary uterine fluid. The patient underwent ovarian stimulation using an antagonist protocol twice, retrieving oocytes and freezing nine embryos. She later had laparoscopic isthmocele repair at another facility.
Upon returning for frozen embryo transfer, irregular bleeding and dysmenorrhea persisted. Repeat ultrasound showed a small cesarean defect (4x3 mm), fluid in the endometrial cavity, and hyperechoic endometrium. Fluid aspiration was performed, followed by the transfer of a grade AA embryo. Although conception occurred, it was a biochemical pregnancy with a βhCG level of 12.43 mIU/ml.
Due to a persistent scar defect and endometrial cavity fluid, the patient underwent hysteroscopy, endometrial biopsy, and minimal resection of the defect. Three months later, a frozen embryo transfer of a grade AB embryo was performed. This resulted in a viable pregnancy, confirmed by a βhCG level of 364 mIU/ml. The pregnancy progressed without complications, and at 36+3 weeks, the patient delivered a healthy baby via planned cesarean section.
This case underscores the importance of diagnosing and managing isthmocele in infertility patients. Surgical repair can restore normal uterine anatomy and enhance the success of assisted reproductive techniques like embryo transfer.
Frozen embryo transfer, Infertility, Assisted reproductive technologies, Isthmocele, Intra-cavitary fluid, Residual myometrial thickness.
In recent decades, the global incidence of cesarean sections has surged, leading to an increased risk of complications. As the rate of cesarean sections continues to rise, there is increasing awareness of the potential complications that can follow the procedure. One such complication is the development of isthmoceles, also known as caesarean scar defects or uterine niches, which are associated with a range of gynecological and obstetrical problems.1 A long-term complication of a cesarean section (CS) is the development of a niche, which results from incomplete healing of the myometrium at the CS scar site. The European Niche Taskforce defines niches as an “indentation of the uterine myometrium of at least two millimeters at the site of the CS scar.” These niches can be evaluated using transvaginal ultrasound.2 The incidence of isthmocele ranges from 24% to 70% in women screened via transvaginal sonography, and from 56% to 84% in those screened via saline instillation sonohysterography.3 An isthmocele can negatively affect a woman’s quality of life and her reproductive abilities. It may impair the uterus’s capacity to safely sustain a pregnancy or even obstruct the ability to conceive.4 The link between isthmocele and infertility is thought to arise from hindered sperm migration and viability. This may result from the accumulation of blood and inflammatory exudate within the niche, along with alterations in myometrial contractility.5 Other associated symptoms include prolonged menstrual bleeding, spotting, persistent pelvic pain, irregular uterine bleeding, and dysmenorrhea. Isthmocele negatively affects embryonic vitality, implantation success, and pregnancy progression. This impact may arise from altered uterine contractility and an unfavorable implantation environment due to the accumulation of blood or fluid within the uterus.6 A large isthmocele has been linked to the development of intra-cavitary fluid (ICF) and negatively impacts in-vitro fertilization (IVF) outcomes in infertile patients undergoing controlled ovarian stimulation cycles. Hysteroscopic resection is a minimally invasive procedure aimed at removing fibrotic tissue, cauterizing vessels, and resecting the edges of the defect. The main aim of the surgery is to excise the niche and reconstruct the uterine defect at the cesarean scar to enhance fertility outcomes in women with secondary infertility.7 Given that most of the available evidence is derived from retrospective case series and case reports, there is no definitive proof to indicate that one approach is superior to others.
A 28-year-old female (para 1, living 1) presented at our fertility center with complaints of prolonged periods, dysmenorrhea, and a history of secondary infertility. She reported having regular menstrual periods but experienced prolonged bleeding that persisted until the next menstrual cycle. Additionally, she expressed significant pain during her menstrual periods. Her previous pregnancy had ended in an emergency cesarean section due to a lack of progress during labor and failed induction. She also had a history of two failed intra-uterine inseminations (IUI) performed one year prior at another facility. A transvaginal ultrasound identified a retroverted uterus with a large isthmocele and fluid in the endometrial cavity ( Figure 1). The patient underwent ovarian stimulation using an antagonist protocol on two occasions, resulting in the retrieval of oocytes and the freezing of nine embryos. Subsequently, she underwent laparoscopic correction of the isthmocele at another facility. Three months post-procedure, she returned to clinic for a frozen embryo transfer (FET). On the tenth day of the FET cycle, a repeat ultrasound showed a small cesarean scar defect measuring 4×3mm with fluid in the endometrial cavity and hyperechoic endometrium as shown in Figure 2. An intra-uterine insemination catheter was used to aspirate the fluid. A single grade AA embryo was transferred, resulting in a biochemical pregnancy with a βhCG level of 12.43 mIU/mL. Given the persistent small cesarean scar defect and fluid in the endometrial cavity, she was referred to our laparoscopic surgeon. Her ultrasound continued to show significant endometrial fluid with a hyperechoic endometrium, leading to a decision to perform a hysteroscopy with endometrial biopsy and minimal resection of the cesarean scar defect as shown in Figure 3. Post-surgery, a single grade AB embryo was transferred during a subsequent FET cycle. This resulted in a pregnancy detected with a positive βhCG level of 364 mIU/mL. The patient was closely monitored throughout her pregnancy, which progressed without complications. At 36+3 weeks of gestation, she delivered a healthy baby weighing 3090 grams via planned cesarean section, considering the previous isthmocele repairs and the presence of a scar niche. This case highlights the challenges and management of secondary infertility associated with isthmocele, prolonged menstrual bleeding, and dysmenorrhea. It underscores the importance of meticulous surgical intervention and careful monitoring in achieving a successful pregnancy outcome. Isthmocele repair, combined with frozen embryo transfer, can lead to a successful pregnancy even in the presence of a cesarean scar defect. Close monitoring and appropriate surgical intervention are crucial in managing such cases to ensure favorable maternal and fetal outcomes.
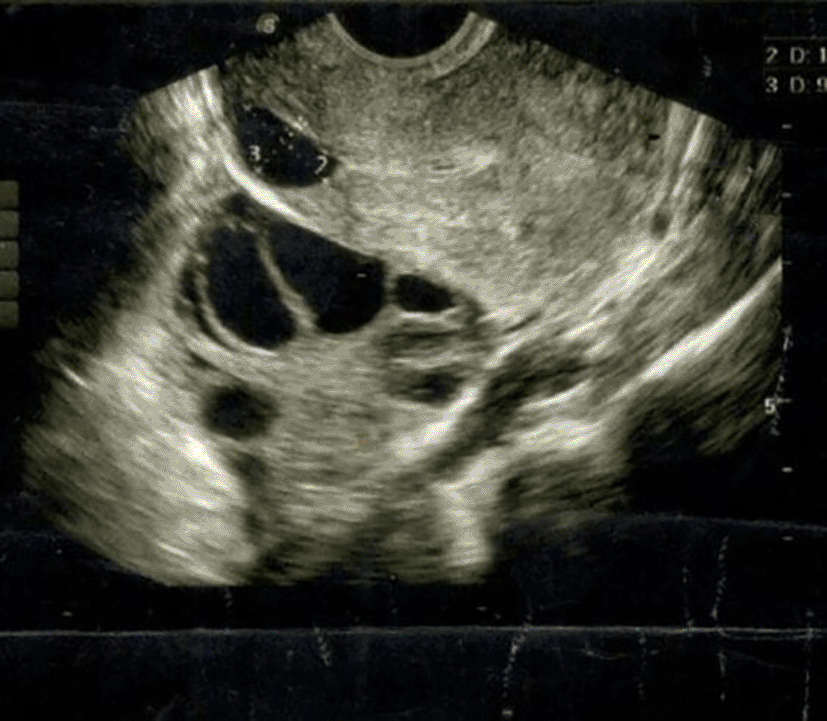
Isthmocele, a known complication in women with a previous cesarean section, can cause symptoms like spotting, dysmenorrhea, pelvic pain, and infertility. Consequently, healthcare providers should be attentive to the possibility of isthmocele in women with these symptoms, especially if they have a history of cesarean deliveries. Transvaginal ultrasound is the primary imaging technique for diagnosing an isthmocele. It is typically seen as a triangular defect in the isthmic portion of the anterior uterine wall, with its base facing the uterine cavity.8 The shape and morphology of the defect can differ, with the anterior isthmus potentially presenting as a round, square, wedge-shaped cavity, or even a cribriform area. In a retrospective cohort study conducted by Vissers J in 2020, surgical hysteroscopic correction of isthmocele in patients with secondary infertility and abnormal uterine bleeding proved effective in restoring fertility within 6–12 months post-surgery.9 Women with isthmocele are at an increased risk of complications in subsequent pregnancies. These complications include miscarriage, cesarean scar pregnancy (CSP) with potentially life-threatening hemorrhage, placenta previa, placenta accreta, and uterine dehiscence or rupture during the third trimester of pregnancy.10
A retrospective case control study conducted by Wang YQ et al., 2017 involving 310 patients undergoing IVF revealed that women with a previous cesarean section had a lower clinical pregnancy rate compared to those with a prior vaginal delivery.11 This is believed to be due to the presence of intra-cavitary fluid creating a hostile environment for embryonic implantation, likely because of the embryotoxic effects of high iron concentrations resulting from hemoglobin degradation. Another study by Zhang Y et al., 2022 explored the relationship between isthmocele size and assisted reproductive technology (ART) outcomes. His findings indicated that women with larger isthmoceles had lower implantation rates, experienced more complications during IVF treatment, and had higher rates of spontaneous abortions and decreased rates of ongoing pregnancies compared to those with smaller isthmoceles.12 These results suggest that the size of an isthmocele can significantly impact implantation capacity and embryo survival.
Hysteroscopy is generally the preferred method for treating isthmocele.13 While hysteroscopic isthmoplasty has been associated with higher pregnancy rates, the observational nature and small sample sizes of existing studies prevent firm conclusions about the superiority of this technique over others. In cases involving infertility, ectopic pregnancy, severe isthmocele, lower parity, and fewer cesarean sections, laparoscopic isthmoplasty is often preferred.14 Current scientific evidence is limited and does not permit a definitive choice of surgical method or approach for isthmocele repair. However, surgery should be considered if fluid is visible within the endometrial cavity, with careful consideration of the risk-benefit balance. If isthmocele is associated with decreased myometrial thickness, laparoscopic surgery might be recommended.15 This technique allows the surgeon to access the isthmocele and reinforce the anterior uterine wall. It is particularly advised for larger defects to minimize the risk of complications such as uterine rupture. Marotta et al. (2013) classified isthmoceles with less than 3 mm of residual myometrium as large and those with 3 mm or greater of residual myometrium as small.16 They suggested that hysteroscopic resection is a safe, quick, and effective method for managing symptoms in patients with small defects. For patients with larger defects who wish to preserve fertility, laparoscopy is considered the preferred approach.
The success of isthmocele repair and subsequent embryo transfer highlights the effectiveness of laparoscopic and hysteroscopic interventions. The combination of isthmocele repair and targeted endometrial management improved the uterine environment, facilitating a successful implantation and pregnancy. Our case highlights several challenges in managing secondary infertility associated with isthmocele. Persistent defects and endometrial fluid can complicate the treatment process, necessitating multiple interventions. In our case the initial FET cycle resulted in a biochemical pregnancy, highlighting the impact of the isthmocele on implantation and early pregnancy maintenance. Despite the initial post-repair ultrasound showing residual fluid and a small cesarean scar defect, the subsequent hysteroscopic intervention was crucial in addressing these issues. The negative impact on IVF outcomes appears to be exacerbated by the accumulation of intra-cavitary fluid before embryo transfer. Resection of the scar niche and aspiration of endometrial fluid helped optimize the uterine environment for embryo transfer.
In the study conducted by Lawrenz et al.,17 it was found that patients developed intra-cavitary fluid during the endometrial preparation phase prior to a FET, compared to during fresh cycles. These findings suggest that opting for an elective FET cycle could be a viable strategy for women who experience intra-cavitary fluid during controlled ovarian stimulation.
The choice of surgical approach for treating isthmocele depends on various factors, including the nature of the defect, the patient’s desire for future fertility, and the surgeon’s expertise with different techniques. Laparoscopic surgery is a more intricate and demanding procedure, and only a limited number of surgeons are trained in this method. Conversely, not all gynecologists are skilled in hysteroscopic resection of isthmoceles. As a result, the choice of technique may often be influenced by the surgeon’s individual skills rather than by the specific requirements of the scar defect. Pregnancy rates following hysteroscopic resection of the isthmocele varied significantly, ranging from 6.6% to 100%. In cases where symptoms continued after the initial hysteroscopic procedure, a second operation was performed, leading to symptom improvement without complications. Bujold et al, 2009 reported no complications after laparoscopic repair, with recurrence rates varying between 0% and 33%. He reported that patients who still experienced symptoms following laparoscopic surgery, can proceed with a hysteroscopic resection.18
Addressing isthmocele is vital in the context of IVF treatment due to its potential impact on reproductive outcomes, which requires personalized management strategies. Non-invasive treatment options for isthmocele have been suggested as possible pretreatment measures for patients preparing for IVF cycles.19 Early detection and effective management of isthmocele are crucial for enhancing IVF success rates and improving overall reproductive outcomes in women with a history of cesarean sections.
When surgical correction of isthmocele is necessary, assessing the residual myometrial thickness is crucial. For patients with a residual myometrial thickness greater than 2.5–3 mm, hysteroscopy is generally preferred. In cases where the residual myometrial thickness is less than this, options such as laparotomy, laparoscopy, or vaginal approaches may be considered as shown in Table 1.20
There is no consensus on the diagnostic criteria or surgical method for isthmocele at present. However, it is evident that symptomatic patients should receive treatment, and surgical intervention is generally required for patients experiencing secondary infertility.
This case illustrates the complex interplay between isthmocele and secondary infertility, highlighting the significant impact of a cesarean scar defect on reproductive outcomes. The patient’s journey, characterized by prolonged menstrual bleeding, dysmenorrhea, and multiple failed IUI attempts, underscores the critical role of accurate diagnosis and targeted treatment in managing secondary infertility. The successful outcome achieved through meticulous laparoscopic isthmocele repair, followed by careful monitoring and a well-timed frozen embryo transfer, demonstrates the potential for positive results even in the face of challenging surgical and reproductive conditions. The combination of advanced surgical techniques and personalized ART protocols was instrumental in overcoming the barriers posed by the isthmocele and cesarean scar defect. This case emphasizes the necessity for an integrated approach involving both surgical and reproductive strategies to optimize fertility outcomes. Ongoing research and clinical experience are continually refining the management of such cases, emphasizing the importance of individualized care and thorough evaluation to achieve successful pregnancies in patients with complex fertility issues.
Written informed consent for publication of the clinical details and the clinical images were obtained from the patient.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)