Keywords
Neuroinflammation, Cytokines, Microglia, Nanobiosensors, Cannabinoids
This article is included in the Neuroinflammation collection.
Neuroinflammation plays a critical role in the progression of neurological disorders such as Alzheimer’s and Parkinson’s diseases, as well as chronic pain. It is characterized by elevated production of pro-inflammatory cytokines, including IL-6, and TNF-α, by microglia, the resident immune cells of the central nervous system. Emerging research highlights the involvement of the endocannabinoid system (ECS) in neuroinflammation, proposing cannabinoids as potential therapeutic agents owing to their immunosuppressive and anti-inflammatory properties.
This study used an in vitro model, specifically the BV-2 murine microglial cell line, to assess neuroinflammation. Cell viability was measured using the MTT assay, and cytokine production was quantified using advanced nanobiosensor technologies, including Surface Plasmon Resonance (SPR). Immunocytochemistry (ICC) was used to evaluate protein expression. Microglial cells were treated with varying concentrations of dexamethasone sodium phosphate (Dex-SP), cannabidiol (CBD), a full-spectrum tetrahydrocannabinol (FSE-THC) extract (70% THC), or a combination of the two at a 1:1 ratio (FSE-THC/CBD).
The treatments did not affect BV-2 microglial cell viability. Nitric oxide (NO) production remained unaltered following treatment with Dex-SP, CBD, FSE-THC 70%, or FSE-THC:CBD in LPS-stimulated BV-2 microglia. However, CBD and FSE-THC:CBD significantly reduced TNF-α levels, whereas Dex-SP and CBD decreased IL-6 expression. Furthermore, Dex-SP, CBD, FSE-THC (70%), and FSE-THC:CBD modulated the activation of Bcl-2, Bax, TNF-α, IFN-γ, NF-κB, and iNOS in concentration- and treatment-specific manners.
Dex-SPs demonstrate acute anti-inflammatory effects in LPS-stimulated BV2 microglial cells. Specific cannabinoids at particular concentrations exhibit comparable or superior efficacy in modulating key inflammatory mediators. These findings suggest the therapeutic potential of cannabinoids in neuroinflammation, and emphasize the need for further investigation to optimize their application.
Neuroinflammation, Cytokines, Microglia, Nanobiosensors, Cannabinoids
A crucial factor in various neurological conditions, including neurodegenerative disorders like Alzheimer’s and Parkinson’s diseases, as well as chronic pain, is neuroinflammation. This process is marked by the release of pro-inflammatory cytokines, which significantly contribute to the development and progression of these disorders.1
Central sensitization, a key mechanism in pain modulation, is significantly influenced by proinflammatory cytokines such as Tumor Necrosis Factor-alpha (TNF-α) and interleukin-6 (IL-6). TNF-α markedly enhances excitatory neurotransmission in the spinal cord. Concurrently, IL-1β and IL-6 contribute to the dysregulation of neurotransmitters in the pain-related neural pathways. This alteration in neurotransmitter balance promotes central sensitization and amplifies pain sensitivity, establishing these cytokines as crucial components in the development of pathological pain.2,3 Microglia, the primary immune cells of the central nervous system (CNS), play a crucial role in the development of neuropathic pain. In recent decades, substantial evidence has highlighted the capacity of microglia to induce dysfunction in the nervous system following nerve injury, thus contributing to pain regulation.4 These cells exert their influence by releasing cytokines such as TNF-a,5 and IL-6,6,7 which drive the process of central sensitization, a state in which CNS neurons become hyper-responsive to stimuli. Therefore, neuroinflammation is a critical factor in the transition from acute to chronic pain.2,4 Cannabinoid receptor type 2 (CB2R) expressed on microglia has emerged as a promising target for therapies addressing pain related to neuroinflammatory conditions, such as chronic pain, owing to its role in mediating analgesia with minimal psychoactive effects. Modulation of CB2R has been shown to affect spinal microglial activity and offers potential for developing novel treatment strategies for neuroinflammation.8 Additionally, microglia, as key regulators of both the central nervous and immune systems, play a crucial role in orchestrating neuroinflammatory responses. Investigating these responses under well-controlled in vitro conditions is essential for elucidating their underlying mechanisms and exploring potential therapeutic strategies.
The endocannabinoid system (ECS) modulates neuroinflammation and may offer potential therapeutic options for the treatment of chronic pain.9 Two types of cannabinoid receptors have been characterized, CB1 and CB2. CB1 is expressed in neural cells and mediates the psychoactive activity of cannabinoids, whereas CB2 is abundant in the immune system and is involved in immunomodulation.10 Cannabinoid derivatives interact with the ECS and have demonstrated immunosuppressive,11 anti-inflammatory,12 pro-apoptotic,13 neuroprotective14 and antitumor properties.13,15 These properties make them promising candidates for the treatment.16 Approximately 120 different cannabinoids have been identified in preparations from the Cannabis plant, including Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). THC is the main psychoactive constituent of cannabis, whereas CBD has no known psychoactive effects.17
Both THC and CBD are widely utilized for therapeutic applications,18 as they reduce cytokine production in human immune cells while inhibiting both T cell proliferation and effector functions.19 When exposed to proinflammatory stimuli, microglia treated with THC or CBD exhibit decreased secretion of cytokines, chemokines, and neurotrophic factors.20 Nevertheless, the precise molecular pathways mediating these cannabinoid effects remain incompletely understood.21 The nuclear receptor peroxisome proliferator-activated receptor gamma (PPAR-γ) is a key intracellular target that mediates cannabinoid-associated immunosuppression unrelated to the cannabinoid CB1 and CB2 receptors.22 Other targets have also been suggested, including G protein-coupled receptors (GPR)55 and GPR18 as well as transient potential (TRP) channels.23
The use of in vitro microglial models, including BV2 cells, facilitates systematic study of neuroinflammation in controlled and reproducible environments. These models enable the manipulation of experimental conditions, permitting the meticulous examination of cellular responses to diverse stimuli. However, accurate quantification of subtle shifts in cytokine secretion and other inflammatory markers within microglial models has historically presented challenges. Conventional techniques are often intricate and time intensive, potentially interrupting the temporal intricacies of the inflammatory process.24,25 In the context of understanding neuroinflammation and its modulation, microglia exhibit a functional dichotomy, with their phenotype having the potential to either support neuronal maintenance or contribute to neuronal harm through low-grade inflammation and reduced neurotrophic support,26 the application of cannabinoids adds a compelling dimension.8,27,28 Active compounds from cannabis have demonstrated anti-inflammatory potential, stimulating interest in their effects on microglial responses.29–31
This study aimed to evaluate the changes in the production of neuroinflammatory mediators in an in vitro model of microglial cells exposed to different concentrations of natural cannabinoids. A Surface Plasmon Resonance imaging (SPRi) nanobiosensor was used for the real-time evaluation of neuroinflammation biomarkers, while immunocytochemistry (ICC) was employed to assess the expression of proteins related to neuroinflammation. This article highlights the role of these techniques in advancing basic research and in developing potential therapeutic approaches for neuroinflammation-associated diseases.
The BV-2 murine microglial cell line (Accegen-ABC-TC212S) were cultured according to a modified protocol32 at 37 °C in a humidified atmosphere of 95% air and 5% CO2 in high-glucose Dulbecco’s modified Eagle’s medium (DMEM, Lonza-12-604F) supplemented with 10% fetal bovine serum (FBS, PANBIOTECH-P30-1402). BV-2 microglial cells were used at passage numbers ranging from P3 to P5 to ensure phenotypic stability and responsiveness to the inflammatory stimuli. Cells were regularly monitored under a microscope to confirm morphological consistency and absence of spontaneous activation, which could indicate phenotypic drift at higher passages.
Plating medium was prepared according to a previously established protocol.33 In summary, BV-2 cells were initially seeded in T75 flasks and maintained in DMEM supplemented with 10% fetal bovine serum (FBS). To prepare BV-2 conditioned medium, microglial cells were cultured in complete growth medium until reaching 70-80% confluence. Subsequently, the cells were transferred to a 12-well plate and incubated overnight before treatment with the indicated compounds. The treatments included Dexamethasone Sodium Phosphate (Dex-SP, Vitalis-0012145) at 4 μM and cannabis-derived products supplied by Clever Leaves in accordance with state regulations. These derivatives were (CBD-23050412) at 5 and 10 μM, full-spectrum extract (FSE-THC-PE-21-78) (THC 70%, CBD <6%) at 5 and 10 μM, or a combination of FSE-THC and CBD at 5 and 10 μM. The cannabinoids were solubilized in ethanol to ensure proper dissolution before being introduced into the culture medium. All treatment and control conditions (with or without LPS) contained ethanol at a final concentration of 100 mM (equivalent to 460 mg/dL or 0.46%). To control potential vehicle effects, all experimental groups, including the untreated controls, received the same ethanol concentration.34
Cells were exposed to these treatments for 2 h in a volume of 0.5 mL, followed by the addition of lipopolysaccharide (LPS, Sigma Aldrich-L2630-10MG) at 100 ng/mL for an additional 4 h. Exposure to LPS for 4 hours in BV-2 cells is a widely utilized protocol as it facilitates the induction of a robust and reproducible inflammatory response, minimizes cytotoxic effects, and enables the investigation of early mechanisms of neuroinflammation.35,36 This methodology aligns with established scientific literature and provides an optimal balance between the induction of inflammation and preservation of cellular viability.37,38 After 6 h, aliquots of the medium were collected and stored at 4 °C until further analysis. The cells were fixed with paraformaldehyde for subsequent cytochemical analysis.
BV-2 cell viability was evaluated using an MTT reduction assay (Life Technologies-1721505).39 The cells (1 × 104 cells/well) were seeded in 96-well plates and incubated for 24 h. The cells were then treated for 6 h with Dex-SP (4 μM), CBD (5 and 10 μM), FSE-THC (70%) (5 and 10 μM), or a combination of FSE-THC and CBD (5 and 10 μM). After treatment, 100 μL MTT solution (5 mg/mL) was added to each well and incubated for 4 h. Subsequently, 50 μL of dimethyl sulfoxide (DMSO) was added, and absorbance was measured for each well was recorded at 570 nm wavelength using a Bio-Rad Model 630 microplate reader (Bio-Rad Laboratories, Richmond, CA, USA).
In 96-well plates, cells were treated with stimuli, and the production of nitric oxide (NO) was evaluated by measuring NO levels in the medium using the Griess reaction (R&D Systems-KGE0001) following a previously described method.40 The process involved combining 50 μl of sample aliquots with an equal volume of Griess reagent (1% sulfanilamide, 0.1% naphthylethylene diamine dihydrochloride, and 2% phosphoric acid) in a 96-well plate. A microplate reader was used to measure absorbance at 550 nm. To determine NO2 concentrations, NaNO2 was used as the standard.
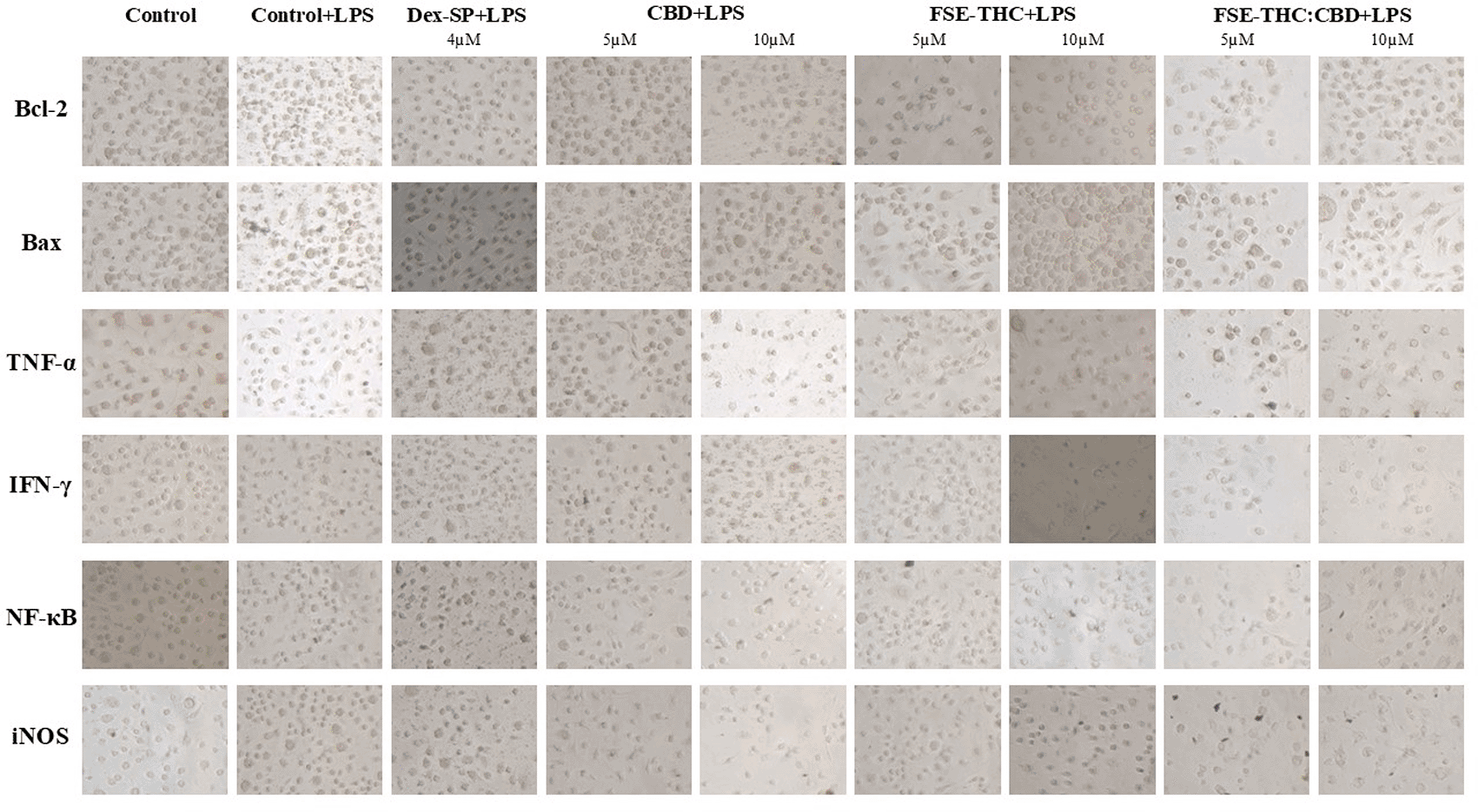
The cells were fixed with 4% (v/v) formaldehyde in PBS for 20 minutes at room temperature, washed twice with PBS, and stored at 4°C. Endogenous peroxidase activity was inhibited and a blocking solution prepared with PBS supplemented with 5% BSA was used to minimize non-specific binding. Samples were then incubated overnight at 4°C with primary antibodies (Invitrogen), including Bcl-2 (MAB8272, 1:200), Bax (AF820, 1:200), TNF-α (52B83, 1:200), IFN-γ (JM10-10, 1:200), RelA/NF-κB p65 (MAB5078, 1:200), and iNOS (NB300-605, 1:200), and then washed with PBS. VisUCyte™ HRP Polymer was applied for 30 min at room temperature, and after additional PBS washes, DAB substrate was added, followed by incubation for 5 min. The reaction was terminated by rinsing it with distilled water. Staining intensity was evaluated under a light microscope, with a minimum of four representative images captured per sample and analyzed using ImageJ 1.54d software. The selected images represented homogeneous areas free of artifacts within each sample, ensuring comparability across experimental groups. Four representative fields were chosen per sample, covering different regions of interest while avoiding overlap to reflect intramural variability. The images were processed in ImageJ 1.54d, converted to grayscale, and analyzed using the auto-threshold tool to identify and quantify the positive areas corresponding to DAB staining. The quantified parameters included the total positive area (in pixels) and the mean pixel intensity within these areas, which were used to calculate the values presented in Table 1.
| Dex-SP (%) | CBD | FSE-THC 70% | FSE-THC:CBD | ||||
|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | |||||
| 4μM | 5μM | 10μM | 5μM | 10μM | 5μM | 10μM | |
| Bcl-2 | ***-22,66 | ***-18,57 | ***-19,28 | ***-14,42 | -24,64 | -7,24 | -16,54 |
| Bax | -16,71 | -12,43 | -12,80 | -8,00 | ***-22,66 | -8,00 | **0,84 |
| TNF-α | ***-21,39 | ***-18,69 | -3,98 | **-11,36 | ***-23,07 | -9,21 | -8,80 |
| IFN-γ | -4,95 | ***-2,60 | **5,40 | 3,36 | ***-21,43 | -0,21 | 0,86 |
| NF- κB | -14,43 | 1,78 | 2,43 | ***8,37 | 2,91 | 14,07 | ***7,56 |
| iNOS | 1,97 | -1,07 | ***21,43 | ***9,90 | -1,62 | 6,06 | ***9,02 |
| |||||||
The selection of the time point for analysis was based on prior literature demonstrating that the key endpoints of interest undergo significant changes within this timeframe under similar experimental conditions.41–43 Furthermore, preliminary experiments conducted in our laboratory confirmed that these endpoints were both detectable and biologically relevant, supporting our decision to perform analyses at this specific time point. In models of injury and neuroinflammation, microglia detect damage-associated or pathogen-associated signals and become activated within 30 min to a few hours, further justifying the chosen timeframe for analysis. While some studies have reported the induction of these endpoints at later time points,44 previous findings indicate that relevant molecular and cellular changes occur within the selected timeframe, ensuring the validity of our approach. Although full time-course optimization was not performed in this study, our experimental design was guided by well-established literature and prior observations in our laboratory.
Cytokine levels were measured using a nanobiosensor-based detection system that allows real-time, label-free quantification with high specificity and minimal sample volume requirements. This technology was selected over conventional methods, such as ELISA, because of its ability to capture dynamic cytokine secretion patterns and its faster processing time, reducing the need for extensive sample handling.45 The nanobiosensor was calibrated using standard cytokine solutions within the expected physiological range for our experimental conditions to ensure the accurate detection of relevant concentrations. Measurements were performed according to the manufacturer’s specifications, with controls included to verify assay performance.
Real-time biomarker quantification was achieved using an OpenPlex system equipped with SPRi technology and a microfluidic setup, which was maintained at 25 °C for all the experiments. This setup facilitated direct interactions between recombinant proteins and monoclonal antibodies against TNF-α (Invitrogen-AMC3012), and IL-6, which were sourced from Abcam and Thermo Fisher Scientific (Waltham, MA, USA), respectively, along with other essential reagents from XanTec Bioanalytics (Düsseldorf, Germany), GE Healthcare (Marlborough, MA 01752 USA), and Sigma-Aldrich (Taufkirchen Germany). The detection method was based on changes in the refractive index as the analytes were bound to their respective ligands,46 quantified in real time, and represented graphically in sensorgrams. Data were analyzed using OriginPro 8.5 (Northampton, MA 01060 USA (A free trial version of Origin/OriginPro is available at https://www.originlab.com/demodownload.aspx), Excel (LibreOffice Calc is an open-access software that provides functionalities similar to Excel. It is available at https://www.libreoffice.org/download/download/), and Python, with carefully controlled parameters such as flow rate and protein concentrations, and standard curves were established using serial antibody dilutions ranging from 1000–20,000 ng/mL. To ensure specificity and repeatability, the culture samples were thawed, diluted, and injected in triplicate, with real-time monitoring of dissociation and a post-analysis regeneration step using a regeneration solution (glycine, pH 2.0) to maintain the integrity of the sensor chips.
In the SPR assays in this study, immobilization of TNF-α and IL-6 monoclonal antibodies was critical, requiring careful consideration of ligand properties, such as isoelectric point, molecular weight, and amino acid sequence, to optimize conditions for attachment to the carboxymethyl dextran (CMD) 200 sensor surface.47,48 Amine-based immobilization, utilizing an N-hydroxysuccinimide (NHS)/1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) mixture to activate a carboxymethyl-dextran layer on the sensor chip, enables a strong covalent bond between the sensor surface and the protein, ensuring high sensitivity and specificity. After activation, the recombinant proteins were injected until the desired immobilization level was reached, with excess active sites blocked using ethanolamine. Regeneration of the surface, which is crucial for reusing the sensor chip, involves injecting a 10 mM glycine hydrochloride solution with 0.00005% sodium dodecyl sulfate (SDS) at a flow rate of 60 μL/min to ensure over 80% recovery, maintain surface integrity, and allow for consistent, repeatable measurements.49–51
Cytokine levels were assessed by measuring the levels of IL-6 and TNF-α, two well-established pro-inflammatory mediators in microglial activation and neuroinflammation. While IL-1β is also a key inflammatory cytokine, the present study focused on IL-6 and TNF-α because of their rapid and robust induction in response to inflammatory stimuli, making them reliable indicators of early microglial activation in this model.52,53
The findings are displayed as average values accompanied by standard deviations (SD), derived from three separate trials. The Shapiro-Wilk test was used to evaluate normality. The majority of groups satisfied the criteria for normal distribution (p > 0.05), one of the experimental groups did not (p < 0.05). Given this, along with the heterogeneity of variances across groups and the presence of nonlinear relationships, non-parametric statistical tests were employed. Comparisons were performed between all experimental groups (Dex-SP, CBD, FSE-THC 70%, and FSE-THC:CBD) and the control (with and without LPS stimulation), as well as between treatment groups. The Kruskal–Wallis test was used to assess the overall group differences. Subsequently, Dunn’s post hoc test with Bonferroni correction was implemented for pairwise comparisons, adjusting p-values to mitigate the Type I error risk. Statistical significance was set at p < 0.05. All analyses were conducted using Python (version 3.12) with the SciPy (version 1.11.2) and Scikit-Posthoc (version 0.7.0) libraries.
In the study of cytotoxic effects on BV2 microglial cells, cell viability was evaluated using the MTT assay. After a 2 h incubation with LPS, pretreatment with Dex-SP (4 μM), CBD (5 and 10 μM), FSE-THC 70% (5 and 10 μM), and FSE-THC:CBD (5 and 10 μM), followed by stimulation with LPS 100 ng/ml for 4 h, did not reduce the viability of the BV-2 microglia ( Figure 1).
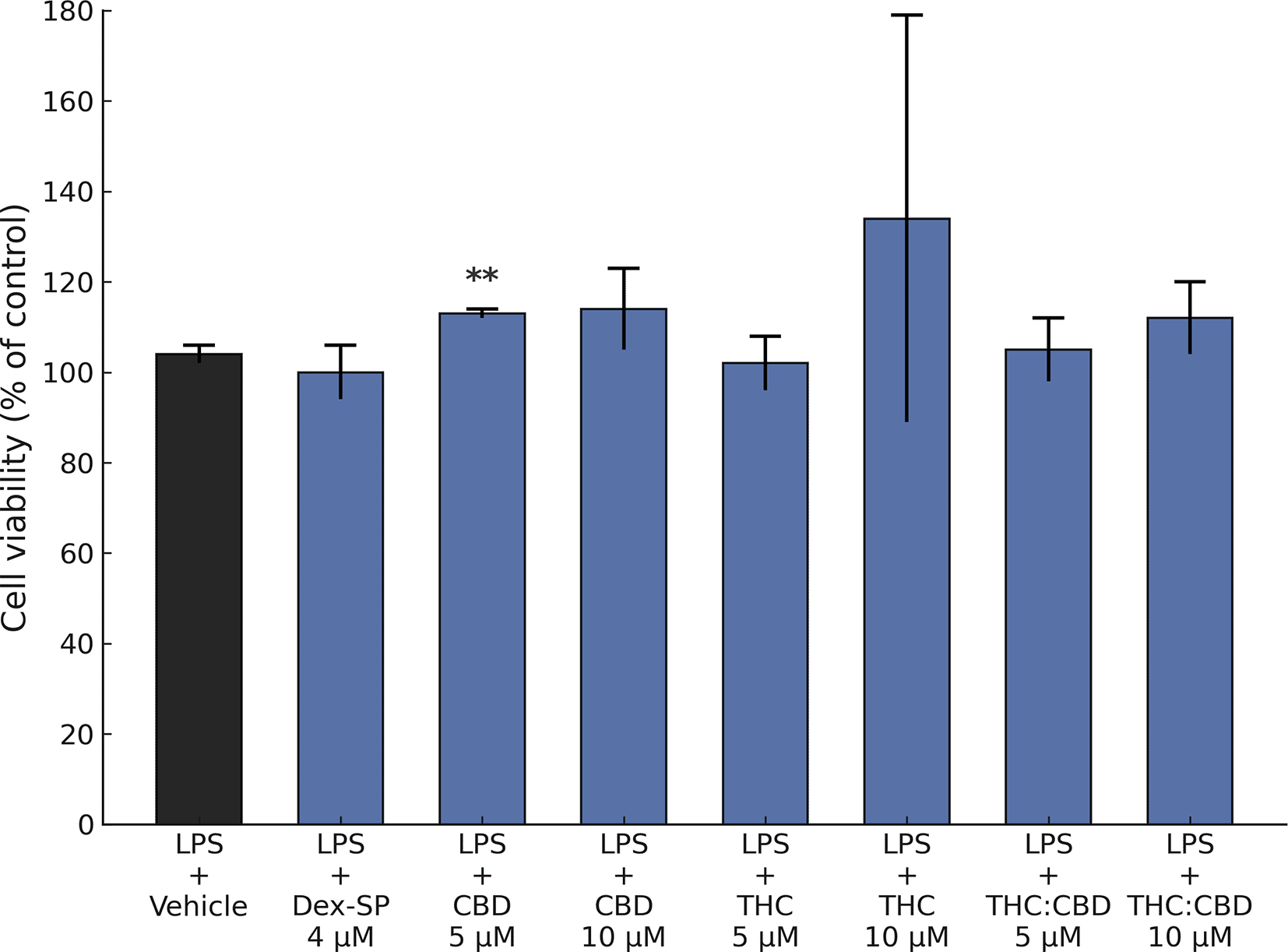
The MTT assay was used to evaluate BV-2 microglial cell viability after 2 h of LPS incubation and 4 h of exposure to various treatments. These treatments included Dex-SP (4 μM), CBD (5 and 10 μM), FSE-THC 70% (5 and 10 μM), and a combination of THC and CBD (50% FSE-THC, standardized to 70% THC, and CBD, 5 and 10 μM). The results indicated that none of the administered treatments had a significant effect on cell viability. The findings were based on three separate experiments, with each condition having six technical replicates. The results were based on three independent experiments, and each condition was tested in six technical replicates. Data are presented as mean ± SD, !!! p < 0.05 versus vehicle, ** p < 0.05 versus LPS + Vehicle, *** p < 0.01 versus LPS + Vehicle.
Studies assessing the impact of Dex-SP (4 μM), CBD (5 and 10 μM), FSE-THC 70% (5 and 10 μM), and FSE-THC:CBD (5 and 10 μM) on nitric oxide (NO) release showed that when BV-2 microglia were exposed to LPS (100 ng/mL) for 4 h, there was no increase in NO production compared to unstimulated cells (p > 0.05). ( Figure 2).
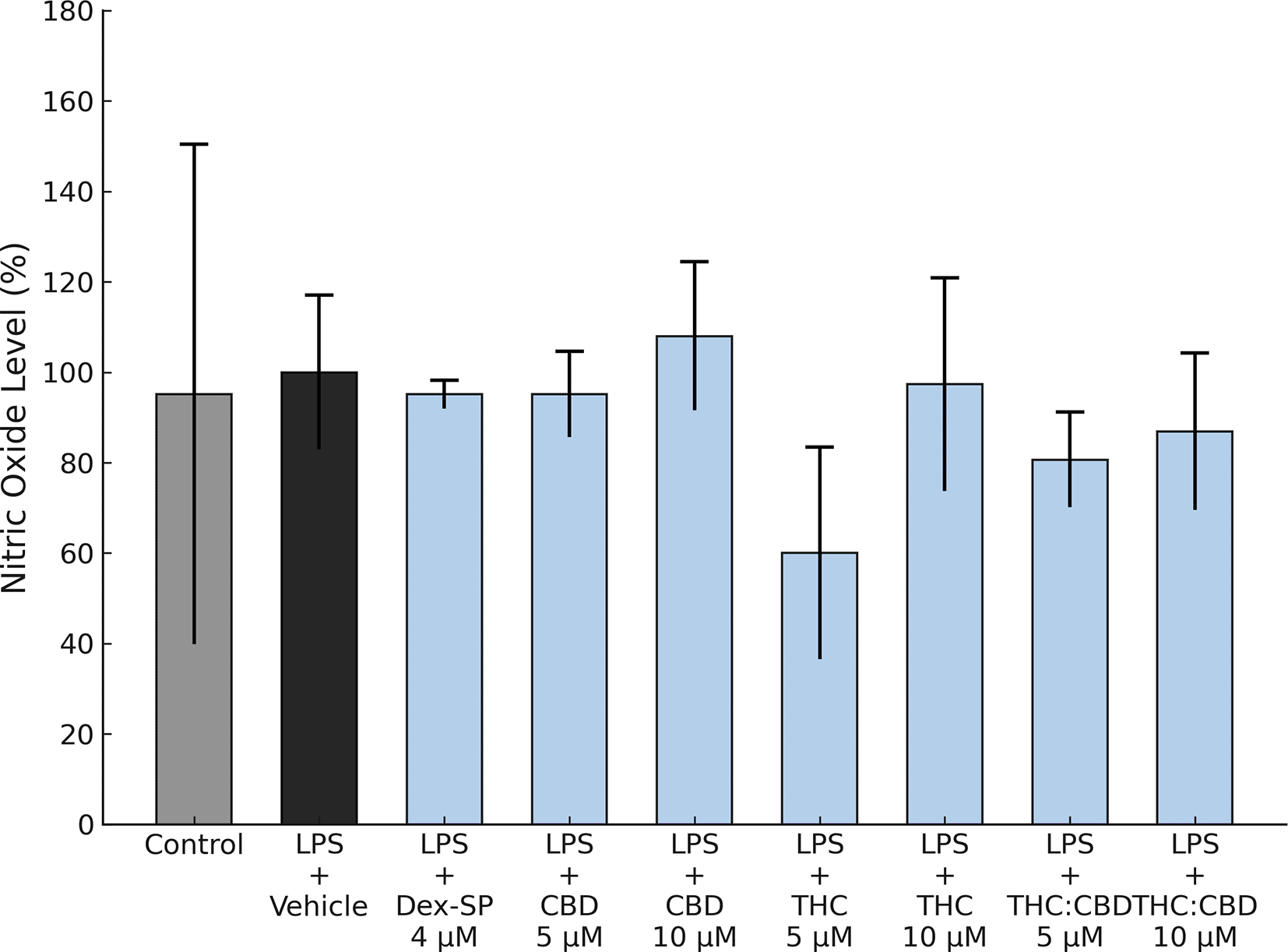
Interactions of Dex-SP (4 μM), CBD (5 and 10 μM), FSE-THC 70% (THC) (5 and 10 μM), and FSE-THC:CBD (a compound containing 50% FSE-THC, standardized to 70%, and CBD) at 5 and 10 μM concentrations in BV-2 microglial cells. Nitric oxide (NO) production was assessed after a 4 h treatment with LPS (100 ng/mL). None of the tested concentrations of Dex-SP or cannabinoids induced significant NO production. The results were based on three independent experiments, and each condition was tested in six technical replicates. Data are presented as mean ± SD, !!! p < 0.05 versus vehicle, ** p < 0.05 versus LPS + Vehicle, *** p < 0.01 versus LPS + Vehicle.
The LPS-induced (100 ng/mL) increase in TNF-α release by BV-2 microglia was significantly attenuated by CBD (5 and 10 μM) and FSE-THC:CBD (5 and 10 μM) (p < 0.001). In contrast, Dex-SP (4 μM) and FSE-THC 70% (5 and 10 μM) did not elicit a significant reduction ( Figure 3A). Similarly, the LPS-induced increase in IL-6 production was significantly diminished by Dex-SP (4 μM) (p < 0.05) and CBD 10 μM (p < 0.01), whereas other cannabinoids did not induce a significant effect ( Figure 3B).
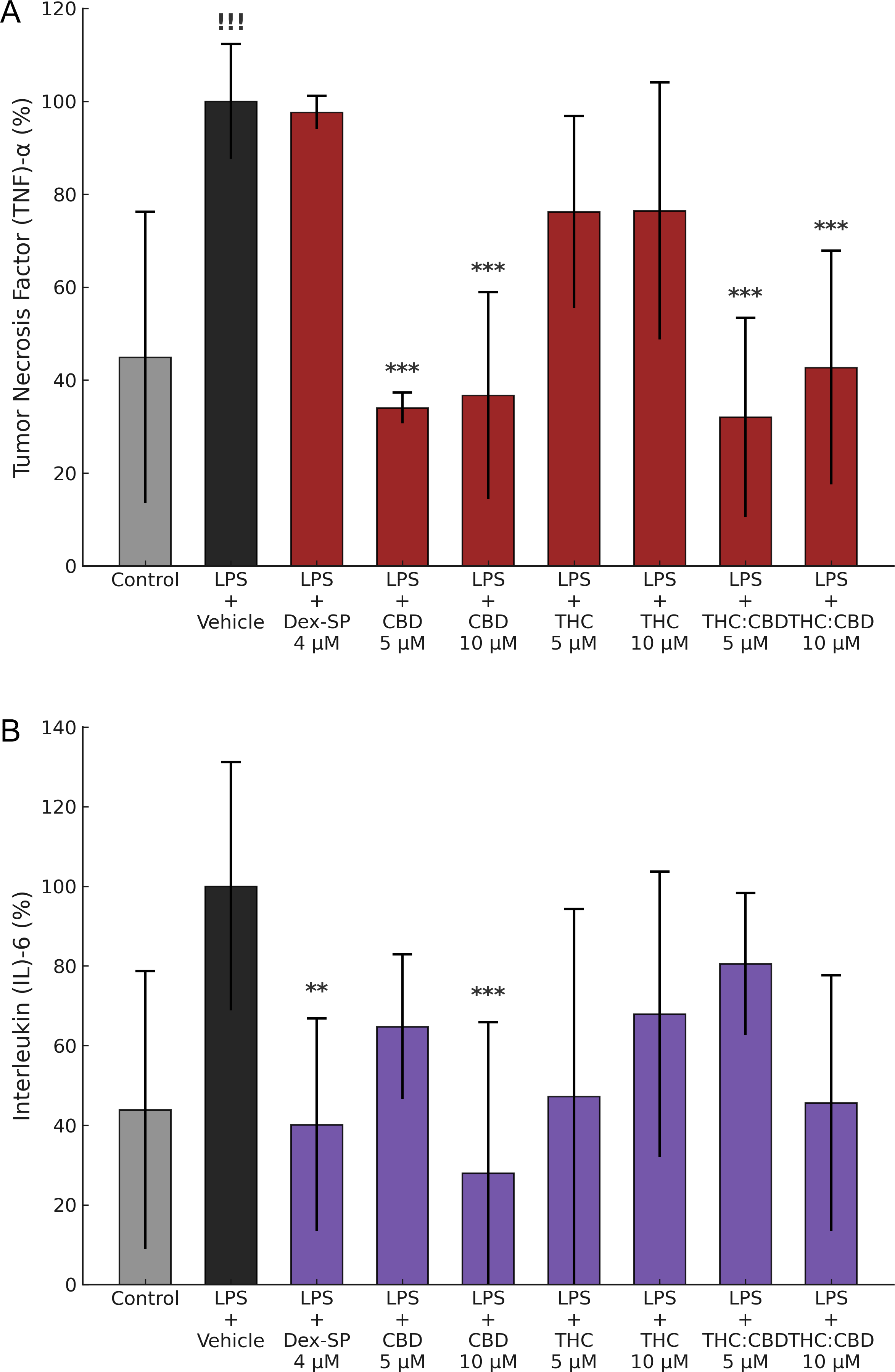
Effects of Dex-SP and cannabinoids on LPS-induced TNF-α and IL-6 production in BV-2 microglial cells. TNF-α and IL-6 levels were measured 4 h after LPS stimulation (100 ng/mL) and treatment with Dex-SP (4 μM), CBD (5 and 10 μM), FSE-THC 70% (5 and 10 μM), and FSE-THC:CBD (5 and 10 μM). (A) CBD (5 and 10 μM) and FSE-THC:CBD (5 and 10 μM) significantly reduced TNF-α production (p < 0.01). In contrast, Dex-SP (4 μM) and FSE-THC 70% (5 and 10 μM) did not inhibit TNF-α production. (B) IL-6 production was significantly reduced by Dex-SP (4 μM) (p < 0.05) and CBD (10 μM) (p < 0.01). The other cannabinoid treatments did not result in statistically significant reductions (p > 0.05). The results were based on three independent experiments, and each condition was tested in six technical replicates. Data are presented as mean ± SD, !!! p < 0.05 versus vehicle, ** p < 0.05 versus LPS + Vehicle, *** p < 0.01 versus LPS + Vehicle.
The results of the immunocytochemistry (ICC) experiments were used to evaluate the effects of Dex-SP (4 μM), CBD (5 and 10 μM), FSE-THC 70% (5 and 10 μM) and FSE-THC:CBD (5 and 10 μM). There was a significant reduction in anti-apoptotic Bcl-2 with Dex-SP (4 μM), CBD (5 and 10 μM), and FSE-THC 70% (10 μM) (p < 0,01) ( Figure 4, Panel A). FSE-THC 70% (10 μM) (p < 0,01), and FSE-THC:CBD (10 μM) (p < 0,05) treatments decreased the levels of pro-apoptotic Bax ( Figure 4, Panel B). Dex-SP (4 μM) (p < 0,01), CBD (5 μM) (p < 0,01), FSE-THC 70% (5 μM) (p < 0,05) and FSE-THC 70% (10 μM) (p < 0,01), treatments were found to significantly decrease TNF-α levels, indicating their anti-inflammatory properties ( Figure 4, Panel C). The response of Interferon Gamma (IFN)-γ expression to treatments varied, with CBD (10 μM) (p < 0,05) minor increases or CBD (5 μM) (p < 0,01) minor decreases, and may decrease with FSE-THC (70%) (10 μM) (p < 0,01) ( Figure 4, Panel D). There was a significant increase in Nuclear Factor Kappa B Subunit 1 (NF-κB) production in the FSE-THC (70%) (5 μM) and FSE-THC:CBD (10 μM) (p < 0,01) groups ( Figure 4, Panel E). iNOS production was significantly altered in the CBD (10 μM), FSE-THC (70%) (5 μM), and FSE-THC:CBD (10 μM) groups (p < 0,01) groups ( Figure 4, Panel F).
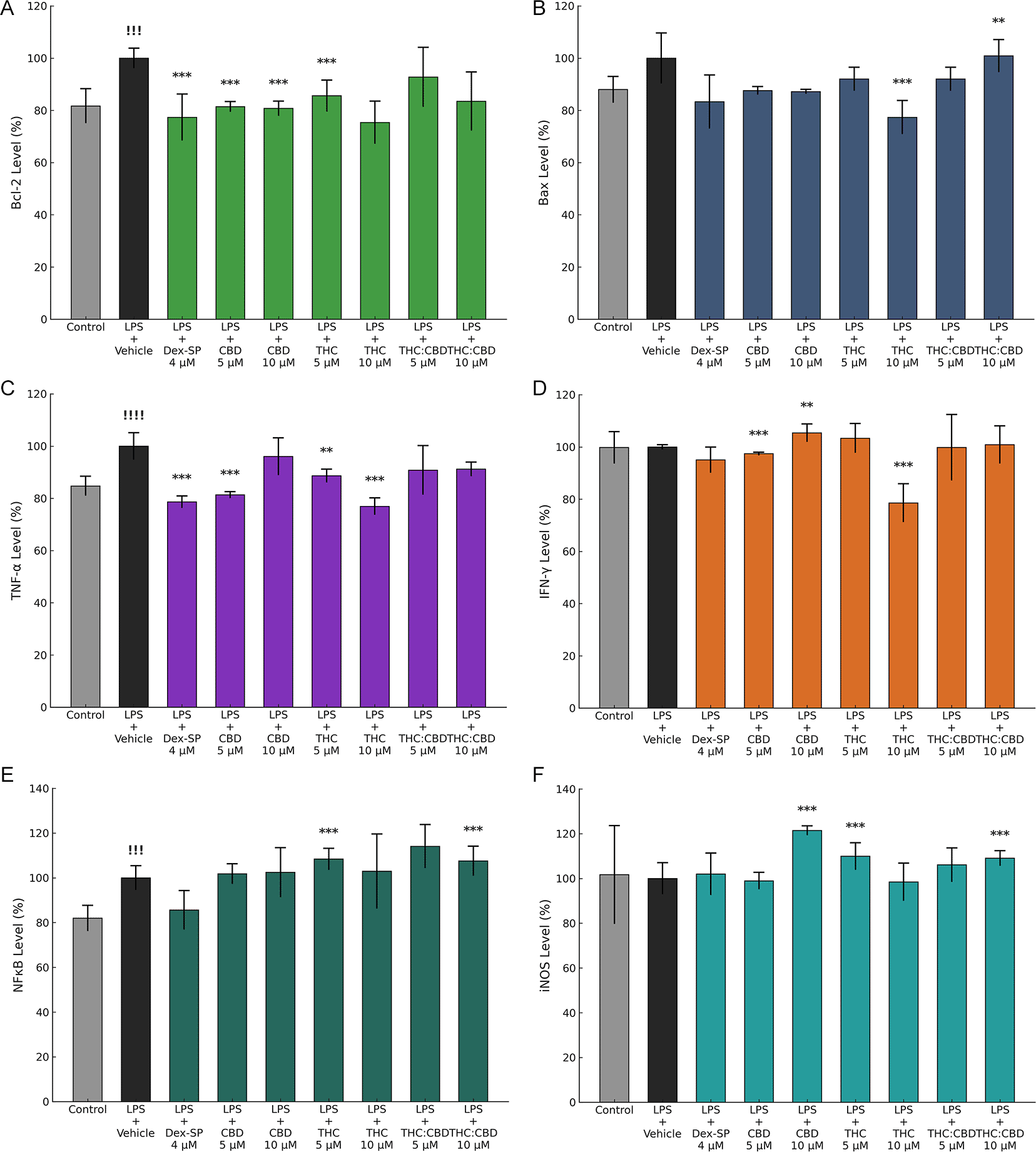
Immunocytochemistry (ICC) analysis revealed significant Bcl-2 reduction with Dex-SP (4 μM), CBD (5 and 10 μM), and FSE-THC 70% (10 μM) (p < 0.01) (Panel A). Bax levels decreased with FSE-THC 70% (10 μM) (p < 0.01) and FSE-THC:CBD (10 μM) (p < 0.05) (Panel B). TNF-α was significantly reduced, indicating anti-inflammatory effects, with Dex-SP (4 μM), CBD (5 μM), FSE-THC 70% (5 μM) (p < 0.05), and FSE-THC 70% (10 μM) (p < 0.01) (Panel C). IFN-γ levels varied; CBD (10 μM) increased (p < 0.05), CBD (5 μM) decreased (p < 0.01), and FSE-THC 70% (10 μM) reduced IFN-γ (p < 0.01) (Panel D). NF-κB was significantly elevated in FSE-THC 70% (5 μM) and FSE-THC:CBD (10 μM) (p < 0.01) (Panel E). iNOS expression was altered in the CBD (10 μM), FSE-THC 70% (5 μM), and FSE-THC:CBD (10 μM) groups (p < 0.01) (Panel F). The results were based on three independent experiments, and each condition was tested in six technical replicates. Data are presented as mean ± SD, !!! p < 0.05 versus vehicle, !!!! p < 0.01 versus vehicle **p < 0.05 versus LPS + Vehicle, ***p < 0.01 versus LPS + Vehicle.
The most efficacious treatments for modulating inflammation are CBD (10 μM) because of their capacity to attenuate IFN-γ, and iNOS, Dex-SP (4 μM) because of their capacity to attenuate TNF-α, and FSE-THC 70% (10 μM), because of their capacity to attenuate Bax, TNF-α, and IFN-γ, which are critical mediators of the inflammatory response. All three treatments demonstrated a reduction in Bcl-2, which may represent a potential mechanism through which they attenuate prolonged microglial activation and facilitate the resolution of inflammation. However, if this reduction is accompanied by a concomitant decrease in Bax (as observed with FSE-THC 70% at 5 μM), it may indicate a shift in the balance between apoptosis and cellular viability ( Table 1).
In our study, none of the tested treatments significantly affected cell viability. Treatment with Dex-SP (4 μM) significantly reduced LPS-induced release of TNF-α (ICC), IL-6, and Bcl-2. In contrast, CBD significantly decreased LPS-induced release of TNF-α (5 and 10 μM), IL-6 (10 μM), Bcl-2 (5 and 10 μM), IFN-γ (5 and 10 μM), and iNOS (10 μM). Additionally, FSE-THC 70% significantly reduced Bcl-2 (5 μM), Bax (10 μM), TNF-α (ICC) (5 and 10 μM), IFN-γ (10 μM), NF-κB (5 μM), and iNOS (5 μM). Finally, FSE-THC:CBD significantly decreased TNF-α (5 and 10 μM), Bax (10 μM), NF-κB (10 μM), and iNOS (10 μM).
We stimulated BV-2 microglial cells using LPS, leading to a notable release of Bcl-2, TNF-α and NF-κB. These compounds are widely acknowledged as critical mediators in inflammatory processes.54 CBD (5 and 10 μM) and FSE-THC 70% (5 and 10 μM) exhibited the greatest ability to modulate inflammatory and apoptotic mediators, significantly reducing TNF-α, IL-6, IFN-γ, NF-κB, iNOS, and Bax, whereas FSE-THC:CBD (5 and 10 μM) showed an intermediate response. In comparison, Dex-SP (4 μM) effectively reduced TNF-α and IL-6 but had no significant impact on IFN-γ, NF-κB, or iNOS, suggesting that phytocannabinoids, particularly CBD and FSE-THC 70%, may represent promising strategies for neuroinflammation modulation.
Previous studies have demonstrated that the endogenous cannabinoid system, which comprises compounds such as anandamide (AEA) and 2-arachidonoylglycerol (2-AG), plays a crucial role in protecting neurons from damage. This protection was achieved by reducing cellular apoptosis and enhancing cell viability.55 Another study highlighted the antioxidant and neuroprotective properties of CBD and Cannabigerol (CBG) in rat astrocytes and cortices, demonstrating their effectiveness in reducing oxidative stress and preventing apoptosis. CBD outperformed CBG, with both impacting the neurokinin 3 receptor, indicating a potential multi-target mechanism.56 To the best of our knowledge, this is the first study to compare the effects of Dex-SP, CBD, and FSE-THC 70%, both individually and in combination, on the viability of BV-2 microglial cells.
The inhibitory effects of CBD 5 and 10 μM on the release of TNF-α were statistically superior to Dex-SP 4 μM. On the other hand, IL-6 release was more effectively inhibited by both Dex-SP 4 μM and CBD 10 μM. THC and CBD have been reported to exert distinct pharmacological actions on known cannabinoid receptors, displaying differential effects on exposed cells. THC functions as a partial agonist for both CB1 and CB2 receptors, while CBD shows a markedly low affinity for these receptors.57 CB2 receptors, found on a range of immune cells, including primary microglia and the BV-2 microglial cell line, are considered key mediators in cannabinoid-driven immunomodulation.58 The immunosuppressive effects of THC have been linked to CB2 receptors, as demonstrated through knockout system studies.59 Consequently, studies have demonstrated that CB2 agonists exhibit immunosuppressive effects in rat primary microglial cultures.60 It has been demonstrated that rat primary microglial cells express CB1 receptors, and the activation of these receptors induces the production of NO.61 To assess whether various compositions of natural cannabinoids mediate immunosuppression in our system, we applied them prior to exposure to LPS and compared this response with that obtained using Dex-SP.
We observed that CBD (5 μM) and FSE-TSH 70% (5 and 10 μM) reduce TNF-α expression at the cytoplasmic level to a similar extent as Dex-SP. The precise mechanisms underlying these findings remain unclear. One potential explanation is that THC acts as a weak agonist for the CB2 receptor62 because that the CB1 is almost absent in BV-2 cells.63 Additionally, several studies indicate that certain terpenoids may interact with components of the cannabinoid signaling system.64
Immunocytochemistry (ICC) analysis revealed that CBD (5 and 10 μM), FSE-THC 70% (5 and 10 μM), and FSE-THC:CBD (5 and 10 μM) exerted an anti-inflammatory effect comparable to Dex-SP (4 μM). Similarly, CBD (5 and 10 μM) and FSE-THC 70% (5 μM) demonstrated a similar ability to Dex-SP (4 μM) in regulating cell survival by suppressing LPS-induced Bcl-2 expression. This reduction in Bcl-2 may reflect a shift in cell fate, promoting a less inflammatory environment and facilitating the clearance of hyperactivated or dysfunctional microglial cells, thereby contributing to inflammation resolution. Likewise, FSE-THC 70% (10 μM) and FSE-THC:CBD (10 μM) exhibited a statistically significant effect in reducing LPS-induced Bax expression.
In our model, ICC analysis of IFN-γ and iNOS showed no detectable changes, nor were any alterations observed in NO production. This may be due to the 4-hour LPS exposure, which might have been insufficient to induce a measurable response. Studies suggest that a 6-hour time point is more appropriate for capturing early microglial activation in neuroinflammatory models.65 Similarly, early production of pro-inflammatory cytokines, such as IL-6 and TNF-α, has been reported shortly after intracerebral hemorrhage, highlighting the rapid onset of neuroinflammatory responses in the central nervous system.66
Proinflammatory cytokines are key mediators of the immune response, often peaking within the first six hours of an inflammatory stimulus. This rapid release is a hallmark of acute inflammation, which occurs in response to infections, injuries, or other inflammatory triggers. For instance, IL-1β and TNF-α are among the earliest cytokines released upon exposure to stimuli such as LPS or ionizing radiation, reflecting the swift activation of the inflammatory cascade.67 Their early secretion is crucial for initiating immune responses by recruiting immune cells to the site of injury and amplifying cytokine production.68 Accurately modeling acute neuroinflammatory responses and assessing pharmacological interventions before secondary changes occur are essential for developing effective therapeutic strategies. LPS-induced models combined with pharmacological agents targeting specific neuroinflammatory pathways provide a valuable framework for studying the acute phase of neuroinflammation.69
Dex-SP, a widely used anti-inflammatory drug, has also been employed clinically to manage various neuroinflammatory processes.70 Dex-SP inhibits inflammatory responses by regulating the activity of transcription factors such as nuclear factor kappa B (NF-κB),71 which was observed in our study. In neuroinflammatory processes, NF-κB plays a crucial role in neurodegeneration mediated by neuroinflammation.72 The upregulation of NF-κB is associated with some neuroinflammatory disease, and targeting its inhibition could potentially serve as an effective therapeutic strategy for treating Alzheimer’s disease,73 Parkinson’s disease,74 and chronic pain.75 Evidence suggests that proinflammatory cytokines, including TNF-α and IL-6 significantly contribute to the NF-κB signaling pathway.76 NF-κB is known to activate these cytokines in cells, thereby triggering an inflammatory response.76 Prior research has shown that in LPS-stimulated BV2 cells, the levels of proinflammatory cytokines TNF-α and IL-6 are elevated.77 Elevated levels of pro-inflammatory cytokines in BV2 cells are believed to play a direct role in the development of neuroinflammation.78 In the present study, Dex-SP (4 μM) significantly decreased the expression of Bcl-2 and TNF-α in LPS-stimulated BV2 cells.
IL-1β is an important pro-inflammatory cytokine in microglial activation; however, in this study, we prioritized the quantification of IL-6 and TNF-α, which are rapidly induced and play central roles in the inflammatory cascade. TNF-α is a key upstream regulator of the inflammatory response, capable of inducing both IL-1β and IL-6 expression,52,53 while IL-6 serves as a crucial modulator of neuroinflammation and is strongly associated with microglial activation. Given their well-documented roles in neuroinflammatory processes, the measurement of IL-6 and TNF-α provided a comprehensive assessment of the inflammatory response in our model. Future studies could complement these findings by including IL-1β measurements to further characterize the cytokine profile over time
On the other hand, cannabinoid derivatives, particularly CBD, have been shown to modulate inflammation, yet there are still gaps in understanding their mechanisms of action or biological responses. It has been reported that the combination of THC and CBD can enhance these properties.79 To date, there are no published studies comparing the anti-inflammatory properties of cannabinoids with Dex-SP in a neuroinflammation model using microglial cells. However, there are studies in other cell types showing that extracts are more potent than CBD alone,80 which aligns with our findings.
The expression of Bax, IFN-γ, NF-κB, and iNOS was significantly reduced in microglial cells exposed to cannabinoids compared to that in cells treated with Dex-SP (4 μM). These differences may be attributed to the distinct modulatory mechanisms of cannabinoids, in contrast to the effects of corticosteroids, such as Dex-SP, on BV-2 microglial cell activity. Previous research has also shown that CBD can exert anti-inflammatory effects on LPS-stimulated microglial cells.81 The specific molecular mechanisms underlying CBD’s actions have yet to be fully elucidated, and more in-depth studies directly comparing the effectiveness and therapeutic potential of CBD, other cannabinoids, and their mixtures are needed. Our study demonstrated a greater anti-inflammatory effect with the presence of EFS-THC, in line with findings reported in other studies.79
The limitation of our study was that no RNA level tests were conducted; hence, the effect of the observed changes on genetic expression was not determined. The exposure duration to LPS was brief, so we were unable to detect changes in the response after the 6-hour period. Moreover, a broader range of inflammatory proteins, such as TLR4 and MyD88, and modulators, including IL-1β, IL-4, IL-10, and Nerve Growth Factor (NGF), are required to further validate the roles of Dex-SP and cannabinoids in the regulation of neuroinflammation in LPS-stimulated BV2 cells.
Our findings indicated that Dex-SP has an anti-inflammatory effect on LPS-stimulated BV2 cells. Furthermore, cannabinoids, particularly at certain concentrations and combinations, demonstrate efficacy comparable to or even superior to that of Dex-SP in reducing certain inflammatory mediators. This includes a reduction in the expression of anti-apoptoic protein as Bcl-2, pro-apoptotic proteins such as and Bax, as well as proinflammatory cytokines such as TNF-α, IFN-γ, NF- κB and iNOS This study will aid in elucidating the therapeutic effects of cannabinoids on processes associated with neuroinflammation. Further research is needed to understand the mechanisms and determine the combinations of cannabinoids that significantly affect inflammation modulation.
Conceptualization J.-M.Q., R.-H. B., L.E.D., and M.X.L.; methodology J.-M.Q., R.-H. B. and L.E.D.; writing—original draft preparation, J.-M.Q., R.-H. B. and L.E.D; writing—review and editing, J.-M.Q., R.-H. B. and L.E.D; supervision, J.-M.Q., R.-H. B. and L.E.D; project administration, R.-H.B.; funding acquisition, R.-H. B., L.E.D., and M.X.L. All authors have read and agreed to the published version of the manuscript.
All data underlying the results are available as part of the article and no additional source data are required.
Zenodo: Assessing neuroinflammation in vitro in a microglial model: advancing cannabinoid-based therapeutic insights. https://doi.org/10.5281/zenodo.14901695.82
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Neuroimmunology, neuropharmacology.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 28 Mar 25 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)