Keywords
Osteoarthritis, injury, exercise, biomarker, saphenous vein, serum
Osteoarthritis (OA) is a common, heterogeneous whole-joint disease contributing to significant morbidity with variable trajectories of symptom progression. Early detrimental joint changes may be identified using molecular biomarkers in serum or synovial fluid. When measuring the former, biomarkers are at risk of systemic dilution, with the latter technically challenging with increased associated risks. In addition, biomarker concentrations are also potentially influenced by extrinsic factors, including exercise. This case-control pilot study aims to understand the influence of sampling location and sub-maximal exercise on serum biomarker concentrations.
Recruited participants either had a recent lower-limb musculoskeletal injury (INJ), established knee OA (KOA) or were controls (CON). Serum was taken from their antecubital fossa (ACF, ‘Arm 1’), their great saphenous vein local to their ipsilateral knee (‘Knee’), and after a ten-minute non-weight-bearing exercise, their ACF again (‘Arm 2’). Serum biomarker assays were performed, with results analysed for differences between condition (CON v KOA v INJ), location (Arm 1 v Knee) and pre-post exercise (Arm 1 v Arm 2).
Thirty-two participants were recruited (CON n=12, KOA n=10, INJ n=10), 81% male, median age 28 (IQR: 25-43) and BMI 23.9 (IQR: 22.5-26.3). Interleukin-(IL)-1β concentration was lower in the KOA group (p=0.004). IL-6 (1.65 and 0.96 ng/L) and leptin (12.65 and 7.01 ug/L) were higher in the Knee sample than Arm 1 (both p<0.001). COMP (173.06 and 190.27 ug/L, p=0.005) and CTX-II (0.68 and 0.83 ug/L, p=0.02) increased following exercise. Further differences were noted when the location and exercise intervention analyses were stratified by condition.
These novel sampling techniques and easy, accessible 10-minute exercise protocol are suitable for a wide range of settings. Both sampling location and precedent non-weight-bearing sub-maximal exercise influenced serum biomarker concentrations in this pilot case-control study. Optimising these sampling variables could improve the sensitivity of biomarker analysis following validation in a larger population.
Osteoarthritis, injury, exercise, biomarker, saphenous vein, serum
The synovial whole-joint disease, osteoarthritis (OA), is extremely common, affecting 600 million people globally, with a significant burden of pain and disability.1 It is the endpoint of interrelated and concurrent heterogenous pathological mechanisms, including a loss of bone and cartilage turnover homeostasis, altered biomechanics, low-grade inflammation, aberrant healing after injury and metabolic dysregulation,2 with a prolonged, asymptomatic, prodromal phase. This period can last for years, potentially enabling recognition of molecular changes prior to radiological and symptomatic changes, which may improve drug-discovery trials and enhance clinical care.3
The joint intra-articular micro-environment is separated by the synovium, which consists of two layers: the outer subintima and the inner intima. The subintima contains loose extra-cellular matrix (ECM) components like type I collagen, adhesins, and fibronectin, as well as adipose and areolar tissues, and is highly vascularised with blood vessels, lymphatic vessels, and nerve fibres.4 Together, the ECM and resident monocytic cells form a three-dimensional structure that acts as an internal immune barrier within the synovial lining, helping to regulate inflammation.5 Understanding the characteristics of the synovial microenvironment is crucial for developing new therapies. Synovial fluid is a hyperfiltration of serum with a maintained equilibrium of urea concentration6 and can offer insight into the joint microenvironment. However, frequent sampling of joint fluid can disturb joint homeostasis, trigger inflammation, and hinder the accurate interpretation of findings. Furthermore, detecting changes in synovial fluid is challenging due to uncertainty about how quickly the joint environment can shift with activity and loading and measurement errors associated with collecting and analysing fluid of varying viscosity.7
The ability to study the joint environment without disturbing joint homeostasis would be ideal, and new imaging techniques have garnered great interest, particularly those capable of detecting early biochemical and microarchitectural changes before structural damage occurs. Techniques such as T2 relaxation time mapping and T1ρ imaging have been validated for detecting early cartilage damage.8 Additionally, methods like sodium imaging and glycosaminoglycan chemical exchange saturation transfer show promise.9 However, the practical application of these techniques, especially for disease stratification, remains limited.
Serum measures of joint-specific molecules have been investigated in many conditions. Markers such as anti-CCP, CRP, and urate in inflammatory or crystal arthropathies reflect systemic dysfunction rather than joint-specific biology. However, in recent years, there has been a concerted research focus on the identification and clinical validation of biological markers (biomarkers) as a proxy for underlying pathological tissue and joint changes, such as cartilage and collagen turnover (cartilage oligomeric protein, COMP, or C-terminal cross-linked telopeptide of type-II collagen, CTX-II), inflammation (interleukin, IL-1ß or tumour necrosis factor-alpha, TNF-α) and adiposity and metabolism (leptin or adiponectin).3,10–12 International consortia involving academia and pharmacological companies, supported by national regulatory bodies, such as the National Institute of Health Biomarker Consortium, have identified a list of potential candidate biomarkers which require translation into the clinical and research sphere.13,14 Several challenges have been identified, including the pathological process (es) identified by specific biomarkers, the range of values perceived to be within acceptable limits, and the form of the biomarker under measurement, with animal and tissue models, normative population values, and multiple biomarker types under investigation (including serum, synovial fluid and urine) to address these.15–18
However, further challenges exist, such as the influence of recent exercise, time from injury and location of sampling. It is well-recognised that certain ‘experimental’ variables must be controlled in clinical settings to ensure the laboratory result is not affected/biased.19 This is particularly relevant when concentration thresholds are used to categorise ‘normal v abnormal’, especially in the early stages of the disease process.20 Examples include early morning testing of hormone levels (to avoid the effect of circadian rhythm) or fasted sampling of blood glucose and lipids.
Within the OA field, previous work has reported that load-bearing alters certain biomarker concentrations, likely due to physiological joint-level mechanical effects, which are not necessarily an indication of pathology.21–23 In addition, increased time from injury can affect biomarker concentrations, which might demonstrate different processes during post-injury healing and early OA, and those during more well-established OA disease.8,24 In addition, when biomarkers are measured in the systemic circulation, such as at the antecubital fossa (ACF), there is likely to be a dilutional effect impacting biomarker(s) concentration. Unpublished data in those with Charcot-Marie-Tooth has previously demonstrated a difference in levels of IL-6 when measured local to the injured joint vs further away in the systemic circulation.
Therefore, this case-control pilot study aims to understand the effect of two ‘experimental variables’, the location of sampling and a short, non-weight-bearing, sub-maximal exercise intervention, on the concentrations of a panel of candidate serum OA biomarkers across three groups (control, recent injury, established OA). The hypothesis of this study is two-fold: serum concentration of biomarkers will be higher nearer to the joint than those measured in the systemic circulation, and precedent sub-maximal exercise will influence biomarker concentrations.
A favourable ethical opinion for this study was granted by the Faculty of Medicine and Health Sciences Research Ethics Committee at the University of Nottingham (FMHS 170-1122) in April 2023. Study participation was voluntary, with each participant signing a written consent form at least 24 hours after being sent the participant information leaflet, with the opportunity to ask further questions prior to enrolment. All researchers were appropriately trained in Good Clinical Practice, phlebotomy and the study protocol (including use of ultrasound). Chaperones and medical staff were always in attendance during the sampling of the lower limb.
Potential participants were identified and recruited through university clubs, local sports teams, and regional running events, using word of mouth, posters and targeted emails to selected local networks. After expressing interest, potential participants were given a verbal explanation and a written participant information leaflet outlining the study and screened for eligibility. If interested and eligible, they were invited for a one-hour testing/data collection study visit, with time for questions and written informed consent beforehand.
Study visits were performed at the Academic Unit of Injury, Recovery, and Inflammation Sciences in the School of Medicine, University of Nottingham. After consent and an additional screening and eligibility questionnaire, basic demographic data (age, sex, ethnicity, body mass) were collected, and any history of recent or chronic injury was recorded.
In brief, the protocol involved the collection of three serum samples from each participant, two from the ACF (‘Arm 1’ and ‘Arm 2’), and one from a vein near their knee (‘Knee’), with the exercise intervention performed between the two ACF samples ( Table 1). After the first sample (Arm 1) had been collected, a paired sample from the same side was drawn from a regional vein near the joint, including the great saphenous vein (GSV), using a novel approach described in more detail here.25 Following this, the participant underwent a short sub-maximal, non-weight-bearing exercise task.
This was performed using a standardised protocol. The participant was asked to use a static cycle ergometer, and after a 1-minute warm-up, they were asked to cycle constantly for 10 minutes, aiming to maintain a rate of perceived effort (RPE) of 12 on the Borg scale (relating to ‘somewhat hard’).26 Following the exercise intervention, a second sample was drawn from the ACF (Arm 2).
Sera were centrifuged for 10 minutes at 3000rpm (Heraeus Biofuge Primo B, Heraeus) before the serum was extracted and aliquoted. Samples were frozen in cryovials at -80 in a temperature-controlled freezer (Model GGU 1500 Premium, Liebherr) before being transferred on ice to undergo analysis for a pre-selected panel of candidate biomarkers by Affinity Biomarker Labs (ABL, London, UK) using enzyme-linked immunosorbent assay (ELISA). The biomarkers chosen were high-sensitivity IL-1β, IL-6, CTX-II, COMP, N-propeptide of collagen IIA (PIIANP), and leptin, which were chosen to offer insights into different pathological mechanisms. Each ELISA plate had two kit controls with three quality control samples. All results underwent internal validation and quality control by ABL.
The pre-specified minimal study sample size was 30, split into 10 participants per arm: control (CON), self-reported established knee OA (KOA) and recent (last 3 months) lower-limb (INJ) ( Table 1). This was based on previously observed differences of IL-6 between local v central venous drainage in unpublished data in Charcot joint disease, which showed a standard deviation of IL-6 to be around 18, and the mean difference between paired samples (local vs more peripheral) of 25. Based on that, this study would require a sample size of 8 (number of paired serum samples) to achieve a power of 80% and a significance level of 5%.
Data were imported into statistical software, cleaned and checked for missing data and significant outliers. The assumptions of normality were assessed and parametric and non-parametric tests were used accordingly. During the initial condition analysis, normality tests were performed using the biomarker concentration levels, when paired data (location and exercise analysis), normality tests were performed using the difference between these paired data. Descriptive analysis was performed initially, reported in mean ± standard deviation, SD, or median (interquartile range, IQR), as appropriate.
Initially, univariate analysis was performed to assess the differences between conditions (CON/KOA/INJ) using analyses of variance (ANOVA) or the Kruskal-Wallis tests using the initial ACF sample (‘Arm 1’), i.e. Arm1: CON vs KOA vs INJ. Differences in sampling location were then analysed: first comparing 'Arm 1' and 'Knee' results, followed by comparisons between pre- and post-exercise using 'Arm 1' and 'Arm 2'. These analyses were performed on the entire cohort and then stratified by condition (CON/KOA/INJ) using paired Student's t-test or Wilcoxon matched-pairs signed-rank test. In addition, the percentage (delta%) change between location and exercise serum collections (|ARM1-Knee|&|ARM1-ARM2|) were calculated. Significance was set at 0.05.
Thirty-two participants were recruited into the study, n=12 CON, n=10 KOA and n=10 INJ ( Table 1). Participants had a median age 28.0 (IQR: 25.0-43.0)(n=1 unrecorded), were 81% male (n=26/32) and had a median BMI of 23.9 (IQR: 22.5-26.3) ( Table 2). The ethnic background of participants was Indian (47%, n=15), Caucasian (34%, n=11), Black, Asian and Afro-Caribbean (3%, n=1 each), with 3 unreported (9%) ( Table 2).
| Demographic | ALL (n=32) | CON (n=12) | KOA (n=10) | INJ (n=10) | p-value |
|---|---|---|---|---|---|
| Age * Median (IQR) | 28.0 (25.0-43.0) | 26.5 (25.5-30.5) | 52.0 (43.0-60.0) | 25.5 (24.0-28.0) | <0.001 |
| Sex (M/F) | 26/6 | 9/3 | 10/0 | 7/3 | 0.18 |
| BMI Median (IQR) | 23.9 (22.5-26.3) | 23.5 (21.9-25.2) | 25.4 (23.5-27.1) | 23.2 (19.6-25.2) | 0.18 |
| Ethnicity * | 0.070 | ||||
| Caucasian | 11 (34%) | 5 (42%) | 5 (50%) | 1 (10%) | |
| Indian | 15 (47%) | 7 (58%) | 1 (10%) | 7 (70%) | |
| Black/Asian | 3 (9%) | 0 (0%) | 2 (20%) | 1 (10%) | |
| Not recorded | 3 (9%) | 0 (0%) | 2 (20%) | 1 (10%) |
The three condition groups (CON/KOA/INJ) were compared initially using Arm 1 values to ascertain any differences ( Table 3, Figure 1). Across the three groups, IL-1β was seen to be significantly lower in those with KOA (0.03ng/L, IQR: 0.02-0.06ng/L) than those with a recent injury (0.14ng/L, IQR: 0.07-4.62ng/L) or control (0.10ng, IQR: 0.50-0.20ng/L), p=0.004. IL-6, CTX-II, leptin, COMP and PIIANP were not significantly different ( Table 3).
| Total | Control | KOA | INJURY | p-value | |
|---|---|---|---|---|---|
| N=32 | N=12 | N=10 | N=10 | ||
| IL-1β ng/L | 0.07 (0.04-0.14) | 0.10 (0.05-0.20) | 0.03 (0.02-0.06) | 0.14 (0.07-4.62) | 0.004* |
| IL-6 ng/L | 0.96 (0.67-2.26) | 1.38 ( 0.74-2.31) | 0.93 (0.72-0.97) | 1.91 (0.48-67.60) | 0.420 |
| CTX-II ug/L | 0.68 (0.37-1.09) | 0.59 (0.34-1.16) | 0.57 (0.27-0.99) | 0.95 (0.46-1.10) | 0.510 |
| Leptin ug/L | 7.01 (2.37-20.62) | 13.04 (3.30-41.67) | 8.12 (5.26-10.65) | 3.53 (1.95-11.33) | 0.230 |
| COMP ug/L | 173.06 (±47.76) | 173.22 (±41.74) | 192.91 (±56.34) | 153.02 (±40.85) | 0.180 |
| PIIANP ug/L | 136.98 (88.78-204.04) | 144.02 (97.58-177.99) | 90.82 (67.05-123.66) | 171.58 (132.91-388.92) | 0.078 |

The dashed circle represents the mean values for all the samples. The numbers represents percentage deviation of individual groups (control, knee osteoarthritis, recent lower-limb injury). compared to the mean.
COMP: Cartilage oligomeric protein, CTX-II: C-terminal cross-linked telopeptide of type-II collagen, IL: Interleukin, PIIANP: N-propeptide of collagen IIA.
Paired samples were taken from the arm (Arm 1) and leg (Knee) on the same side before exercise ( Figure 2). Differences between these were assessed initially at a whole study population level and then stratified by condition. Due to the technical difficulties of the novel technique employed, only 26 participants had blood drawn from their lower limbs (CON n=10, KOA n=10, INJ n=6).
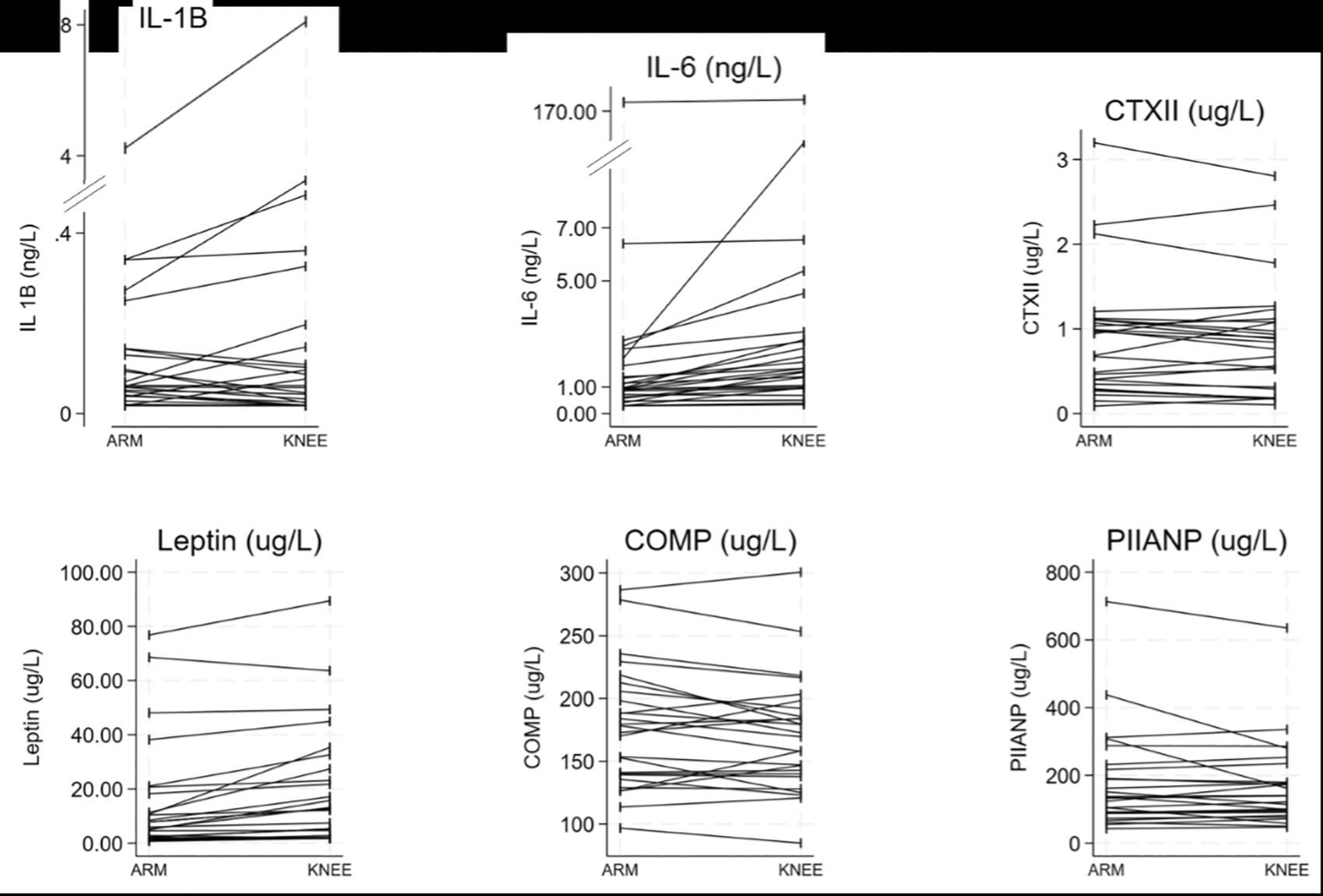
At a group level, CON, KOA and INJ, ( Table 4), significant differences in biomarker concentrations were observed based on the sampling location. Both IL-6 and leptin concentrations were found to be 72–80% higher in serum collected around the knee compared to the ACF. No other significant differences were observed between biomarker concentrations at the group level.
| Arm 1 | Knee | p-value | |
|---|---|---|---|
| IL-1β (ng/L) | 0.07 (0.04-0.14) | 0.06 (0.02-0.15) | 0.670 |
| IL-6 (ng/L) | 0.96 (0.67-2.26) | 1.65 (0.97-2.79) | <0.001* |
| CTX-II (ug/L) | 0.68 (0.37-1.09) | 0.80 (0.29-1.08) | 0.377 |
| Leptin (ug/L) | 7.01 (2.37-20.62) | 12.65 (2.75-27.34) | <0.001* |
| COMP (ug/L) | 173.06 (±47.76) | 171.16 (±45.36) | 0.170 |
| PIIANP (ug/L) | 136.98 (88.78-204.04) | 130.22 (90.64-179.89) | 1 |
When stratified for condition, four biomarkers exhibited differences between sampling locations ( Table 5). IL-6 was seen to differ across all three conditions between Arm 1 and Knee, increasing for CON and KOA and decreasing for INJ participants; leptin was significantly increased between locations in CON and KOA participants; PIIANP significantly increased between location in KOA individuals; and COMP significantly decreased between sampling locations in those with INJ ( Table 5).
| CON | KOA | INJ | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Arm | Knee | p-value | Arm | Knee | p-value | Arm | Knee | p-value | |
| IL-1β ng/L | 0.10 (0.05-0.20) | 0.09 (0.06-0.33) | 0.211 | 0.03 (0.02-0.06) | 0.02 (0.02-0.02) | 0.156 | 0.14 (0.07-4.62) | 0.15 (0.04-0.36) | 0.688 |
| IL-6 ng/L | 1.38 (0.74-2.31) | 2.33 (1.07-5.37) | 0.002* | 0.93 (0.72-0.97) | 1.37 (0.95-2.15) | 0.004* | 1.91 (0.48-67.60) | 1.63 (0.51-3.08) | 0.031* |
| CTX-II ug/L | 0.59 (0.34-1.16) | 0.91 (0.56-1.27) | 0.777 | 0.57 (0.27-0.99) | 0.53 (0.18-0.90) | 0.155 | 0.95 (0.46-1.10) | 0.91 (0.31-1.23) | 0.333 |
| Leptin ug/L | 13.04 (3.30-41.67) | 18.70 (2.75-44.89) | 0.027* | 8.12 (5.26-10.65) | 12.88 (7.40-23.08) | 0.002* | 3.53 (1.95-11.33) | 2.00 (1.84-4.64) | 0.219 |
| COMP ug/L | 173.22 (±41.74) | 167.08 (±38.37) | 0.510 | 192.91 (±56.34) | 189.55 (±55.84) | 0.545 | 153.02 (±40.85) | 147.30 (±25.12) | 0.088 |
| PIIANP ug/L | 144.02 (97.58-177.99) | 127.61 (97.96-176.70) | 0.322 | 90.82 (67.05-123.66) | 97.26 (76.80-172.96) | 0.004* | 171.58 (132.91-388.92) | 213.19 (138.96-279.92) | 0.313 |
When the serum biomarker concentration percentage change was calculated between locations to see if the participant condition affected the joint response, only PIIANP differed (CON: 4.10%, (IQR: -5.9,23.17%); KOA: -4.04% (IQR: -9.01,-1.13); INJ 9.10%, (IQR: -7.69,17.06), p=0.007). No other biomarkers displayed any significant percentage change differences.
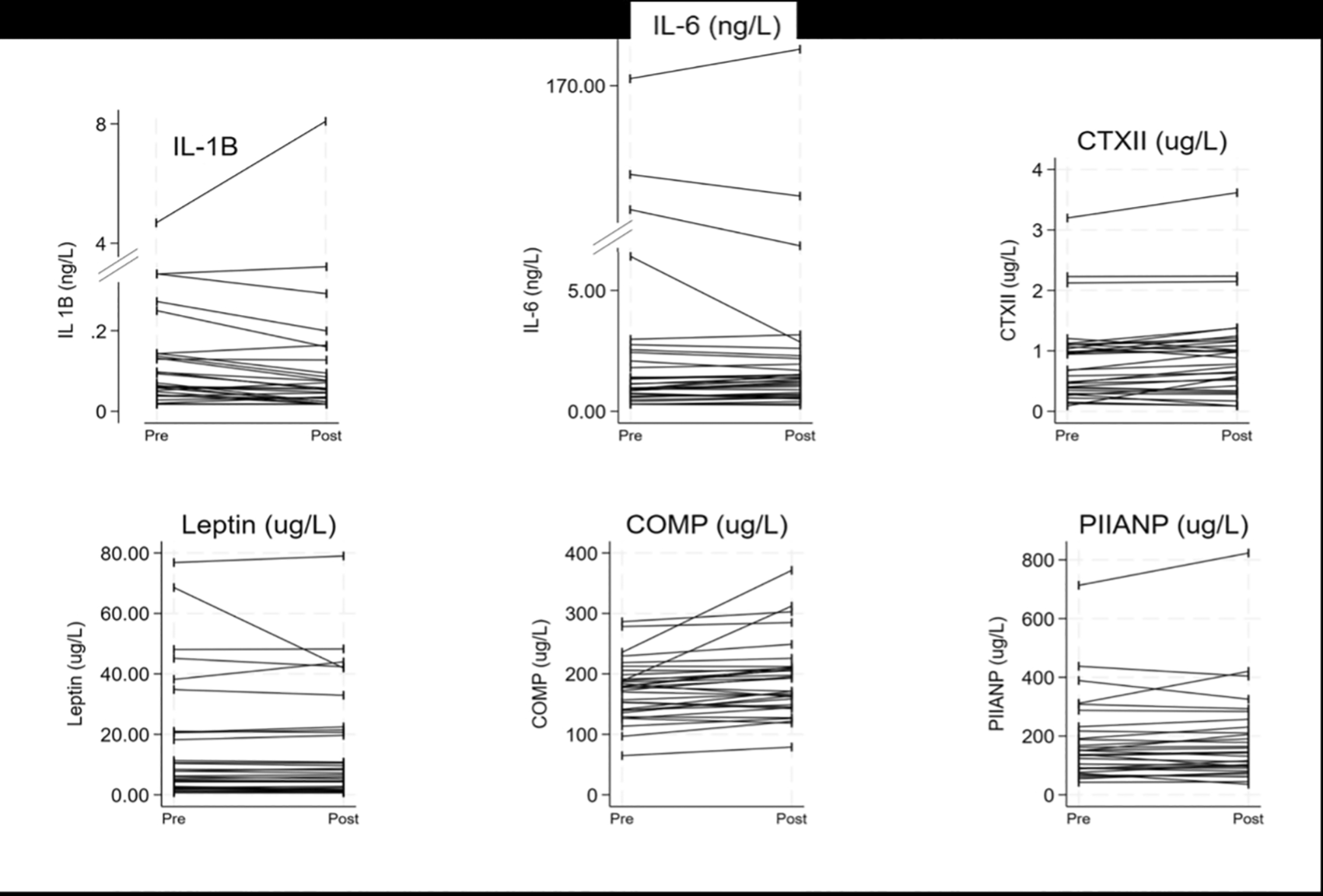
All 32 participants had a blood sample taken before and after the exercise intervention (Arm 1 and Arm 2, Figure 3). At the whole group level of the study, three biomarkers were significantly different after the intervention ( Table 6). These were IL-1β which significantly decreased, and CTX-II and COMP which significantly increased ( Table 6). The three other biomarkers, IL-6, leptin and PIIANP, showed no differences at a group level following the exercise intervention.
The dashed circle represents the mean values for all the samples. The numbers represents percentage deviation of individual groups compared to the mean.
COMP: Cartilage oligomeric protein, CTX-II: C-terminal cross-linked telopeptide of type-II collagen, IL: Interleukin, PIIANP: N-propeptide of collagen IIA.
| Pre-exercise | Post-exercise | p-value | |
|---|---|---|---|
| IL-1β (ng/L) | 0.07 (0.04-0.14) | 0.06 (0.02-0.14) | 0.542 |
| IL-6 (ng/L) | 0.96 (0.67-2.26) | 1.29 (0.68-2.07) | 0.566 |
| CTX-II (ug/L) | 0.68 (0.37-1.09) | 0.83 (0.38-1.16) | 0.013* |
| Leptin (ug/L) | 7.01 (2.37-20.62) | 6.94 (2.10-21.04) | 0.692 |
| COMP (ug/L) | 173.06 (±47.76) | 190.27 (±61.99) | <0.001* |
| PIIANP (ug/L) | 136.98 (88.78-204.04) | 137.09 (93.28-220.46) | 0.309 |
| Control | KOA | Injury (INJ) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-exercise | Post-exercise | p-value | Pre-exercise | Post-exercise | p-value | Pre-exercise | Post-exercise | p-value | |
| IL-1β ng/L | 0.10 (0.05-0.20) | 0.07 (0.04-0.16) | 0.031* | 0.03 (0.02-0.06) | 0.03 (0.02-0.05) | 0.081 | 0.14 (0.07-4.62) | 0.09 (0.07-2.60) | 0.646 |
| IL-6 ng/L | 1.38 (0.74-2.31) | 1.51 (0.94-2.12) | 0.444 | 0.93 (0.72-0.97) | 1.06 (0.77-1.25) | 0.041* | 1.91 (0.48-67.60) | 1.85 (0.56-37.68) | 0.609 |
| CTX-II ug/L | 0.59 (0.34-1.16) | 0.72 (0.31-1.13) | 0.328 | 0.57 (0.27-0.99) | 0.86 (0.42-1.09) | 0.030* | 0.95 (0.46-1.10) | 0.95 (0.52-1.17) | 0.420 |
| Leptin ug/L | 13.04 (3.30-41.67) | 14.19 (3.48-42.15) | 0.569 | 8.12 (5.26-10.65) | 7.73 (5.34-10.50) | 0.432 | 3.53 (1.95-11.33) | 3.52 (1.63-10.81) | 0.322 |
| COMP ug/L | 173.22 (±41.74) | 184.27 (±40.36) | 0.034* | 192.91 (±56.34) | 225.34 (±87.04) | 0.065 | 153.02 (±40.85) | 162.40 (±37.29) | 0.049* |
| PIIANP ug/L | 144.02 (97.58-177.99) | 138.89 (100.39-184.26) | 0.408 | 90.82 (67.05-123.66) | 85.77 (61.78-112.40) | 0.248 | 171.58 (132.91-388.92) | 218.05 (142.72-325.53) | 0.331 |
There were further significant differences when the exercise intervention results were stratified by the participant condition ( Table 7). Both IL-6 and CTX-II significantly increased in those with KOA post-exercise. COMP significantly increased in both INJ and CON, and IL-1β also significantly decreased in CON participants ( Table 7). PIIANP and leptin displayed no difference following the exercise intervention at a condition level.
No significant differences were noted across the condition groups in any serum biomarker percentage change following the exercise intervention, suggesting all groups of participants responded to exercise uniformly.
This pilot study aimed to test the hypothesis that the location of serum sampling or a short preceding sub-maximal exercise intervention would significantly influence serum concentrations of candidate OA biomarkers and has several notable findings. Leptin and IL-6 concentrations differed between sampling locations, with PIIANP also differing when stratified by condition. A short volume of non-weight-bearing exercise altered the concentrations of IL-1β, CTX-II and COMP, with IL-6 varying within the KOA group. These novel results have relevance for future clinical and research work.
Given the vast burden of OA morbidity and disability, molecular biomarkers are being investigated to see if they offer the ability to differentiate between good and poor joint health (both acute and chronic).11 In non-inflammatory joint conditions, there are well-established processes in joint biology associated with cartilage, bone, or synovial inflammation that are clinically useful. An abnormal molecular pattern might be the first sign of OA development, especially in those with a traumatic injury at risk of post-traumatic OA.27,28 L-1β, known to be related to OA,29 was lower in KOA during the condition-specific analysis; however, it has not yet found a role as a sensitive OA biomarker,11,14,24 perhaps due to its non-specific nature as a pro-inflammatory cytokine. Despite that, this result is notable given the very modest sample size and is in part likely due to the high-sensitivity assay used, which should be considered when future studies are designed.
Whilst synovial fluid offers the best insight into the joint microenvironment, repeated sampling of synovial fluid can cause inflammation and disturb joint homeostasis. This study explores the idea that the joint-specific biomarkers may differ in blood collected near the joint. Sampling closer to the joint could provide a clearer view of the joint environment and improve test accuracy by minimising dilution in the bloodstream.25 We observed that leptin and IL-6 concentrations were 70-80% higher in the circulation near the joint compared to the upper arm, regardless of condition. When this analysis was stratified by condition, more differences were demonstrated, with IL-6, leptin and PIIANP all tending to be higher near the joint compared to the arm.
This might change our understanding of the microenvironment of the joint, but also the relative importance of these biomarkers changes in the lower limb circulation. The mechanisms driving these discrepancies are not clear but potentially could be attributed to several factors, including greater adipose tissue presence around the lower limb joints, which produces more leptin, increased mechanical stress on weight-bearing joints like the knees, and heavy loading of muscles in weight-bearing limbs, with IL-6 being one of the first cytokines released in response. These findings suggest that local venepuncture might offer an insight into the microenvironment and local inflammatory, metabolic and cartilage turnover due to injury and OA development.24,30–32 These findings require validation in other populations, with similar studies planned elsewhere,33 but offer an exciting new avenue for diagnostic and predictive biomarker studies.
Another proposition is that exercise can increase synovial fluid filtration, leading to a more distinct expression of OA or injury-related biomarkers in serum samples. The effect of exercise on serum biomarker concentration is important to understand for two reasons. Firstly, if the results of the biomarkers are negatively influenced by preceding or recent exercise, especially if this is strenuous, then any clinical outcomes might be impacted by a potential false positive or false negative test result. Previous work has demonstrated that load-bearing exercise can alter biomarker concentration,21–23 so in the clinical settings, this needs to either be accounted for or the participants asked to refrain from exercise for a period of time before the test. One previous study did not show an increase in biomarker concentration,34 which could have resulted due to the sample size; however, a similarly sized study did show changes, but this followed a very different activity,35 so the amount of load may also be relevant. Secondly, if exercise increases serum biomarker concentration, this might offer an additional way to increase test sensitivity.21–23,35 However, not all individuals with or at risk of OA can weight-bear pain-free. Consequently, this study deliberately adopted a non-weight-bearing exercise to determine if exercise-related biomarker concentration changes were due to mechanical stress and to identify if this technique could be used as an alternative for those unable to fully weight-bear.
Following the exercise intervention, two ECM turnover biomarkers, CTX-II and COMP, significantly increased, suggesting either increased cartilage turnover and cartilage matrix degradation or remodelling. These findings support that exercise might increase test sensitivity and offer anti-inflammatory effects,36 as well as giving an insight into exercise-related joint changes. These findings were further supported during the condition analysis, with both IL-6 and CTX-II increased in those with OA (suggesting a potentially aberrant ECM and inflammatory response), COMP increasing in those with a recent injury and control participants, and IL-1β decreasing in control participants. The CTX-II and COMP findings complement those described previously21–23; however, those related to IL-1β and IL-6 in this setting are novel, potentially giving us an insight into acute mechanoinflammation.37
This pilot study has identified some new findings which require external validation in much larger populations. The strengths are the paired samples for both interventions and the well-trained, consistent research team (all venepuncture was performed by OOS or SK). In accordance with open data science principles, anonymized data and code are made available through the GitHub website.38 There are potential significant biases, including a Type II error due to the small sample size, which may not allow for the detection of significant changes. Additionally, recruitment strategy and differences in age, sex, exercise levels, and BMI were not accounted for in this research due to the small sample size. Those factors, including participants' age could eliminate differences between groups but would not account for differences between the sample collection sites or the variations observed before and after exercise. Future studies comparing synovial fluid, femoral vein and venous dynamics, using advanced techniques like technetium-99m tracing, gamma scintigraphy, or single-phton emission computed tomography, could validate the use of the GSV or other sites.
This unique pilot study has identified differences in serum biomarker concentration as a result of the location from which the venous sample was drawn and a ten-minute sub-maximal non-weight-bearing exercise task. These findings have relevance for future clinical and research work but require validation in a larger, external population.
Data and software codes are freely-available and accessible. They are posted Open Source via the GitHub platform, with a digital objector identifier created using Zenodo.38 Data is under a CC BY license. STATA 18.5 (STATACorp, LLP), was used for analysis, free software analysis alternative include R (https://www.r-project.org/).
Repository: GitHub. Name: SORE Study.38
Link: https://github.com/stefankluzek/SOREstudy
This link contains the following underlying data:
README.md – Authors, contents and key variables
SORE analysis 21102024.do – code used to analyse date (for STATA, version 18.5)
SORE lab data.xlsx – raw results of serum biomarker assays
SORE participant information leaflet template.docx
SORE consent form 2021 v1.1.docx
SORE pre-screening questionnaire.docx
STROBE checklist.docx
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Ethical approval was granted by The Faculty of Medicine and Health Sciences Research Ethics Committee, University of Nottingham (UoN FMHS 170-1122) in April 2023. Participants provided informed, written consent. Consent was granted by the researchers featured in the photographs within the manuscript. This study was conducted in line with the Declaration of Helsinki.
OOS performed study conceptualization, data acquisition, curation and analysis, and writing (drafting and editing), supported by SK. MMR conducted study visits, analysis and study administration. AA processed all samples and assisted with analysis. RP and SK assisted in conceptulization analysis and study logisitics. ANB and SK provided senior review, supervision and expert input with SK additionally leading on funding acquisition. All authors agreed the final version. OOS acts the guarantor.
Zenodo: stefankluzek/SOREstudy: SORE Study paper release, DOI: https://doi.org/10.5281/zenodo.14454447
This project contains the following underlying data:
README.md
SORE analysis 21 10 2024.do
SORE lab data.xlsx
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The authors would like to thank all the participants for their involvement, you were vital to this study and its success, and we are very thankful for your commitment, time and feedback to improve future research and clinical care.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Jordan J, Luta G, Stabler T, Renner J, et al.: Ethnic and sex differences in serum levels of cartilage oligomeric matrix protein: The Johnston county osteoarthritis project. Arthritis & Rheumatism. 2003; 48 (3): 675-681 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: osteoarthritis translational research
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Knee osteoarthritis, Molecular Biology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 04 Apr 25 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)