Keywords
anti-proliferative, anti-migratory, anti-invasive, EMT,Wnt/β-catenin signaling pathway
Hepatocellular carcinoma (HCC) is the most invasive liver cancer, with high incidence rates and resistance to chemotherapy. Polyphenols from red rice bran extract (RRBE) exert diverse anti-cancer effects on certain cancers. This study, for the first time, examined the suppressive effects of RRBE on proliferation, migration, and invasion in HepG2 cells with a preliminary investigation into how its inhibitory effects are linked to reversing the EMT process via the Wnt/β-catenin pathway.
The anti-proliferative and cytotoxic effects of RRBE on HepG2 were determined by the resazurin cell viability assay. Its effects on the cell cycle distribution and the apoptosis induction in HepG2 cells were assessed by flow cytometry. Its ability to impede migration was tested using the scratch assay and the transwell migration assay. An initial study was conducted to explore the link between its inhibitory effect and the reversal of EMT via the Wnt/β-catenin signaling pathway using Western blot analysis. Polyphenols and flavonoids were quantified using the Folin-Ciocalteu assay and the aluminum chloride colorimetric assay, respectively.
RRBE exhibited specific anti-proliferative and cytotoxic effects against HepG2 liver cancer cells compared to normal BNL CL2 liver cells in a dose- and time-dependent manner. RRBE caused a halt in the progression of HepG2 cells at the G2/M phase, resulting in subsequent apoptosis with variations in DNA content. The non-toxic doses of RRBE fully blocked the invasive migration of HepG2 cells after 24 h of its exposure as opposed to the untreated cells. Moreover, E-cadherin protein levels in HepG2 cells dose-dependently increased after 48 h of RRBE exposure, while the levels of β-catenin, vimentin, and MMP-9 proteins dose-dependently decreased compared to their respective untreated cells. The results indicated that RRBE-treated HepG2 cells showed a transition to epithelial characteristics along with diminished malignant features. Preliminary findings from the analysis of these proteins suggest that the inhibitory effects of RRBE may be connected to reversing EMT by regulating the Wnt/β-catenin signaling pathway. The phytochemical analysis revealed that RRBE contained high levels of polyphenols and flavonoids.
Red rice bran polyphenols demonstrated anti-proliferative, anti-migratory, and anti-invasive effects, which are possibly through Wnt/β-catenin-driven EMT reversal.
anti-proliferative, anti-migratory, anti-invasive, EMT,Wnt/β-catenin signaling pathway
According to Global Cancer Statistics 2020, liver cancer ranks at the top in terms of global incidence and mortality, and hepatocellular carcinoma (HCC) is the most common form of liver cancer.1 In Southeast Asia (e.g., Thailand, Cambodia, and Vietnam), the incidence and mortality rates of HCC are the highest.1 Despite the significant progress in HCC research, the prognosis for HCC remains poor because patients' early symptoms are often subtle, leading to diagnosis at an advanced stage, which is ineligible for curative surgery. In advanced HCC cases, the recommended therapy consists of sorafenib and Lenvatinib as the initial chemotherapy, but most patients do not achieve higher survival rates due to the resistance of HCC to chemotherapy and the onset of cirrhosis in later treatment stages.2,3 Moreover, considering the complexity of HCC pathogenesis, target therapies might present a more therapeutic alternative for HCC patients. These therapies are designed to target specific dysregulated signaling pathways during the progression of HCC, employing small molecules or monoclonal antibodies.2,3 The aim is to impede the growth of cancer cells and trigger apoptosis. However, the number of approved targeted drugs for HCC treatment remains inadequate.2,3
Recent accumulating evidence indicates that epithelial-mesenchymal transition (EMT), a prominent biological process, plays a pivotal role in the metastatic progression of HCC.4 EMT enables tumor cells to suppress their epithelial features and acquire the characteristics of mesenchymal cells, ultimately resulting in the loss of cell adhesion, increased motility, and invasiveness. EMT is characterized by the loss of epithelial cell markers, such as E-cadherin and cytokeratins, and enhanced levels of mesenchymal cell markers, such as vimentin, N-cadherin, and fibronectin. EMT activation in HCC significantly accelerates metastasis and is mediated by multiple molecular pathways, including the Wnt/β-catenin signaling pathway, where β-catenin acts as a critical nuclear mediator. The significant impact of the aberrant Wnt/β-catenin pathway on multiple processes is crucial for the progression of HCC.5,6
Using polyphenols, which are abundant phytochemicals in plants, as a method to combat HCC by preventing, delaying, or suppressing tumor growth and metastasis is becoming a promising strategy. Polyphenols are commonly found in many plant-based foods, such as vegetables, fruits, legumes, and whole grains. Several experiments, performed in vitro and in vivo, have indicated that dietary individual polyphenols have diverse anti-cancer properties, such as anti-proliferative, anti-migratory, anti-invasive, anti-metastatic, and pro-apoptotic effects.7,8 These anti-cancer properties are related to reversing EMT through the modulation of the Wnt/β-catenin signaling cascade.7,8
Polyphenols are highly concentrated in the bran layers of Asian pigmented rice varieties, mainly black, red, and dark purple. This colored rice bran is viewed as a safe, inexpensive, and easily accessible option for different dietary supplements.9 Studies have shown that pigmented rice bran polyphenols can suppress proliferation and induce apoptosis in particular cancer types.10–14 Their ability to inhibit migration and metastasis has also been evidenced, albeit to a lesser extent, particularly in connection with the EMT process and Wnt/β-catenin signaling pathway.15–17 Our earlier findings indicate that polyphenols from RRBE, which is derived from bran extract of Red Jasmine Rice, a Thai non-glutinous red rice, can lower oxidative stress and inflammation in neutrophils and macrophages18 and exert protective effects against oxidative stress-induced cytotoxicity in murine hepatocytes.19 Oxidative stress influences the progression of HCC by affecting gene expression, signaling pathways, transcription factors, and the tumor microenvironment.20 This study thus aimed to assess the inhibitory effects of RRBE on the proliferation, migration, and invasion of hepatocarcinoma cells, a topic that has not been explored yet. Moreover, a preliminary study was conducted to explore the connection between RRBE's inhibitory effects and the reversal of the EMT process via the Wnt/β-catenin pathway.
Ethanol was an analytical grade of RCI Labscan Limited (Thailand); Gallic acid, (±)-Catechin hydrate, dimethylsulfoxide (DMSO), Folin-ciocalteau reagent, sodium carbonate (Na2CO3) were purchased from Sigma-Aldrich (USA); Dulbecco's Modified Eagle's Medium (DMEM), Minimal Essential Medium (MEM), and phosphate buffer solution (PBS) were purchased from Caisson Laboratories (USA); fetal bovine serum (FBS) was purchased from Capricorn Scientific (Germany); resazurin sodium was purchased from Himedia (India); sodium hydroxide, aluminum chloride, and sodium nitrite were obtained from Ajax Finechem Pty Ltd (Australia). All reagents were analytical grade.
Rice bran (10 kg), Red Jasmine rice or Hawm Dawk Mali Daeng in Thai, from Chiang Mai, Thailand, was macerated with 40% (v/v) ethanol in water at room temperature (RT), and the supernatant was collected after 7 days. The remaining bran was macerated again, and this procedure was repeated twice. Then, the collected supernatants were combined, filtered through Whatman filter paper No.1 (Whatman, UK), and concentrated in a rotary evaporator and a freeze dryer. Red rice bran extract (RRBE) was obtained, weighed, and stored at -20°C until use.
Cell culture
The human hepatoma cell line HepG2 and the mouse hepatocyte cell line BNL CL2 were purchased from the American Type Culture Collection (ATCC, USA). HepG2 cells were cultured in MEM (Minimum Essential Medium). BNL CL2 cells were cultured in DMEM (Dulbecco's Modified Eagle's Medium). Both media were supplemented with 10% inactivated fetal bovine serum (FBS). Both cell lines were maintained at 37 °C in a 5% CO2 atmosphere.
Resazurin cell viability assay
HepG2 cells (2x106 cells/well) and BNL CL2 cells (1.2x106 cells/well) were seeded in 6-well plates and incubated overnight at 37 °C in an atmosphere of 5% CO2. HepG2 cells were treated with 3 mL of RRBE in completed media at concentrations ranging from 62.5 to 375 μg/mL; BNL CL2 cells were treated with 3 mL of RRBE in completed media at concentrations ranging from 62.5 to 500 μg/mL. Both cell lines were further incubated for 72 h (for selective effects). In addition, HepG2 cells at the same density were treated with RRBE at equal concentrations and incubated for 24, 48, and 72 h (for dose- and time-dependent effects). The untreated cells were used as the control group. After the removal of the old media, 2 mL of fresh media containing 0.5 mM resazurin (Himedia laboratories, India) was added and then incubated for 2 h. Fluorescence intensity at 488 nm (excitation) and 570 nm (emission) was measured, and the percentage of cell survival was calculated by the following formula:
T = RFU of treated cells with RRBE
C = RFU of control cells with only media
T0 = RFU of cells before the addition of RRBE (Day0 plate)
(RFU is relative fluorescence unit)
HepG2 cells (2x106 cells/well) were seeded in 6-well plates and incubated overnight at 37 °C in an atmosphere of 5% CO2. Thereafter, HepG2 cells were treated with 3 mL of 375 μg/mL RRBE and further incubated for 24, 48, and 72 h. The untreated cells were used as the control group. The cells were harvested, pelleted by centrifugation, and permeabilized with 80% ice-cold ethanol at -20 °C. Next, permeabilized cells in a single-cell suspension were stained with propidium iodide (PI)/RNase staining buffer (BD bioscience, USA) at RT for 30 min. These stained cells were collected using a flow cytometer (Becton-Dickinson FACScan, BD Biosciences, SanJose, USA) and analyzed for cell cycle phases with ModFit LTTM trial version (Verity Software House, USA, available at https://www.vsh.com/products/mflt/). The sub-G1 population was calculated to estimate the apoptotic cell population
Scratch assay
HepG2 cells (2x106 cells/well) were seeded in 6-well plates and incubated overnight at 37 °C in an atmosphere of 5% CO2. Next, cell monolayers were scratched with a sterile 200 μL pipette tip, creating the gap area, and then incubated with 3 mL of RRBE at concentrations of 125 and 167 μg/mL in MEM with 2% FBS. The untreated cells were used as the control group. The gap area was captured at 0, 24, 48, and 72 h and measured in μm2 by using the T.bar program (Nikon Eclipse TS100). The migration ability of cells, with or without treatment, was quantified by the change in gap area over time relative to the initial gap area at time point 0 h. The migration ability of untreated cells at 24 hours was used as a reference point with a value of 1, and the migration ability of untreated cells at 48 and 72 h was determined relative to this reference point.
Transwell migration assay
HepG2 cells (2x106 cells/well) were seeded in 6-well plates and incubated overnight at 37 °C in an atmosphere of 5% CO2. The cells were then treated with 3 mL of RRBE at concentrations of 125 and 167 μg/mL in MEM with 10% FBS for 24 h. The untreated cells were used as the control group. The cells were collected and seeded onto the upper compartment of the transwell inserts (24 mm Transwell with 8.0 μm pore polycarbonate membrane insert sterile, Corning, USA) in MEM with 2% FBS. The lower compartment contained MEM with 10% FBS as chemoattractants. After 24 h incubation, non-migrated cells on the upper surface of the insert membrane were removed. The migrated cells that moved and attached to the lower surface of the insert membrane were fixed with 3% paraformaldehyde for 15 min and stained with crystal violet for 25 min. The total number of migrated cells in each of the random fields within an insert was counted under an inverted microscope (Nikon Eclipse TS100) and averaged.
HepG2 cells (2x106 cells/well) were seeded in 6-well plates and incubated overnight at 37 °C in an atmosphere of 5% CO2. Thereafter, HepG2 cells were treated with 3 mL of RRBE at concentrations of 125 and 167 μg/mL. The untreated cells were used as the control group. After further incubation for 24, 48, and 72 h, the cells were harvested, and total cell lysates were prepared. HepG2 cell lysates containing 50 μg of total protein were separated by SDS-PAGE on 7.5% polyacrylamide gels. After electrophoresis with SDS-PAGE, the separated proteins from the gel were transferred onto the immobilon-FL PVDF membrane (Merck Millipore, USA) and immunoblotted with anti-E-cadherin polyclonal antibody (Merck Millipore, Germany), anti-β-catenin polyclonal antibody (Merck Millipore, Germany), anti-Vimentin polyclonal antibody (Sino Biological, China), and anti-MMP-9 polyclonal antibody (Cell signaling technology, USA). Goat anti-rabbit secondary antibody (LICOR Biotechnologies, USA) was used to detect these target proteins. The signals were then measured using the Odyssey CLx Imaging System (LI-COR Biotechnologies, USA). β-actin was used as a loading control. The anti-beta actin monoclonal antibody (Cell Signaling Technology) and goat anti-mouse secondary antibody (LICOR Biotechnologies, USA) were used.
Determination of total phenolic content
The total phenolic content (TPC) of RRBE was determined by the Folin-Ciocalteu method. The mixture contained 525 μL of Folin-Ciocalteu reagent, 525 μL of 7.5% (w/v) sodium carbonate, and 70 μL RRBE. After incubation at RT for 30 min, the absorbance of the mixture was measured at 725 nm. Gallic acid was used as the standard curve for the quantitation of TPC. TPC was presented as mg of gallic acid equivalent (GAE) per 1 g of RRBE.
Determination of total flavonoid content
The total flavonoid content (TFC) of RRBE was determined by the aluminum chloride colorimetric assay. The mixture contained 75 μL of 5% (w/v) sodium nitrite, 150 μL of 10% (w/v) aluminum chloride, and 500 μL RRBE. After incubation at RT for 5 min, 500 μL of 1 M sodium hydroxide and 275 μL of DI water were added to the mixture. After further incubation at RT for 30 min, the absorbance of the mixture was measured at 510 nm. Catechin was used as the standard curve for the quantitation of TFC. TFC was presented as mg of catechin equivalent (CE) per 1 g of RRBE.
The results were presented as mean ± SD from at least three separate experiments. All statistical analyses were carried out using IBM SPSS Statistics 22.0 software. The one-way analysis of variance (ANOVA) followed by Duncan or Dunnett's T3 for multiple comparisons was used to determine significant differences between groups. A level of significance was set at p-value <0.05. For the anti-proliferation and cytotoxicity assay, the IC50 value was extrapolated from the dose inhibition curve obtained by plotting the percentage of Inhibition versus RRBE concentrations using GraphPad Prism version 5.0 for Windows, serial number license GPS-2690594-LDVW-660EC (GraphPad Software, USA, available at https://www.graphpad.com).
This study assessed how RRBE affected the viability of HepG2 liver cancer cells in comparison to BNL CL2 normal liver cells. The resazurin cell viability assay, conducted after 72 h of incubation, revealed a reduction in the number of viable HepG2 and BNL CL2 cells as the concentrations of RRBE increased, indicating the dose-dependent anti-proliferative effects (see Fig. 1a). Moreover, non-lethal effects were observed in BNL CL2 cells with all concentrations of RRBE, but 250 and 375 μg/mL RRBE induced approximately 25% and 50% reduction in HepG2 cell viability, respectively, suggesting a dose-dependent cytotoxic effects. The IC50 values of RRBE against the two cell lines are depicted (see Fig. 1b). The selectivity index (SI) was based on the IC50 ratio of RRBE in the non-tumor BNL CL2 cell line to the tumor HepG2 cell line, which was found to be > 2. SI values above 2 are considered as high selectivity towards cancer cells. Overall, RRBE exhibited strong anti-proliferative and cytotoxic effects, specifically targeting liver cancer cells.
After noting the specific impacts of RRBE on anti-proliferative and cytotoxic effects against HepG2 cells, we then investigated the effects of different incubation periods (24, 48, and 72 h). After 24 hours of incubation, the resazurin cell viability assay displayed a decrease in the percentage of viable HepG2 cells as RRBE concentrations increased (see Fig. 2a). After 48 h of incubation, a decrease in viable HepG2 cells and a negative percent viability were noted at 375 μg/mL RRBE, indicating cell death of around 20%. After being exposed to 250 and 375 μg/mL RRBE for up to 72 h, the largest decline in viable cells, along with about 30% and 60% cell death, were recorded, respectively. The overall results demonstrated that RRBE only exerted an anti-proliferative effect that varied with the dosage within a 24-hour period. RRBE caused a complete halt in growth and subsequent cell death in HepG2 cells after 48 and 72 h, indicating a dose and time-related effect. In addition, IC50 values of RRBE decreased progressively over time, with a twofold reduction in an IC50 value at 72 h in comparison to 24 h (see Fig. 2b). The more potent anti-proliferative and cytotoxic effects were associated with lower IC50 values.
Studies conducted before revealed that the growth of HepG2 cells was inhibited, and cell death was induced by 375 μg/mL RRBE in a time-related fashion ( Figure 2a). Consequently, 375 μg/mL RRBE was selected to study how it impacted the cell cycle distribution of HepG2 cells. Flow cytometry showed an elevated proportion of cells in the G2/M phase in the treated group compared to the control group at 24, 48, and 72 h of incubation ( Figure 3). Within the next 24 hours, RRBE treatment caused the G2/M phase population to surge by 50% (see Fig. 3a, 3b). Following 48 and 72 h, there was a gradual decline in the number of G2/M cells, accompanied by a 2 and 3-fold rise in apoptotic cells, respectively, displaying a sub-G1 peak with diminished DNA content, in comparison to the 24-hour period ( Figures 3c, 3d, 3e, and 3f). Before reaching a sub-G1 peak, these arrested cells gradually underwent apoptosis, resulting in varied DNA contents due to variations in DNA degradation levels during apoptosis. By 72 h, a high portion of these cells acquired DNA content resembling that of the S phase, leading to a significant rise in the S phase population.
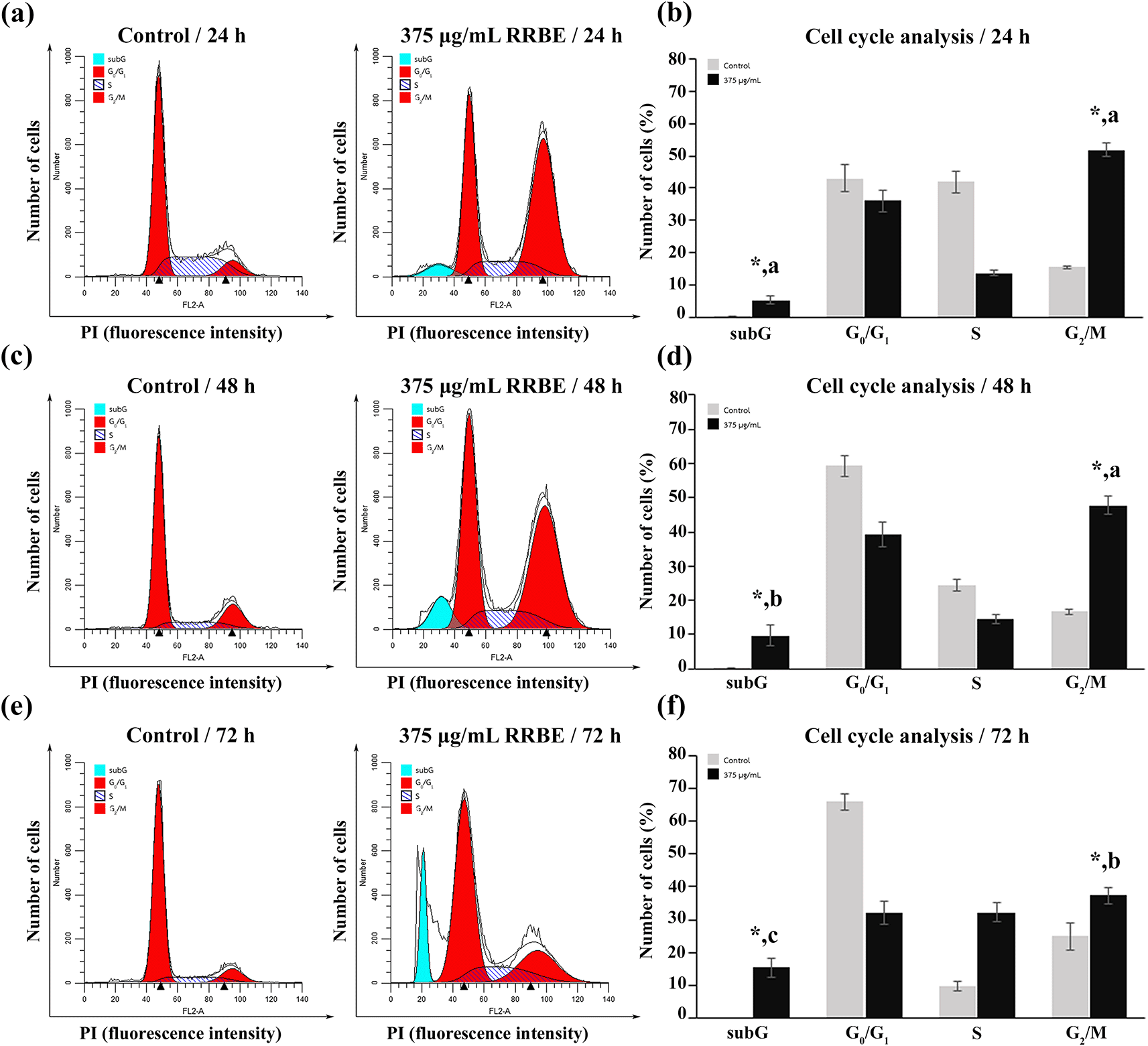
Representative DNA distribution histograms (a, c, e) and bar graphs (b, d, f ) displaying the percentage of HepG2 cells in different phases after the indicated time points. Asterisks (*) indicate significant differences in relation to the corresponding control (p < 0.05). Different letters indicate significant differences compared to the same phase at different time points (p < 0.05).
The previous findings demonstrated that RRBE at concentrations of 125 and 167 μg/mL exhibited a mild to moderate ability to inhibit cell proliferation. As a result, the two doses were employed to assess the anti-migration effect of RRBE on HepG2 cells, excluding its influence on cell proliferation. In the scratch assay, HepG2 cells were exposed to 125 and 167 μg/mL RRBE in a medium containing 2% FBS rather than the standard 10% for durations of 24, 48, and 72 h, as opposed to control cells that were left untreated. Of note, 1-2% FBS is used to sustain cells, not to stimulate their proliferation. The results demonstrated that untreated HepG2 cells in a medium with 2% FBS could migrate into an artificial gap on a cell monolayer, and their relative migration ability to fill the gap increased by 4-fold at 48 hours and 9-fold at 72 hours compared to 24 h (see Fig. 4a, 4b). In comparison to the untreated cells, the ability of HepG2 cells to migrate was fully blocked after 24 h of exposure to 125 and 167 μg/mL RRBE.
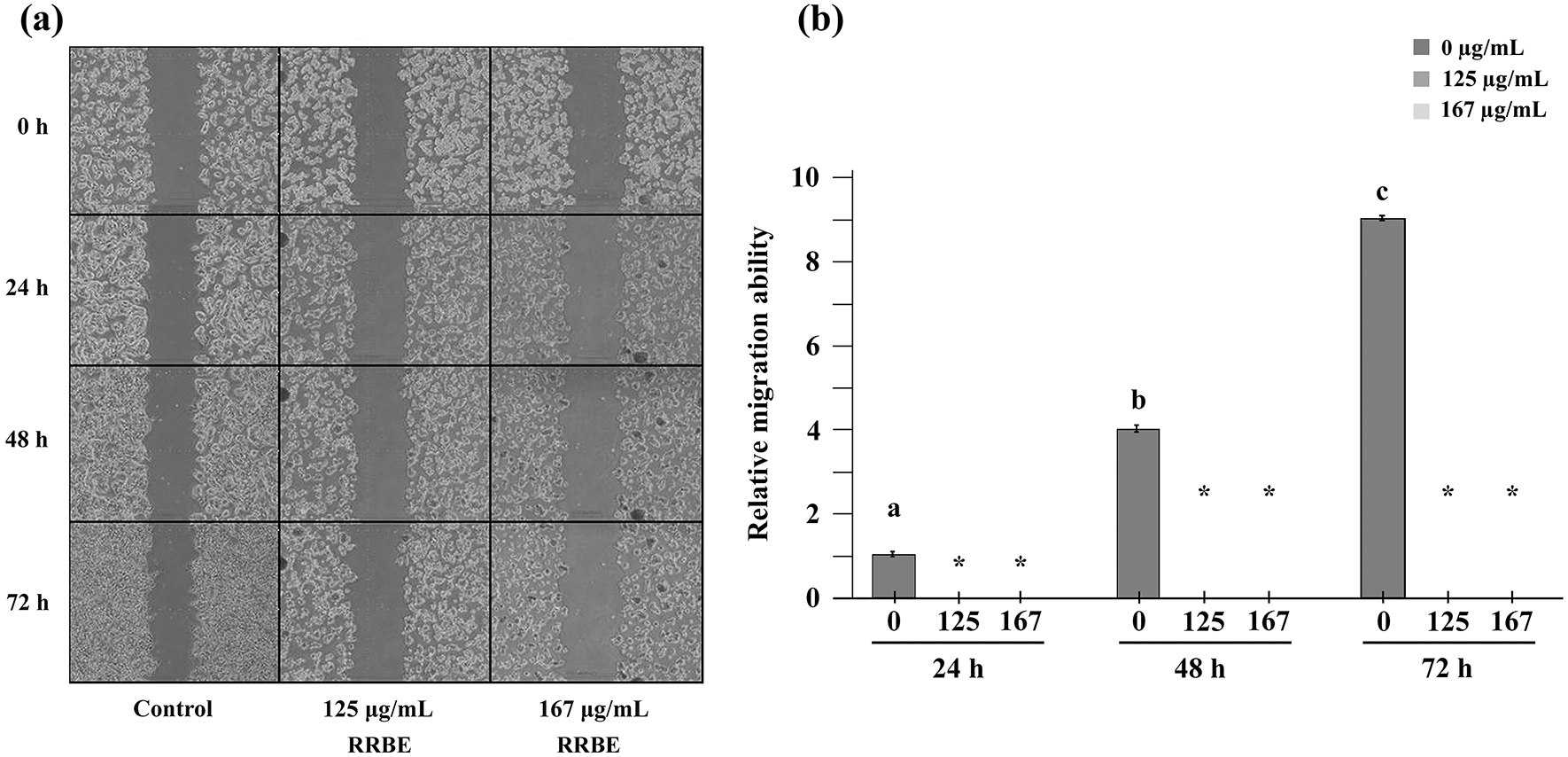
Representative images of the scratch assay after the specified doses and time points (a). A bar chart displaying the relative ability of HepG2 cells to migrate over time compared to the migration ability of control cells at 24 h, which was marked as 1 (b). Asterisks (*) indicate significant differences compared to the corresponding control at the same time (p < 0.05). Different letters in the control groups at different time points indicate significant differences relative to the control at 24 h (p < 0.05).
In contrast to the scratch assay, the transwell migration assay quantifies cell motility towards a chemoattractant gradient set up across the insert membrane with different FBS concentrations in the upper and lower chambers. After analyzing the scratch assay data, we opted to expose HepG2 cells to 125 and 167 μg/mL RRBE for a duration of 24 h while the control cells remained untreated. Next, we harvested the cells and placed them on the upper compartment of the transwell inserts that held MEM with 2% FBS. Concurrently, MEM with 10% FBS was added into the lower compartment of the chamber to function as chemoattractants. The transwell migration assay was performed for an additional 24 h. The results revealed that about 100 untreated cells per field, on average, moved through pores and reached the underside of the insert membrane (see Fig. 5a, 5b). In contrast, cells treated with RRBE at 125 and 167 μg/mL failed to migrate through transwell inserts, indicating the anti-migratory effect (see Fig. 5a, 5b).
Upon identifying the anti-migration effect of RRBE on HepG2 cells, we subsequently tested its ability to inhibit invasion. The Western blot technique was utilized to examine changes in the levels of E-cadherin, β-catenin, vimentin, and MMP-9 proteins in HepG2 cells following treatment with RRBE for 24, 48, and 72 h. The results showed that E-cadherin, vimentin, β-catenin, and MMP-9 proteins were undetectable in HepG2 cells after 24 hours, with or without RRBE. However, a clear indication of β-actin protein as a loading control was detected (data not shown). These results suggested that up to 50 μg of total protein lysates was sufficient for detecting only β-actin protein but not the proteins of interest, likely because of their low expression levels and/or the limited sensitivity of antibodies in this context. After 48 h, RRBE induced a dose-dependent increase in the E-cadherin levels, although this effect did not amplify with time (see Fig. 6a, 6 b). Conversely, RRBE resulted in a dose-dependent reduction in the levels of β-catenin, vimentin, and MMP-9 proteins when compared to their respective controls (see Fig. 6a, 6b). In this context, vimentin expression levels were time-dependently inhibited by a lower RRBE dosage, and MMP-9 protein levels were time-dependently suppressed by a higher RRBE dosage. The results indicated that HepG2 cells exhibited epithelial features and lost the mesenchymal phenotypes following RRBE treatments.
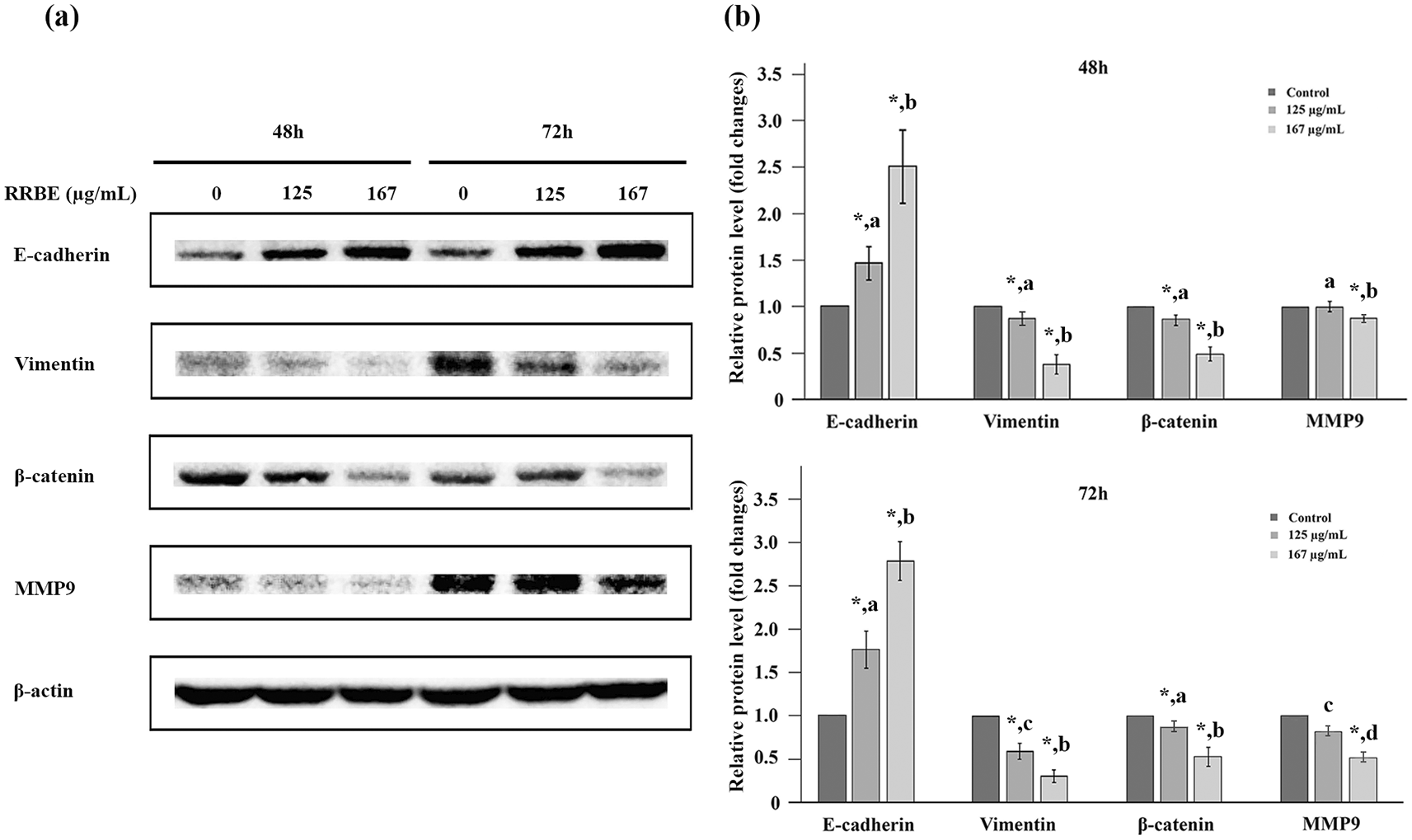
Representative western blot images (a) and bar charts (b) showing EMT marker levels in HepG2 cells after the specified doses and time points. Asterisks (*) indicate significant differences compared to the corresponding control. For each protein, different letters indicate significant differences compared to the same and different time points (p < 0.05).
A high yield of RRBE was achieved, and 1 g of RRBE contained high amounts of polyphenols and flavonoids (see Table 1). These bioactive compounds are likely significant contributors to the anti-cancer effects of RRBE.
In the past years, researchers have become interested in black, red, and dark purple rice for its significant polyphenol content. This study employed the two widely used spectrophotometric protocols, the Folin-Ciocalteu assay and the aluminum chloride colorimetric assay, to determine the levels of polyphenolics and flavonoids, a large family class of polyphenols in red rice bran extract (RRBE) from Red Jasmine Rice, a variety of Thai non-glutinous red rice. The findings showed that RRBE contained significant levels of these compounds (any amount exceeding 100 mg/g extract is considered substantial). Many studies have been conducted on various anti-cancer aspects of overall polyphenols or their specific groups in pigmented rice bran.9,21 However, limited research has been carried out on the quantity of polyphenols and their associated anti-cancer effects in the bran of various pigmented rice varieties.11
Colored rice bran polyphenols have demonstrated their ability to inhibit cell proliferation, alter cell cycle progression, and induce apoptosis in particular cancers.10,13,14,22 In this study, the cell viability graph revealed that RRBE dose-dependently exhibited a higher selectivity for hepatocarcinoma HepG2 cells over normal liver BNL CL2 cells. Moreover, RRBE arrested the cell cycle at the G2/M phase and promoted cell apoptosis, as demonstrated by cells with hypodiploid DNA content in the sub-G1 phase. Limited data is available on the effects of red rice bran on liver cancer, but current findings indicate that proanthocyanidin-rich fraction (PRFR) in red rice bran extract hindered the proliferation of HepG2 cells by inducing a pause in their division at the G2/M phase through the reduction of cyclin B1 and cell division cycle (Cdc25) proteins.12 During the transition from the G2 phase, cyclin B1 combines with cyclin-dependent kinase 1 (Cdk1) to drive cells into mitosis by phosphorylating key proteins. The activation of the cyclin B1-Cdk1 complex for cell progression into mitosis relies on Cdc25, a specific phosphatase, which removes inhibitory phosphate groups from Cdk1. PRFR also stimulated the apoptosis pathway in HepG2 cells by enhancing the levels of active apoptotic proteins.12 Apart from liver cancer, recent studies have shown that red rice bran extract can inhibit cell growth, cause cell cycle arrest in the G2/M phase and promote cell apoptosis in colon cancer cells.14,22 Of note, the p53 tumor suppressor arrests the cell cycle at two separate stages: before DNA replication (between G1 and S phases) and before cell division (between G2 and M phases), as well as induces apoptosis. HepG2, a highly aggressive type of cancer, expresses the normal and more functional form of p53.23 Regarding this aspect, recent studies have shown that the p53-wild type HCT116 colon cancer cells were more susceptible to apoptosis induction by RRBE than p53R273H HT29 cells, suggesting that the p53 pathway is likely involved in the apoptosis induction due to RRBE treatment.14
Considering that cell migration is a pivotal factor in cancer progression and metastasis, the anti-migration effects of RRBE were examined using both the scratch assay and the transwell migration assay. The scratch assay assesses the ability of treated HepG2 cells to migrate and subsequently close a gap compared with untreated cells. A constraint of this assay is its inability to measure cell chemotaxis. Conversely, the transwell cell migration assay provides a comprehensive analysis of the response of HepG2 cells to specific chemoattractants, such as 10% FBS in this study, as they move through a barrier towards them. The impact of pigmented rice bran on migration has been studied in specific types of cancer, including red rice bran in breast cancer24 and cholangiocarcinoma,17 black rice bran in breast cancer,16 and purple rice bran in prostate cancer.15 Our study is the first to document the anti-migration effects of RRBE on HCC.
Recent studies have revealed that abnormal activation of the Wnt/β-Catenin signaling pathway, where β-catenin serves as a vital nuclear mediator, is a major cause of HCC tumorigenesis, progression, and therapy resistance, and this pathway is linked to EMT. The most frequent mutations in HCC involve changes in CTNNB1, responsible for β-catenin, making it resistant to breakdown, followed by mutations in AXIN1, responsible for Axin-1, a key protein in β-catenin degradation, resulting in increased stability and buildup of β-catenin in the cytoplasm.5,6 The accumulated β-catenin moves to the nucleus, where it improperly triggers Wnt-target genes linked to cell cycle regulation, such as c-Myc and CCND1, promoting the growth and division of HCC cells. Additionally, it activates EMT-inducing genes, such as vimentin and MMP-9, promoting the invasion and metastasis of HCC cells. Vimentin, categorized as a type III intermediate filament, contributes to the metastatic progression of HCC cells by participating in the restructuring of the cytoskeleton in EMT-related signaling pathways.25 Matrix metalloproteinases (MMPs) are active in HCC cells and facilitate their movement and invasion by breaking down extracellular matrix (ECM) proteins and basement membrane during EMT.26 Particularly, MMP-9, also called gelatinase B, is a critical regulator in ECM degradation and is considered a reliable marker for the invasion and metastasis of cancer cells. Furthermore, the loss of E-cadherin often results in an up-regulation of β-catenin's signal transduction activity, further increasing EMT levels.
The findings indicated that RRBE was able to effectively impede the migration of HepG2 cells. A preliminary study on the link between RRBE's inhibitory effects and EMT reversal revealed that RRBE resulted in a decrease in levels of mesenchymal markers such as vimentin and MMP-9 but an evident increase in E-cadherin levels in HepG2 cells, indicating a shift towards an epithelial phenotype. The presence of E-cadherin is vital for maintaining the physical integrity of epithelial cells by supporting the adherens junctions that link neighboring cells; its depletion is a key event in EMT and a defining characteristic of metastatic cells. It can be inferred from the data that RRBE can hinder the migration and invasion of HepG2 cells by reversing EMT, thereby diminishing their malignant features, as observed by reduced vimentin and MMP9 levels, along with the inability of HepG2 cells to migrate and invade. Moreover, the decreased levels of vimentin and MMP9 point to the possibility that RRBE can suppress EMT by modulating the Wnt/ β-catenin signaling pathway via β-catenin. Further analysis into the β-catenin levels demonstrated that treatment with RRBE resulted in a decrease in these levels, hinting at its ability to hinder EMT by lowering Wnt/ β-catenin pathway activity. Nonetheless, the results of the initial study were inconclusive, which prompted additional research into the detailed molecular mechanisms. The anti-migration and anti-invasion effects of colored rice bran extract through EMT suppression have been scarcely explored.15–17 Also, it is important to mention that RRBE's ability to reduce β-catenin levels may be responsible for its anti-proliferative effects by inhibiting the hyperactive Wnt/β-catenin signaling that enhances uncontrolled and rapid proliferation of HCC. Investigating additional targets of β-catenin signaling that regulates the cell cycle, such as c-Myc and CCND1, will enhance our understanding of the involvement of the Wnt/β-catenin pathway.
RRBE polyphenols, extracted from the bran of Red Jasmine Rice, a Thai non-glutinous red rice, demonstrated anti-proliferative, anti-migratory, and anti-invasive effects, which could be related to the reversal of EMT by modulating the Wnt/β-catenin signaling pathway.
• Source code available from: URL (https://www.vsh.com/products/mflt, https://www.graphpad.com) to Version Control System.
• Archived software available from: (https://doi.org/10.5281/zenodo.14868405) where archived source code can be accessed.
• License: OSI approved open license software is under CC0.
Zenodo: Red rice bran polyphenols suppress invasive properties of HepG2 cells, possibly through Wnt/β-catenin-mediated EMT reversal.
https://doi.org/10.5281/zenodo.14868405.27
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
This study was financially supported by the Research Fund from the Faculty of Medicine, Thammasat University. Nattawan Thaolipo was financially supported by the Research Grants for Graduate Students from the Faculty of Medicine, Thammasat University.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Rukmana R, Silfarohana R, Putra A, Devi Safrina, et al.: Phytochemical Profile, Antioxidant Activity and Anticancer Activity of Gamma-Irradiated Black Rice Bran (Oryza sativa L.) Ethanolic Extract: In-Vitro and In-Silico Study. Science and Technology Indonesia. 2025; 10 (2): 628-643 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: medicinal plants and traditional medicines, anticancer activity, antibacterial activity
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 07 Apr 25 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)