Keywords
Hematology, coagulation, clinical chemistry, disease progression, ICU, Covid-19
This article is included in the Coronavirus (COVID-19) collection.
The surge of COVID-19 cases in Indonesia has markedly heightened the hospital demand for ICU care. Patients infected by SARS-CoV-2 exhibit discernible changes in laboratory results throughout their infection. This study aimed to assess the progression of COVID-19 infection, ICU admission rate, and patient outcomes by analyzing the profile of laboratory parameters in hospitalized COVID-19 patients.
This retrospective cohort involved six hospitals in Jakarta. The inclusion criteria consisted of PCR-confirmed hospitalized COVID-19 patients who underwent laboratory examinations for hematology, hemostasis, clinical chemistry, and infection markers during the pandemic from 2020 to 2021.
Total of 1865 COVID-19 patients participated in this study, with the majority of 54.16% male patients. Of these patients, 24.23% required ICU care, and 46.23% were aged >60 years old. The mortality rate among COVID-19 patients was 21.87%, with 58.68% of male patients, and 54.9% aged >60 years. Comorbidities such as hypertension, diabetes mellitus, lung diseases, and liver diseases, which aggravate the severity of COVID-19 infection and increase the mortality rate, were observed (p<0.05). The median values of leukocytes, neutrophils, Neutrophil-to-Lymphocyte Ratio (NLR), prolonged prothrombin time (PT), D-dimer, aspartate aminotransferase (AST), lactate dehydrogenase (LDH), urea, creatinine, C-reactive protein (CRP), procalcitonin (PCT), and interleukin-6 (IL-6) increased significantly. Conversely, median glomerular filtration rate (eGFR) and lymphocyte values declined with the progression of COVID-19 infection, ICU admission, and non-survival patient outcomes (p< 0.001).
Patients aged >60 years, with length of stay exceeding 8 days, low Hemoglobin levels, high NLR, low platelet levels, prolonged PT, high D-dimer, high CRP, and elevated IL-6 were correlated with severe COVID-19 infection, ICU admission, and unfavorable patient outcomes. The values of leukocyte count, neutrophil%, NLR, PT, D-dimer, AST, LDH, urea, creatinine, CRP, PCT, and IL-6 increased, while eGFR and lymphocytes decreased in severe COVID-19 infection, ICU admission, and non-survival patient outcome evaluations.
Hematology, coagulation, clinical chemistry, disease progression, ICU, Covid-19
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus-2, which targets the human respiratory tract1,2 In Indonesia, the majority of COVID-19 cases were concentrated in the DKI Jakarta province, followed by West Java, Central Java, and East Java.3 Most patients with COVID-19 exhibit either asymptomatic conditions or mild symptoms that do not require hospitalization. However, approximately 14% of the cases manifest moderate and severe symptoms requiring hospital treatment and oxygen support. In addition, 5% of these cases require intensive care unit (ICU) treatments.4,5 Severe cases of COVID-19 can be further exacerbated by acute respiratory distress syndrome, sepsis and septic shock, and multi-organ failure, including acute kidney or heart failure.5,6 The presence of comorbidities and advanced age elevates the risk of mortality.
Laboratory examinations are of significant importance in COVID-19 patient care, encompassing evaluation such as hematology, hemostasis, clinical chemistry, and infection markers. These laboratory parameters can either be utilized independently or in combination to discern disease progression, the necessity for ICU care, as well as clinical outcomes prediction.7,8
This study endeavors to explore the hematological profile, clinical chemistry, hemostasis, and prominent markers of COVID-19 infection in correlation with the severity of disease progression, ICU admission, and the assessment of clinical outcomes in adult COVID-19 patients across several hospitals in Jakarta, Indonesia. Through a comprehensive examination of these parameters, we aimed to identify pivotal factors that substantially influence the clinical course and prognosis of COVID-19 patients. A profound comprehensive understanding of these associations possesses a significant contribution to more effective management and treatment strategies for patients in critical conditions.
The present study took place at six hospitals in Jakarta utilizing retrospective cohort study data. The subjects included in this study were PCR-confirmed adults in patients with COVID-19 infection. During the COVID-19 pandemic in Jakarta, this study compiled data from a total of 1865 patients between December 2020 and September 2021. Only individuals with complete, recorded data relevant to this study were included. Both online and paper-based hospital databases were used. This study adhered to the principles of the Helsinki Declaration. The research protocol was reviewed and approved by the Ethics Committee of Yarsi University in Jakarta, Indonesia (reference number of approval: 285/KEP-UY/BIA/VIII/2021). The approval was granted on August 16, 2021. Due to the retrospective nature of the data, the Ethics Committee provided a consent waiver.
Statistical analysis was carried out using MedCalc v22.013 (https://www.medcalc.org) and Prism v8.4.2 tools (GPS-1837002-L, Machine ID: 5BBA7C9B19C). Free alternatives to these softwares are JASP (https://jasp-stats.org/) or R with RStudio. A p-value of 0.05 or less was deemed statistically significant. Categorical variables were assessed utilizing the chi-square test to determine significant associations, while continuous variables were analyzed using the non-parametric Kruskal-Wallis test due to non-normality distributed data.
A total of 1865 patients with COVID-19 met the inclusion criteria. No significant differences were observed in terms of disease progression, ICU admissions, and clinical outcomes between genders. Of 43.8% of COVID-19 patients fell within the 40-60 age range, with 52.07% experiencing a moderate severity of COVID-19 within this age group. Notably, 24.23% of patients required ICU care, and 46.23% of them were >60 years old. The mortality rate among COVID-19 patients was 21.87%, with 58.68% of them being male, and 54.9% aged >60 years.
A total of 52.43% underwent a treatment duration exceeding 8 days, especially notable in patients exhibiting moderate (29.06%) and severe symptoms (13.88%). Common comorbidities among COVID-19 patients included hypertension (44.0%), diabetes mellitus (37.65%), lung disease (8.48%), liver disease (1.55%), and malignancy (4.01%). These comorbidities exacerbated the severity of COVID-19 infection and contributed to an increased mortality rate ( Table 1).
| Characteristics | n | Degree of COVID-19 Progression | ICU Admission | Clinical Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | p | Non ICU | ICU | p | Survival | Non Survival | P | |||
| 1865 | 429 | 966 | 470 | 1413 | 452 | 1457 | 408 | |||||
| Gender | Male (%) | 1010 (54.16) | 214 (11.47) | 531 (28.47) | 265 (14.2) | 0.113 a | 759 (40.69) | 251 (13.45) | 0.533 | 778 (41.71) | 232 (12.43) | 0.214 |
| Female (%) | 855 (45.84) | 215 (11.52) | 435 (23.32) | 205 (10.99) | 654 (35.06) | 201 (10.77) | 679 (36.4) | 176 (9.43) | ||||
| Age (Years) | 18-40 (%) | 409 (21.99) | 145 (7.77) | 226 (12.11) | 38 (2.03) | <0.001 a | 356 (19.08) | 53 (2.84) | <0.001 a | 375 (20.1) | 34 (1.82) | <0.001 a |
| 40-60 (%) | 818 (43.8) | 186 (9.97) | 426 (22.84) | 206 (11.04) | 628 (33.67) | 190 (10.18) | 668 (35.81) | 150 (8.04) | ||||
| >60 (%) | 638 (34.21) | 98 (5.25) | 314 (16.83) | 226 (12.11) | 429 (23) | 209 (11.2) | 414 (22.19) | 224 (12.01) | ||||
| Length of stay (days) | 1-3 day(s) (%) | 112 (6.01) | 15 (0.8) | 48 (2.57) | 49 (2.62) | <0.001 a | 81 (4.34) | 31 (1.66) | <0.001 a | 41 (2.19) | 71 (3.8) | <0.001 a |
| 4-7 days (%) | 775 (41.55) | 237 (12.7) | 376 (20.16) | 162 (8.68) | 634 (33.99) | 141 (7.56) | 593 (31.79) | 182 (9.75) | ||||
| >8 days (%) | 978 (52.43) | 177 (9.49) | 542 (29.06) | 259 (13.88) | 698 (37.42) | 280 (15.01) | 823 (44.12) | 155 (8.31) | ||||
| Comorbidities | Hypertension (%) | 1662 (100) | 393 (23.64) | 883 (53.12) | 386 (23.22) | <0.001 a | 1283 (77.19) | 379 (22.8) | 0.067 | 1315 (79.12) | 347 (20.87) | <0.001 |
| Yes (%) | 732 (44.04) | 159 (9.56) | 365 (21.96) | 208 (12.51) | 545 (32.79) | 187 (11.25) | 536 (32.25) | 196 (11.79) | ||||
| No (%) | 930 (55.95) | 234 (14.07) | 518 (31.16) | 178 (10.7) | 738 (44.4) | 192 (11.55) | 779 (46.87) | 151 (9.08) | ||||
| Diabetes Mellitus (%) | 1628 (100) | 374 (22.97) | 872 (53.56) | 382 (23.46) | <0.001 a | 1250 (76.78) | 378 (23.21) | 0.042 | 1280 (78.62) | 348 (21.37) | <0.001 | |
| Yes (%) | 613 (37.65) | 130 (7.98) | 287 (17.62) | 196 (12.03) | 453 (27.82) | 160 (9.82) | 437 (26.84) | 176 (10.81) | ||||
| No (%) | 1015 (62.34) | 244 (14.98) | 585 (35.93) | 186 (11.42) | 797 (48.95) | 218 (13.39) | 843 (51.78) | 172 (10.56) | ||||
| Lung Disease (%) | 1414 (100) | 309 (21.85) | 817 (57.77) | 288 (20.36) | <0.001 a | 1091 (77.15) | 323 (22.84) | <0.001 | 1136 (80.33) | 278 (19.66) | 0.001 | |
| Yes (%) | 120 (8.48) | 4 (0.28) | 65 (4.59) | 51 (3.6) | 64 (4.52) | 56 (3.96) | 83 (5.86) | 37 (2.61) | ||||
| No (%) | 1294 (91.51) | 305 (21.57) | 752 (53.18) | 237 (16.76) | 1027 (72.63) | 267 (18.88) | 1053 (74.46) | 241 (17.04) | ||||
| Liver Disease (%) | 1097 (100) | 305 (27.8) | 565 (51.5) | 227 (20.69) | <0.001 a | 838 (76.39) | 259 (23.6) | 0.004 | 910 (82.95) | 187 (17.04) | 0.001 | |
| Yes (%) | 17 (1.54) | 2 (0.18) | 3 (0.27) | 12 (1.09) | 9 (0.82) | 8 (0.72) | 9 (0.82) | 8 (0.72) | ||||
| No (%) | 1080 (98.45) | 303 (27.62) | 562 (51.23) | 215 (19.59) | 829 (75.56) | 251 (22.88) | 901 (82.13) | 179 (16.31) | ||||
| Malignancy (%) | 797 (100) | 84 (10.53) | 517 (64.86) | 196 (24.59) | 0.8 a | 568 (71.26) | 229 (28.73) | 0.055 | 635 (79.67) | 162 (20.32) | 0.117 | |
| Yes (%) | 32 (4.01) | 4 (0.5) | 19 (2.38) | 9 (1.12) | 18 (2.25) | 14 (1.75) | 22 (2.76) | 10 (1.25) | ||||
| No (%) | 765 (95.98) | 80 (10.03) | 498 (62.48) | 187 (23.46) | 550 (69) | 215 (26.97) | 613 (76.91) | 152 (19.07) | ||||
In the context of hematological parameters, the values of hemoglobin, leukocyte, and platelet exhibited considerable variation across COVID-19 disease progression, ICU admission, and patient outcome assessment. However, hemoglobin levels above 10 g/dL were observed in 1724 cases (92.43%), while levels <10 g/dL were present in 141 cases (7.56%) ( Figure 1).
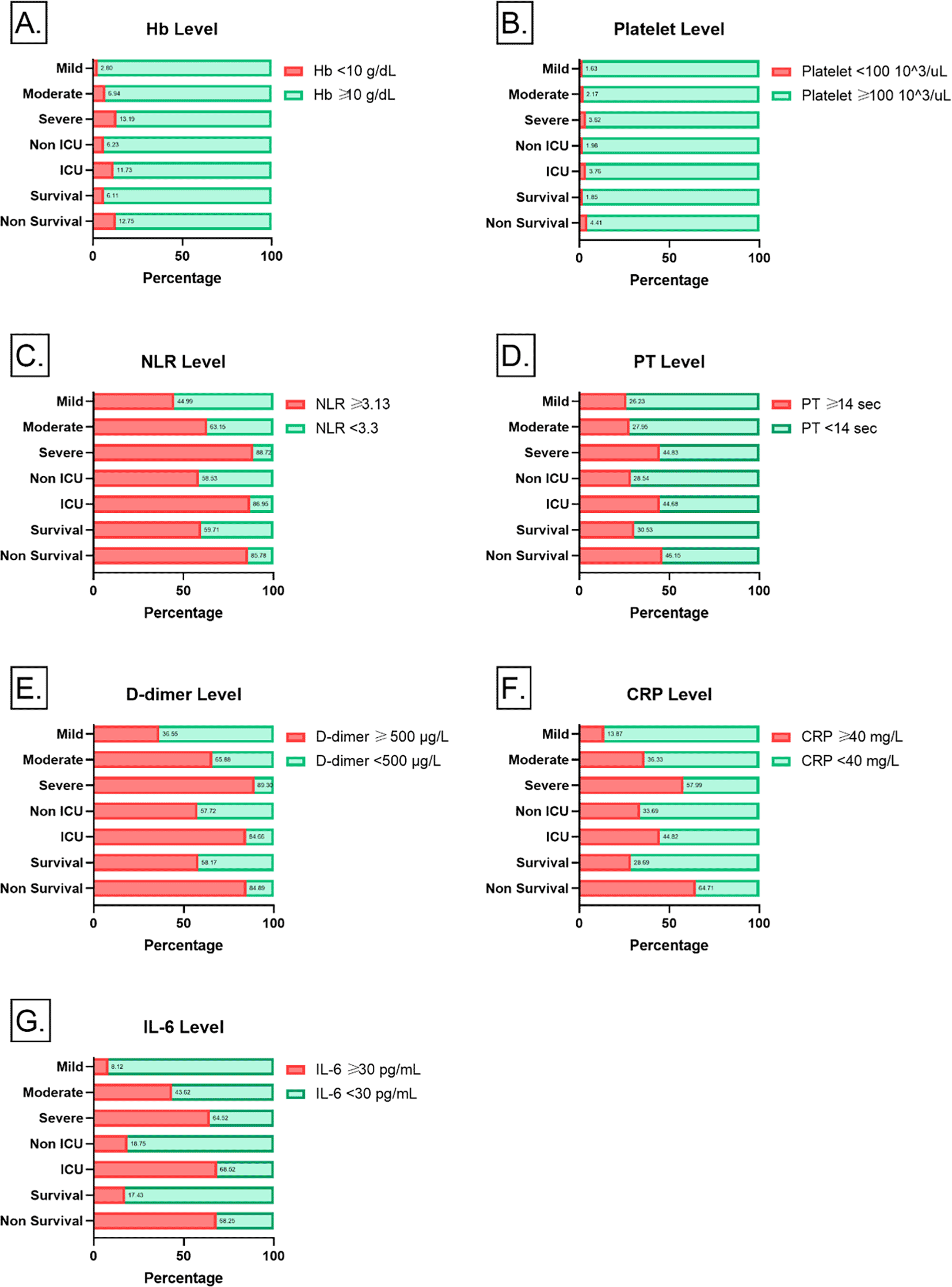
These are associated with the Degree of Disease progression, ICU Admission, and Patient outcome assessment.
In this study, the determination of low and high platelet counts was based on a meta-analysis by Lippi et al,7 defining thrombocytopenia as a platelet count of < 100 x103/μL. Low platelet counts were observed in 45 cases (2.41%). The median NLR value exhibited a significant increase in correlation with the progression of COVID-19 infection, as well as in response to ICU requirements and the assessment of patient outcomes among non-survivors. D-dimer levels demonstrated a noteworthy rise corresponding to the advancement of COVID-19 severity, ICU admissions, and the clinical outcomes of non-surviving patients.
Median levels of several clinical chemistry parameters including AST, LDH, and urea showed a substantial elevate in correlation to the progression of COVID-19, the necessity of ICU care, and in non-surviving patients. Conversely, renal clearance function indicated by eGFR values declined significantly when correlating it with the progression of COVID-19 severity, ICU admissions, and the clinical outcome of non-surviving patients.
In regard to infection markers such as CRP, PCT, and IL-6, the median values demonstrated a notable increase in accordance with the severity of COVID-19, ICI admissions, and the clinical outcomes of non-surviving patients ( Table 2).
| Parameter | Unit | n | Degree of COVID-19 Severity | ICU Admission | Clinical Outcome | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | Pa | No ICU | ICU | Pb | Survival | Non Survival | Pb | |||
| HEMATOLOGY | ||||||||||||
| Hemoglobin 1 | g/dL | 1865 | 13.6 (3.7-19.7) | 13.2 (5.1-20.9) | 13.2 (4.2-20.7) | <0.001 | 13.4 (3.7-20.9) | 13 (4.2-20.8) | <0.001 | 13.3 (3.7-20.9) | 13.2 (4.8-20.8) | 0.029 |
| Leukocyte | 103/μL | 1865 | 6.4 (1.6-31.7) | 7.6 (0.78-74.3) | 9.3 (0.89-50.6) | <0.001 | 7.12 (0.78-74.3) | 9.3 (1.34-70.8) | <0.001 | 7.2 (0.78-74.3) | 9.795 (0.89-70.8) | <0.001 |
| Thrombocyte | 103/μL | 1865 | 247 (22.9-842) | 241 (10-910) | 236.5 (8-799) | 0.099 | 243 (10-910) | 235 (8-703) | 0.0709 | 245 (10-910) | 230 (8-799) | 0.008 |
| Erythrocyte 1 | 106/uL | 1865 | 4.8 (2.4-7.2) | 4.625 (1-8.24) | 4.595 (1-6.91) | <0.001 | 4.7 (1-7.3) | 4.51 (1-8.24) | <0.001 | 4.7 (1-7.3) | 4.59 (1-8.24) | 0.005 |
| Hematocrit 1 | % | 1865 | 41 (21-56) | 39 (15.8-68.8) | 38 (7.7-65.9) | <0.001 | 40 (15.8-56) | 38 (7.7-68.8) | <0.001 | 39.8 (7.7-56) | 38.5 (15-68.8) | <0.001 |
| Diff Count | ||||||||||||
| Basophil1 | % | 1865 | 0 (0-2) | 0.2 (0-4) | 0.1 (0-2) | <0.001 | 0.1 (0-4) | 0 (0-2) | <0.001 | 0.1 (0-4) | 0.1 (0-2) | 0.4702 |
| Eosinophil | % | 1865 | 1 (0-13) | 0.2 (0-9) | 0 (0-77) | <0.001 | 0.2 (0-77) | 0 (0-4.8) | <0.001 | 0.2 (0-77) | 0 (0-7.8) | <0.001 |
| Neutrophil | % | 1865 | 66 (2-94) | 72.80 (0.7-98.2) | 82.70 (8.6-98) | <0.001 | 71 (0.7-96.3) | 82.5 (8.6-98.2) | <0.001 | 71.2 (0.7-96.3) | 82 (8.6-98.2) | <0.001 |
| Lymphocyte | % | 1865 | 23 (1-57) | 17.7 (0.6-86) | 10.40 (0.7-68.91) | <0.001 | 19 (0.7-86) | 10.2 (0.6-46.9) | <0.001 | 19 (0.7-86) | 10.6 (0.6-57.2) | <0.001 |
| Monocyte1 | % | 1865 | 13.6 (3.7-19.7) | 13.2 (5.1-20.9) | 13.2 (4.2-20.7) | <0.001 | 13.4 (3.7-20.9) | 13 (4.2-20.8) | <0.001 | 13.3 (3.7-20.9) | 13.2 (4.8-20.8) | <0.001 |
| NLR | 1865 | 2.96 (0.28-67.8) | 4.18 (0.1-209) | 8.07 (0.34-139.29) | <0.001 | 3.7 (0.1-209) | 8.2 (0.93-163.67) | <0.001 | 3.77 (0.1-209) | 7.8 (0.56-163.67) | <0.001 | |
| COAGULATION | ||||||||||||
| PT 1 | sec | 680 | 13 (10.1-31) | 13.2 (9.9-138.85) | 13.7 (10.8-120) | <0.001 | 13.1 (9.9-138.85) | 13.7 (10.4-120) | <0.001 | 13.2 (9.9-138.85) | 13.8 (11.1-82.9) | <0.001 |
| APTT | sec | 783 | 31.95 (20.1-180) | 32.5 (11.6-161.8) | 32.5 (14.2-180) | 0.357 | 32 (11.6-180) | 33.4 (14.2-180) | 0.012 | 32.3 (11.6-180) | 32.9 (14.2-180) | 0.192 |
| D-Dimer | ng/mL | 1429 | 400 (100-16900) | 716.5 (41-87826) | 1426 (223-108323) | <0.001 | 603 (41-87826) | 1400 (198-108323) | <0.001 | 650 (41-108323) | 1400 (127-87826) | <0.001 |
| CLINICAL CHEMISTRY | ||||||||||||
| AST | Unit/mL | 1055 | 29.7 (10-523) | 33 (9-866) | 49.1 (11.2-1454) | <0.001 | 32 (10-866) | 46 (9-1454) | <0.001 | 33 (10-866) | 52.45 (9-1454) | <0.001 |
| ALT | Unit/mL | 1096 | 34.9 (5-605) | 30 (3-2038) | 37.8 (5-904) | 0.014 | 32.95 (3-2038) | 35.4 (5-904) | 0.156 | 32 (3-2038) | 36.9 (6-904) | 0.02 |
| LDH | Unit/L | 575 | 192.5 (72-1233) | 275 (11.2-1503) | 445 (106-1821) | <0.001 | 216.5 (11.2-1503) | 406 (20-1821) | <0.001 | 231 (11.2-1503) | 483.5 (106-1821) | <0.001 |
| Ureum | mg/dL | 998 | 22.45 (0-265.8) | 28.9 (5.7-305) | 47.9 (0-372) | <0.001 | 28.4 (5.7-305) | 44 (0-372) | <0.001 | 28.6 (5.7-305) | 49 (0.42-372) | <0.001 |
| Creatinine | mg/dL | 1394 | 0.9 (0.41-14.25) | 0.97 (0.14-44) | 1.11 (0.3-25.55) | <0.001 | 0.9 (0.3-44) | 1.1 (0.14-27.93) | <0.001 | 0.9 (0.14-44) | 1.2 (0.42-25.55) | <0.001 |
| eGFR | mL/min/ 1.73m2 | 591 | 95 (8-138) | 17 (4-139) | 26 (5-114) | <0.001 | 63 (4-139) | 23.5 (5-114) | <0.001 | 68.5 (5-139) | 21 (4-109) | <0.001 |
| CRP | ug/mL | 1341 | 6 (0.08-285.2) | 20 (0.01-348) | 62.4 (0.01-445) | <0.001 | 15.2 (0.01-437) | 26.48 (0.01-445) | <0.001 | 11.55 (0.01-437) | 67 (0.02-445) | <0.001 |
| PCT | ug/L | 414 | 0.07 (0.045-32) | 0.11 (0.02-121.11) | 0.34 (0.037-78.8) | <0.001 | 0.105 (0.02-121.11) | 0.3 (0.037-78.8) | <0.001 | 0.115 (0.02-121.11) | 0.55 (0.02-78.8) | <0.001 |
| IL-6 | pg/mL | 383 | 2.455 (1.5-879.7) | 21.715 (1.5-8065) | 44.57 (1.5-5000) | <0.001 | 4.13 (1.5-8065) | 61.26 (1.5-5000) | <0.001 | 3.9 (1.5-8065) | 54.15 (3.37-5000) | <0.001 |
This study highlighted the association of comorbidities such as hypertension and diabetes with the severity of COVID-19 infections. Notably, severe cases leading to ICU admissions were characterized by low hemoglobin and platelets while at the same time exhibiting elevated leukocytes, neutrophils, and NLR. In addition, prolonged PT, elevated D-dimer, CRP, PCT, and IL-6 indicated disease severity and poor outcomes. In regard to severe cases, AST, LDH, urea, and creatinine exhibited a notable increase, while eGFR values declined. These findings underscore the importance of comprehending various hematological, clinical chemistry, and inflammatory markers in COVID-19 patients.
Our observations indicate a higher prevalence of COVID-19 infection among men; however, gender differences did not influence the severity of COVID-19, ICU admission rates, or patient outcomes. This aligns with findings from other studies.9,10 The predominant age group in the current study was 40-60 years, with the majority experiencing moderate COVID-19 symptoms. Notably, this age group comprised an active and mobile population, making them more susceptible to virus exposure. Other studies have observed a similar age distribution within the age range of 31-59 years (57.5%),11 and the average age of severe COVID-19 cases was 60.4 years.9
We observed that COVID-19 patients aged >60 were associated with clinical progression of COVID-19, ICU admission, and the clinical outcome of mortality. Another study has revealed that the median age of ICU-admitted patients was higher at 62.4 years in comparison to non-ICU patients (46 years),12 with the highest likelihood of death occurring in the age group of >60 years, reaching 43.6% of cases,11 while our study indicated a 35% mortality rate.
The expression of ACE2 increases with age, leading to potentially excessive ACE2 expression in patients over 50 years old. It seems that compromised immune response, reduced organ function, comorbidities, and various other factors elevate the risk of mortality. Advanced age also seems to correspond to a diminished immune system, heightening the susceptibility to ARDS and death.13
Within our study, 24.23% of patients required ICU treatment, which is in contrast with an 11.8% rate in an Iranian study,10 and a 32% rate in China.14 ICU admission in COVID-19 patients is associated with adverse clinical conditions characterized by dyspnoea, decreased O2 saturation, the requirement of oxygen support, and administration of broad-spectrum antibiotics.10 Furthermore, this study indicated that 52.43% of patients required prolonged treatment exceeding 8 days, particularly those with moderate and severe conditions. Another study has demonstrated an escalating average length of stay corresponding to disease severity as the following: 12.88 days for mild, 14.12 days for moderate, and 16.36 days for severe cases.15 We also noticed that ICU-admitted patients required hospitalization for more than 8 days at the rate of 61.9%. In line with our results, the average length of stay in the ICU at approximately 12.34 was observed in comparison to that of non-ICU admission at 5.73 days.16
The most prevalent comorbidities identified in this study were hypertension and diabetes mellitus. The presence of these comorbidities is associated with a heightened severity of COVID, and increased mortality.9,17 In addition, our investigation revealed that diabetes mellitus was specifically linked to higher frequency ICU admissions. Patients with comorbid diabetes mellitus were 3.12 times more likely admitted to ICU.12
Our analysis uncovered that hemoglobin levels in non-surviving COVID-19 patients were significantly lower than in those who survived. Anemia conditions can compromise oxygen supply to various tissues including the lungs and impact the quality of life after recovery.18 It is evident that Hb levels <10 g/dL significantly affect the severity of COVID-19, the necessity for ICU care, and the likelihood of non-survival outcomes. A similar trend was also observed when platelet count <100x103/μL.
A significant increase in the number of leukocytes and neutrophils was noticed in correlation with COVID-19 infection progression ICU admission, and non-survival. Conversely, platelets and lymphocytes exhibited the opposite pattern. Altered complete blood count (CBC) in COVID-19 infection has been extensively documented, featuring an increase in leukocytes, particularly neutrophils, and a concurrent decline in lymphocytes.19 The phenomenon is explained by the decline in CD4 and CD8 in patients with severe infections. While SARS virus implies the crucial role of lymphocytes in eliminating virus-infected cells, COVID-19 raises a hypothesis that patient survival depends on the body's ability to replenish lymphocytes lost to viral attack. The lymphocyte count, especially CD4 cell count, may serve as a clinical predictor for gauging disease progression and prognosis.20 Various factors underlying these hematological changes included cytopathic viral effects on lymphocytes, the suppressive impact of cytokine storms, lactic acidosis, and atrophy of lymphoid organs.21,22,23
NLR increased significantly corresponding to the severity of COVID-19 progression, ICU admission, and non-survival. NLR serves as an indicator of systemic inflammation which is commonly used to predict the progression of COVID-19 infections.24 Thus, implicating NRL could be a prognostic factor for potential shifts in clinical symptoms from moderate to severe. Moreover, NLR stands out as an independent biomarker signal to indicate unfavorable clinical outcomes,25 with non-surviving patients showing higher NLR values compared to those who survive post-COVID-19 infection.26 The elevation in NLR throughout the treatment period emerges as a predictive factor for mortality.
Evaluation of hemostasis parameters revealed prolongation PT values and elevated D-dimer levels in patients with severe COVID-19, those who were admitted to the ICU admission, and non-surviving patients. This observation aligns with findings from other studies.19,27 Patients with acute respiratory distress syndrome (ARDS) and progression to death exhibited elevated D-dimer levels, suggesting a significant correlation between elevated D-dimer and complications leading to mortality in COVID-19.28
The mechanisms behind the activation of the coagulation system by the COVID-19 virus remain poorly understood, giving rise to the term COVID-19-associated coagulopathy (CAC). Several hypotheses have been proposed, including CAC being triggered by cytokine storms in COVID-19 patients, endothelial dysfunction leading to causing microthrombi, particularly in the pulmonary system, and thrombotic microangiopathy induced by the release of vWF due to inflammation.29 Prolonged PT and activated partial thromboplastin time (aPTT) were more prevalent in severe COVID-19 compared to mild symptoms with noted that the elevations are not as pronounced as in bacterial sepsis and disseminated intravascular coagulation (DIC).20,30 A combination of pulmonary thrombotic microangiopathy and low-grade DIC potentially leads to coagulopathic symptoms in COVID-19 patients. Prolonged PT and increased D-dimer levels may serve as predictors, offering insight into the progression of COVID-19 in patients and indicating the possibility of occurring hemostatic cascade triggered by the virus.
In regards to inflammatory parameters assessed in this study, remarkable elevation has been observed in CRP, PCT, and IL-6 levels, exhibiting a discernable correlation with the severity of COVID-19, ICU admission, and outcome of non-surviving patients. This finding strengthens the hypothesis that monitoring CRP, PCT, IL-6, and D-dimers is imperative for gauging the clinical progression and recovery of COVID-19 patients. Consistent with our results, previous studies have observed heightened levels of inflammatory parameters including CRP, procalcitonin, IL-6, and ferritin in severe cases of COVID-19 infection.14,31,32 A meta-analysis conducted by Huang et al,33 concluded that elevated serum CRP, PCT, D-dimer, and ferritin were linked to unfavorable outcomes including death, ARDS, and ICU admission.
Clinical chemistry parameters unveiled increased CRP and IL-6 levels in patients with severe COVID-19, those requiring ICU admission, and those who did not survive. In severe cases particularly, the inflammatory response triggered by the SARS Cov-2 virus leads to cytokine storms, escalating vascular permeability multi-organ failure, and potential fatality if the cytokine storm persists. There is suspicion that the SARS-CoV-2 virus may induce both the inflammatory and the coagulation systems.32 PCT levels rising up to five times the upper limit of normal level (ULN) are associated with more severe COVID-19 symptoms, attributed to heightened PTC production and release from extrathyroidal sources due to bacterial infection. Moreover, interleukin-1β (IL-1β), tumor necrosis factor (TNF)-α, and IL-6 contributed to the elevation of PTC levels, though increased interferon (INF)-γ during the infection period inhibits the synthesis of these proinflammatory markers. In uncomplicated COVID-19 cases, it is unsurprising that PCT remains within the normal range. Conversely, elevated PCT levels in COVID-19 may also stem from bacterial coinfection, intensifying clinical symptoms and contributing to complications in patients.7
The clinical chemical parameters such as AST, LDH, urea, and creatinine exhibited a noteworthy escalation in correlation with the severity of COVID-19, ICU admission, and non-survival outcomes. In contrast, the renal filtration function indicated by eGFR demonstrated a significant reduction corresponding to the severity of COVID-19, ICU admission, and non-survival cases as observed by other studies.14,20 Increased LDH as a liver function marker and kidney function are associated with multiorgan failure triggered by a cytokine storm in COVID-19 infection. This cascade reaction is anticipated to induce acute lung injury and ARDS, subsequently causing damage to various organs, including the livers and kidneys. As a consequence, these parameters are observed to increase, especially in patients with severe conditions.20
Patients aged over 60 years and those with a length of stay exceeding 8 days exhibited a significant correlation with severe COVID-19, heightened ICU admission rates, and unfavorable clinical outcomes. The presence of comorbidities including hypertension, diabetes mellitus, lung and liver disease is also linked remarkably to severe COVID-19 and adverse patient outcomes. Furthermore, certain laboratory markers, including leukocyte count, neutrophil, NLR, PT, D-Dimer, AST, LDH, urea, creatinine, CRP, PCT, and IL-6, demonstrated noteworthy increases in severe COVID-19, ICU admission, and non-survival cases, whereas levels of Hb, platelet, eGFR, and lymphocyte count declined considerably.
Conceptualization, A.I., P.P., L.S., L.W., A.S., A.S.K.; Methodology, A.I., P.P., L.S., L.I., L.W., A.S.K.; Validation, A.I., P.P., L.S., L.W., A.S; Resources and Investigation, A.I., P.P., L.S., L.I., S.B., L.U.; Data Curation, A.I., P.P., L.S., L.I., S.B., L.U., A.S; Formal Analysis, A.I., L.S., P.P., L.W., L.I. A.S.; Writing Original Draft Preparations, L.W., L.S., A.S.K., A.I., A.S.; Writing, Review and Editing, A.I., P.P., L.S., L.W., L.I.; Supervision, A.S.K., A.I., P.P., L.S.; Project Administration, A.S.K., A.I.; Founding Acquisition, A.S.K., A.I. All authors have read and agreed to the published version of the manuscript.
The research protocol was reviewed and approved by the Ethics Committee of Yarsi University in Jakarta, Indonesia (reference number of approval: 285/KEP-UY/BIA/VIII/2021). The approval was granted on August 16, 2021. Due to the retrospective nature of the data, the Ethics Committee provided a consent waiver.
Mendeley data repository- “Indonesian Laboratory Data of Covid19”
This project contains following underlying data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0)(https://creativecommons.org/licenses/by/4.0/).
We would like to thank the following hospital managements: Jakarta Islamic Pondok Kopi Hospital, Abdi Waluyo Hospital, Yarsi Hospital, Harapan Kita Cardiac and Vascular Center, Mitra Keluarga Kemayoran Hospital, and Fatmawati Central General Hospital.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Therapeutic interventions, Clinical diagnosis, Cardiovascular diseases, miRNAs research, Biosensors
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 14 Apr 25 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)