Keywords
Foot-and-Mouth Disease, Foot-and-Mouth Disease Virus, Mus musculus, CD4+ T Cells, CD8+ T Cells, IgG-specific antibody, Vaccine
This article is included in the Preclinical Reproducibility and Robustness gateway.
Foot-and-mouth disease (FMD) is caused by the FMD virus (FMDV) in cloven-hoofed animals and has an economic impact on the livestock industry. On April 28, 2022, the re-emergence of this disease in Indonesia was confirmed. Commercial FMD vaccine containing the Manisa O1 strain is labile, and cannot provide cross-protection against other serotypes and circulating FMDV strain in Indonesia. Currently, no FMD vaccine in Indonesia contains locally isolated viruses. Therefore, it is imperative to develop FMD vaccines containing local isolates, given the nature of this virus, which lacks cross-protection and has low immunogenicity. This study aimed to evaluate the immune response in mice (Mus musculus) post-immunization with an FMDV master seed candidate for vaccine development.
The study employs Flow cytometry was used to measure the activation of helper T cells and cytotoxic T cells, and indirect ELISA was used to measure immunoglobulin G (IgG)-specific antibodies. This study was divided into four groups and five-point observations were conducted (D0, D14, D21, D28, and D56). The first treatment was on D0, and the second was on D14. All treatments were administered intramuscularly to the thigh muscles.
The inactivated FMD virus elicited a diverse immune response across all groups for various observations (P<0.05). MontanideTM ISA O/W adjuvant added to inactivated FMD virus titers of 106 TCDID50 and 107 TCID50 can provide a better post-immunization immune response than the other two groups. The optimal formulation for immune responses is a combination of inactivated FMD virus titers of 107 TCID50 with MontanideTM ISA O/W. The activation of helper T cells dominates the cellular immune response compared to cytotoxic T cells. The IgG-specific antibody response was stronger than the cellular immune response. Further research is needed to investigate the potency of master seed candidate in animal models high level (Guinea pig).
Foot-and-Mouth Disease, Foot-and-Mouth Disease Virus, Mus musculus, CD4+ T Cells, CD8+ T Cells, IgG-specific antibody, Vaccine
Foot-and-mouth disease (FMD) is a disease in animals, especially ruminants and cloven-hoofed wild animals, with a rapid transmission rate and economic impact on the livestock industry (Knight-Jones and Rushton, 2013; Stenfeldt et al., 2015; Wong et al., 2020; Chepkwony et al., 2021). Direct effects include decreased milk production, body weight, mortality, reproductive disorders, changes in genetic structure, and delays in trading livestock and animal products. Indirect impacts include additional costs of vaccination, early culling, and restrictions on local and international livestock traffic (Knight-Jones & Rushton, 2013). Therefore, this disease is a severe threat to the livestock industry, especially the international trade of livestock and animal products, and the World Organization for Animal Health (WOAH) classifies this disease in list A (Jo et al., 2019; Li et al., 2021).
Several countries have reported the loss and impact of FMD. The United Kingdom has experienced losses of 2.7 billion pounds (Davies, 2002). In 1977, Taiwan lost USD 6.617 billion, Uruguay in 2001 lost USD 80 million in 2010 lost USD 550 million, and South Korea in 2010-2011 lost USD 2.8 billion (Knight-Jones et al., 2017). According to an analysis by the International Federation of Red Cross and Red Crescent Societies/IFRC (2023), the potential losses experienced by Indonesia owing to FMD reached Rp. 9.9 trillion. According to data from the FMD Task Force of the Ministry of Agriculture of the Republic of Indonesia, as of August 3, 2023, reached 11,785 cattle (FMD Task Force, 2023).
The first FMD case in Indonesia was confirmed on April 28, 2022. The incident is contained in the Decree of the Minister of Agriculture of the Republic of Indonesia on the establishment of FMD outbreak areas: 403/KPTS/PK.300/M/05/2022 in several districts in East Java and 404/KPTS/PK.300/M/05/2022 in Aceh Tamiang District, Aceh Province (Directorate General of Animal Husbandry and Animal Health, 2022a, 2022b). The latest data on FMD incidence in Indonesia are 613,955 sick animals spread across 18 provinces in Indonesia, with a total of 15,709,725 vaccinated animals (FMD Task Force, 2023).
FMD is classified as a re-emerging disease in Indonesia. This was because Indonesia had an 1884 FMD outbreak, and in 1986, it was declared free of FMD (Silitonga, 2017; Yuan et al., 2020). FMD cases in Indonesia belong to the serotype O lineage Middle East-South Asia (ME-SA), topotype Ind-2001, sublineage e, and show a nucleotide sequence similarity of 95.3% with the Ind-2001e FMD virus (FMDV) originating from other Southeast Asian (Susila et al., 2023).
Vaccination is a preventive and control measure against FMD (Zhang et al., 2011; Park, 2013). Various studies on FMD vaccine development have been conducted, including inactivated vaccines, modified inactivated vaccines (differentiating infected from Vaccinated Animals/DIVA), and adenovirus vaccine vectors to virus-like particles (VLP) based (Uddowla et al., 2012; Xiao et al., 2016; Sitt et al., 2019). However, inactivated vaccines are the majority and have been in use since 1930 (Gupta et al., 2017; Kamel et al., 2019; Lu et al., 2022). However, inactivated vaccines produce short-term humoral immunity ± 4-6 months, periodic boosters are needed (Diaz-San Segundo et al., 2017; Choi et al., 2020).
The Directorate General of Animal Husbandry and Animal Health of the Ministry of Agriculture, Republic of Indonesia, responded to the FMD outbreak by importing vaccines from several countries. This is because Indonesia does not have a vaccine that contains local isolates. The importation of the FMD vaccine policy in the Decree of the Minister of Agriculture of the Republic of Indonesia Number 517/KPTS/PK.300/M/6/2022. Commercial FMD vaccines containing the Manisa O1 can provide partial immunity against the FMDV circulating in Indonesia (Fishbourne et al., 2017). The success of a vaccination program is determined by the vaccine’s efficacy and matching with the virus circulating in the field (Mitoma et al., 2021). Therefore, studies of FMD vaccines containing locally isolated vaccines are required.
The roadmap for veterinary vaccine development by the UK Vaccine Research and Development Network is divided into several stages, including the Target Product Profile (TPP), Discovery/Feasibility, and Development, including the early and late phases of registration. These stages of vaccine development must follow applicable regulations and ethical rules (Francis, 2020). The FMD vaccine developed in this study entered the discovery/feasibility stage. One aspect of the FMD vaccine development process is immunogenicity, which includes cellular and humoral immune responses produced (Lee et al., 2020b; Chathuranga et al., 2022). The mouse model of immunogenicity of the FMD vaccine has been widely used and continues to be used even more than cattle (Lee et al., 2016; Gnazzo et al., 2020). Similarities in the genomes of mice, cattle, pigs, and humans can be used as animal models to study basic qualitative and quantitative genetics and breeding (Muff et al., 2019). This study focuses on cellular and humoral immune responses in mice (Mus musculus) post-immunization with the FMD vaccine master seed candidate.
All research methodologies, procedures, and utilization of experimental animals were approved by the Animal Care and Use Committee (ACUC) at the Faculty of Veterinary Medicine, Airlangga University, Surabaya, Indonesia on June 20, 2023, with certificate number 2.KEH.101.06.2023. The inclusion criteria of the ethics board that approved the research were Dr. Nusdianto Triakoso, M.Ag., and DVM. The terminal procedure (cardiac puncture) was used to acquire blood samples. The animals were under terminal anesthesia to prevent the suffering of the animals. This article reports compliance with the ARRIVE (Animal Research: Reporting of in vivo Experiments) guidelines (Percie du Sert, et al., 2020).
This study was conducted from October to December 2023. The entire research process was conducted at the Animal Development & Research Facility, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia; Laboratory of Virology, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia; Research Center for Vaccine Technology and Development (RCVTD), Institute of Tropical Disease, Universitas Airlangga, Surabaya, Indonesia; and Laboratory of Animal Structure, Anatomy, and Development, Faculty of Mathematics and Natural Sciences, Universitas Brawijaya, Malang, Indonesia.
Antigens and adjuvants in this study were conducted by the Research Center for Vaccine Technology and Development (RCVTD), Institute of Tropical Disease, Universitas Airlangga, Surabaya, Indonesia. In this study, FMD serotype O was isolated from naturally FMD-infected cattle in Lamongan, East Java, Indonesia. FMD serotype O was inactivated based on the research conducted by Kurniawan et al. (2024) and Tobing et al. (2024). The biological titer of the antigen stock was calculated as 109 TCID50. The choice of oil-in-water emulsion adjuvants (MontanideTM ISA, SEPPIC, France) was based on the research conducted by Dar et al. (2013).
Two-month-old male and female BALB/c mice (Mus musculus), weight 20-30 gr, were housed five per cage and kept at 20 ± 2°C under a 12-hr light–dark schedule, with ad libitum access to water and feed. The number of sample sizes and replicates refers to the Resource Equation method (Arifin & Zahirudin, 2017). Eighty BALB/c mice (Mus musculus) with an equal sample size of males and females (n=40) were used. All mice were housed at the Animal Development & Research Facility, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia. All mice were confirmed to be healthy, as indicated by clear eyes, clean fur, and active. Acclimatization was performed for seven days (Burkholder, 2012).
The research design employed four groups (G1, G2, G3, G4) and two treatments (D0 and D14) for each group. G1 was treated with MontanideTM ISA O/W (adjuvant alone). G2 cells were treated with an inactivated FMD virus titer of 108 TCID50 (antigen only). G3 was treated with MontanideTM ISA O/W and inactivated FMD virus titer of 107 TCID50 (vaccine prototype I). G4 was treated with MontanideTM ISA O/W and inactivated FMD virus titer of 106 TCID50 (vaccine prototype II). The vaccine prototype was formulated with 20% antigen, 70% adjuvant, and 10% water for injection. The treatment design is shown in Table 1.
Mice were acclimatized for seven days. Treatment was given twice with five observations. The first treatment was conducted as Day 0 (D0), and the second treatment was 14 days after the first treatment (D14). All treatments were administered intramuscularly to the thigh muscle. Five separate observations (D0, D14, D21, D28, and D56). Each observation involved euthanasia of BALB/C mice (Mus musculus), males (n=2), and females (n=2) in each group to take serum and spleen for analysis by indirect-ELISA and flow cytometry. A schematic of the research design is shown in Figure 1.
The terminal procedure (cardiac puncture) was used to acquire blood samples. The animals were under terminal anesthesia. The anesthesia protocol used a combination of ketamine hydrochloride (Ilium Ketamil®, Australia) (60 mg i.p.) and xylazine hydrochloride (Xyla®, Netherlands) (16 mg i.p.). A blood sample will be slowly withdrawn from the ventricle to prevent the heart from collapsing (Parasuraman et al., 2010). A cardiac puncture using a 26-gauge syringe (TERUMO®, JAPAN) was used to collect blood into an oblique collection tube until the serum was separated. Serum was purified by centrifugation for 15 min at 3,000 rpm. The supernatant was serum purified and collected into a 2 ml (EPPENDORFTM, GERMANY).
The spleen-collecting protocol reported by Grosjean et al. (2021) was followed. The skin was sterilized using 70% ethanol. The skin and muscle layers were cut through by using sterile scissors. The spleen was visualized next to the stomach on the left side of the abdominal cavity. The spleen was removed as was any non-specific tissue (e.g. fat).
Checkerboard titration
The antigen stock was serially diluted with the coating buffer (). The total volume required for each dilution was 1,000 μL. The serum reference (data not shown) was diluted two-fold with PBS 1x (1/10–1/1280). The total volume required for each dilution was 750 μL. Checkerboard titration was performed using a 96-well microplate (SPL LIFE SCIENCE, SOUTH KOREA). Antigen-coated plates were added to the diluted antigen stock at 100 μL/well (antigen-coated layout not shown). Incubated samples were shaken and incubated at 4°C overnight and covered with aluminum foil (KLINPAK®) on the microplate. Discard steps after coating with the antigen. The washing steps were repeated three times with PBST 0.05%, 200 μL/well in all wells. Discard the steps after each washing step. Blocking steps were performed with creamer 4%, 100 μL/well in all wells. Incubation was carried out at 37°C for 1 h and the microplate was covered with aluminum foil (KLINPAK®). The steps are discarded after the blocked steps. The washing steps were repeated three times with PBST 0.05%, 200 μL/well in all wells. Discard the steps after each washing step. The primary antibody steps were performed with serum reference, all diluted at 50 μL/well (primary antibody layout not shown). Incubation was performed at 37°C for 1 h, and the microplate was covered with aluminum foil (KLINPAK®). The steps were discarded after adding the primary antibody. Secondary antibody steps were performed with Mouse Immunoglobulin G (IgG) (H&L) Antibody Peroxidase Conjugated (ROCKLAND®, USA) at a concentration of 1:50,000, 50 μL/well, in all wells. These steps were discarded after adding the secondary antibody. Washing steps were performed with PBST 0.05%, 200 μL/well, and repeated thrice. Discard the steps after each washing step. Substrate steps were performed with TMB ELISA Peroxidase Substrate (ROCKLAND®, USA) in the dark at 100 μL/well in all wells. Observations were made continuously until changes occurred. Stopped reaction steps were performed with H2SO4 2M, 100 μL/well, in all wells. Absorbance was measured using an ELISA reader (BIOBASE, CHINA) at a wavelength of 450 nm to obtain the Optical Density (OD) (Ran et al., 2019). OD values <1.5 and a regression value (R2) approaching “1” was observed by curve, indicating that the deviations were minor (data not shown). The checkerboard titration results were optimized as the antigen and serum diluted were 10−3 and 1/160, respectively.
Serum sample
The antigen stock was diluted with the coating buffer performed 10−3. The total volume required for dilution was 9,000 μl. The serum reference (data not shown) as a serum standard was diluted two-fold with PBS 1x (1/10–1/1280). The total volume required for each dilution was 50 μL. Serum samples were diluted with PBS 1x performed 1/160. The total volume required was 50 μL per well. Serum samples were analyzed using a 96-well microplate (SPL LIFE SCIENCE, SOUTH KOREA). The antigen-coated steps were added to the antigen diluted 10−3 at 100 μL/well (antigen-coated layout not shown). Incubated samples were shaken and incubated at 4°C overnight and covered with aluminum foil (KLINPAK®) on the microplate. Discard steps after coating with the antigen. The washing steps were repeated three times with PBST 0.05%, 200 μL/well in all wells. Discard the steps after each washing step. Blocking steps were performed with creamer 4%, 100 μL/well in all wells. Incubation was carried out at 37°C for 1 h and the microplate was covered with aluminum foil (KLINPAK®). The steps are discarded after the blocked steps. The washing steps were repeated three times with PBST 0.05%, 200 μL/well in all wells. Discard the steps after each washing step. The primary antibody steps were performed with serum samples, all diluted at 50 μL/well (serum sample layout not shown). Incubation was performed at 37°C for 1 h, and the microplate was covered with aluminum foil (KLINPAK®). The steps were discarded after adding the primary antibody. Secondary antibody steps were performed with Mouse IgG (H&L) Antibody Peroxidase Conjugated (ROCKLAND®, USA) at a concentration of 1:50,000, 50 μL/well, in all wells. These steps were discarded after adding the secondary antibody. Washing steps were performed with PBST 0.05%, 200 μL/well, and repeated thrice. Discard the steps after each washing step. Substrate steps were performed with TMB ELISA Peroxidase Substrate (ROCKLAND®, USA) in the dark at 100 μL/well in all wells. Observations were made continuously until changes occurred. Stopped reaction steps were performed with H2SO4 2M, 100 μL/well, in all wells. Absorbance was measured using an ELISA reader (BIOBASE, CHINA) at a wavelength of 450 nm to obtain the Optical Density (OD) (Ran et al., 2019).
Flow cytometry procedure
Single-cell suspensions were separated from the spleen were performed using by mechanical dissociation. The single-cell suspension was transferred 200 μl into a 1.5 ml centrifuge tube. The suspension was resuspended in 300 μl of PBS 1x and homogenized in a vortex mixer. The suspension was centrifuged for 5 min at 2,500 rpm at 10°C. The supernatant was discarded and the cell pellet was resuspended in 50 μL of cell fluorescence solution for staining with FITC (Fluorescein) anti-mouse CD4 (BIOLEGEND®, CALIFORNIA) and PE (Phycoerythrin) anti-mouse CD8 (BIOLEGEND®, CALIFORNIA) antibodies at 4 °C in the dark for 20 min. Cells were resuspended in 400 μl PBS 1x and transferred into a cuvette FCM for analysis using a FACScan flow cytometer (BD BIOSCIENCES, USA). Flow cytometry was used to determine the percentage of CD4+CD8- and CD4-CD8+ T lymphocytes. Detection of FITC anti-mouse CD4 (BIOLEGEND®, CALIFORNIA) used blue laser excitation at 488 nm wavelength and PE anti-mouse CD8 (BIOLEGEND®, CALIFORNIA) used yellow-green laser excitation at 561 nm wavelength.
Data analysis was done using SPSS version 26 (IBM Corp.,Armonk, NY, USA) to statistically analyze the percentages of CD4+ CD8- and CD4- CD8+ T lymphocytes and OD450 IgG-specific (P<0.05) (Lee et al., 2023). The groups within each observation were distinguished using Duncan’s post-hoc test (P<0.05) (Lee et al., 2023; Dahlan, 2014). The R2 value of the checkerboard titration was statistically analyzed using Microsoft Excel® 2019 (Microsoft Office, Washington, USA).
Representative flow cytometry dot plots of CD4/CD8 T lymphocytes in male and female BALB/C mice (Mus musculus) post-treatment are shown in Figures 2 and 3, respectively.
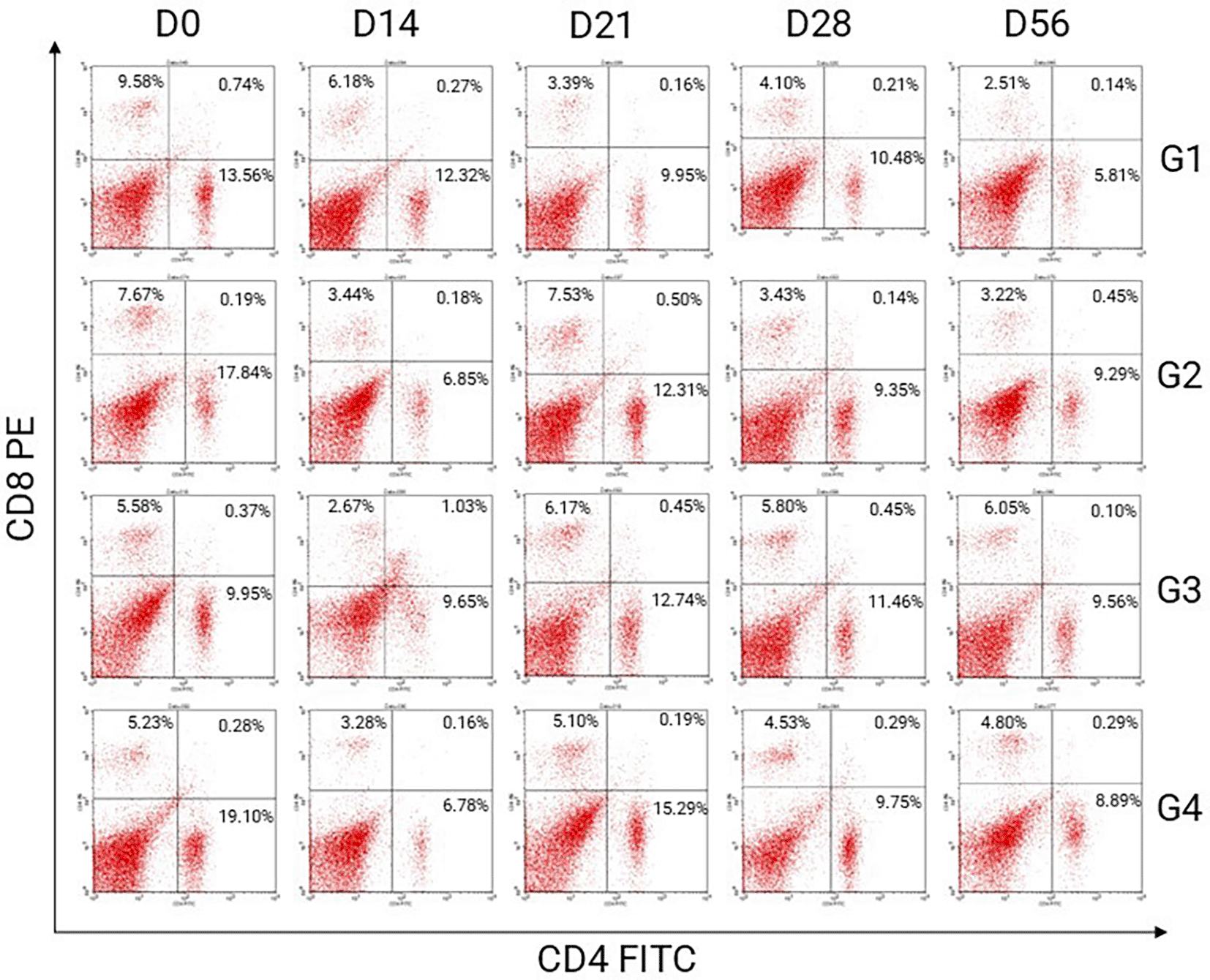
The percentage used in this research is single positive quadrant presented for CD4+CD8- T lymphocytes. There are four quadrants in dot blot analysed by flow cytometry. LL quadrant is the lower right, presented for CD4-CD8- T lymphocytes or double negative. LR quadrant is the lower right, presented for CD4+CD8- or single positive. UL quadrant is the upper left, presented for CD4-CD8+ or double positive. UR quadrant is the upper right, presented for CD4+CD8+ or double positive. Acronyms G presented for group treatments. Acronyms D present for day observations.
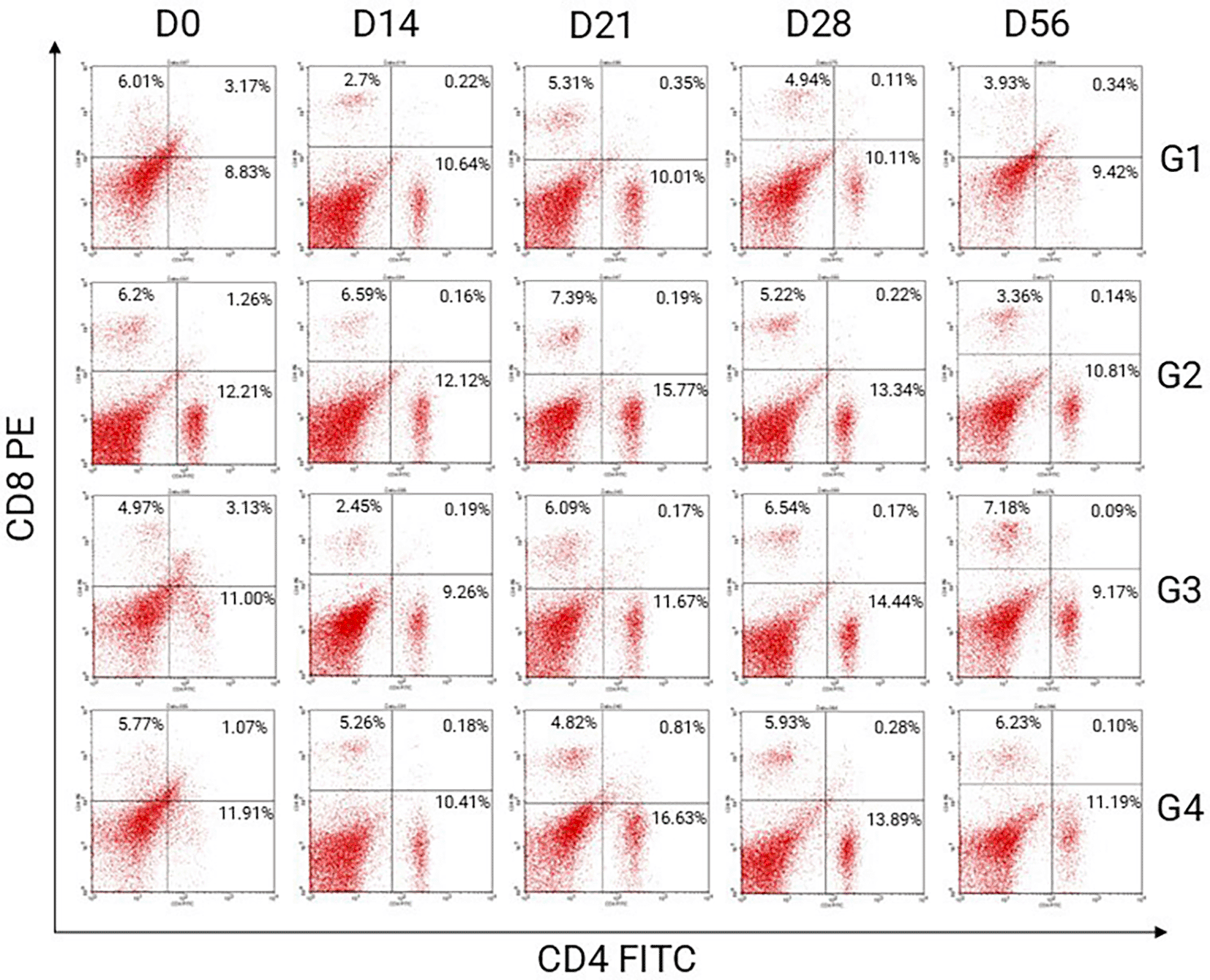
The percentage used in this research is single positive quadrant presented for CD4+CD8- T lymphocytes. There are four quadrants in dot blot analysed by flow cytometry. LL quadrant is the lower right, presented for CD4-CD8- T lymphocytes or double negative. LR quadrant is the lower right, presented for CD4+CD8- or single positive. UL quadrant is the upper left, presented for CD4-CD8+ or double positive. UR quadrant is the upper right, presented for CD4+CD8+ or double positive.
The helper T cell activation is the percentage (%) of CD4+CD8- T lymphocytes analyzed by flow cytometry as a single positive in the Lower Right Quadrant in the dot blot. This study used fluorescein isothiocyanate (FITC)-conjugated antibodies at a blue excitation wavelength of 488 nm. The percentage of CD4+CD8- T lymphocytes was analyzed using flow cytometry, as shown in Table 2.
The helper T cell activation in mice (Mus musculus) post-treatment in all groups decreased significantly until D14. Observations on D21 increased significantly compared with those on the previous day. This increase was not significantly different from that of the pre-treatments (D0). Observations on D28 decreased compared with those on the previous day. The decrease was not significantly different from that of the pre-treatments (D0). Observations on D56 decreased compared with those on the previous day. The decrease was not significantly different from that of the pre-treatments (D0). The helper T cell activation profile in this study in all groups is shown in Figure 4.
Cytotoxic T cell activation is the proportion (%) of CD4-CD8+ T lymphocytes identified by flow cytometry as a single positive in the upper left quadrant in the dot blot. This study used phycoerythrin (PE)-conjugated antibodies with a yellow green excitation wavelength of 561 nm. The percentage of CD4-CD8+ T lymphocytes analyzed by flow cytometry is shown in Table 3.
The cytotoxic T cell activation in mice (Mus musculus) post-treatment in all groups decreased significantly until D14. Observations on D21 increased significantly compared with those on the previous day. This increase was significantly different compared to the pre-treatments (D0). Observations on D28 decreased compared to those on the previous day. This decrease was significantly different compared to the pre-treatments (D0). Observations on D56 increased compared with those on the previous day. This increase was significantly different compared to the pre-treatments (D0). The cytotoxic T cell activation profile of all groups in this study is shown in Figure 5.
IgG-specific antibodies were analyzed using indirect ELISA at a wavelength of 450 nm. Peroxidase-conjugated antibodies were used in this study. The OD450 values were analyzed using indirect indirect-ELISA according in Table 4.
The IgG-specific antibodies in the mice (Mus musculus) post-treatment in all groups increased significantly until D14. Observations on D21 increased significantly compared with those on the previous day. The increase that occurred was significant compared to pre-treatment (D0). Observations on D28 were significantly increased compared to those on the previous day. The increase that occurred was significant compared to the pre-treatments (D0). Observations on D56 decreased compared with those on the previous day. The decrease was significantly different from that before treatment (D0). The IgG-specific antibody profiles in all groups in this study are shown in Figure 6.
Several factors can influence FMD vaccines to induce an immune response, including potency, matching with the field virus, and 146S integrity (Sutmoller et al., 2003; Paton et al., 2018). Vaccine potency includes antigen, dosage, and adjuvant (WOAH, 2022; Maree et al., 2015; Peta et al., 2021).
Matching with field virus serotypes is a possible factor that could impact vaccine efficacy, as serotypes O and SAT FMDV are more unstable than other serotypes (Lu et al., 2022). This factor, which affects FMD, cannot induce cross-protection with one serotype against the other FMDV serotypes. Consequently, ongoing vaccination is essential (Kotecha et al., 2015).
The approaching dose in the FMD vaccine is a protective dose (PD). This method provides immunity against viral challenges in groups of animals immunized with different amounts of vaccine. A protective dose of 50% (PD50) represents the vaccine dose required to provide immunity to 50% of vaccinated animals (WOAH, 2022). According to the WOAH standards, doses are classified into two categories: standard and high, depending on the intended use (WOAH, 2022). The standard protective dose contains an antigen concentration that produces a minimum potency level of 3 PD50 for prophylactic purposes. The high protective dose contained an antigen concentration of >6 PD50 for control purposes during an outbreak.
In this study, the different treatments did not show a significant pattern in the percentage of CD4+CD8- T lymphocytes in any of the groups. However, the percentage of CD4-CD8+ T lymphocytes in G3 mice showed a significant pattern. IgG-specific antibodies in G3 and G4 showed a significant pattern. In this group, the treatment consisted of antigens with an adjuvant.
Adjuvants in inactivated vaccine formulations can enhance the immune response to antigens by reducing the antigen dose (Cao, 2014; Jo et al., 2021). Reducing the antigen dose aims to reduce vaccine production costs (Lu et al., 2022). Mineral oil emulsion adjuvants in these formulations of prototype FMD vaccines act by forming a depot at the injection site, which then slows the release of the antigen (Dar et al., 2013). The use of oil-in-water adjuvants is highly recommended for inactivated FMD vaccines. This adjuvant can induce neutralizing antibodies earlier and more effectively, leading to higher cellular immunity and good immunity in cattle (Dar et al., 2013; Choi et al., 2020). Another adjuvant, such as a water-in-oil-in-water adjuvant for FMD vaccines, can degrade 146S particles, which are immunogenic components of FMDV (Harmsen et al., 2015).
The FMD vaccine must induce a robust immune response and match that of the field virus (Paton et al., 2005). FMDV is not known to provide cross-protection against other serotypes (Lee et al., 2020a). The World Reference Laboratory for FMD recommends the widespread use and prioritization of the Manisa O1 serotype in the most currently produced FMD vaccines (Doel, 2003; Lee et al., 2020a). Matching and challenge tests (Fishbourne et al., 2017) showed that vaccines with this serotype could only partially protect against the FMDV lineage O/ME-SA/Ind-2001. According to Paton et al. (2005) and Mitoma et al. (2021), alignment between vaccine virus and field virus whether by serological assay such as ELISA or VNT and even further by capsid gene sequence is crucial for adequate protection and success of the vaccination program.
The principles of FMD vaccine development must be able to trigger an immune response mediated by Th1 (T helper 1) and Th2 (T helper 2) to increase the immune response, which at the same time protects against a broader range of diseases with few side effects (Guzman et al., 2010; Carr et al., 2013). Most FMD vaccine development has focused on humoral immunity rather than cellular immunity (Jo et al., 2021). Humoral immunity formed after FMD vaccination can only produce short-term humoral immunity for approximately 4-6 months (Diaz-San Segundo et al., 2017). As a result, the FMD vaccine requires a booster one month after the primary vaccination and another booster at least 4–6 months later (Diaz-San Segundo et al., 2017; Choi et al., 2020).
Innate and adaptive immune responses work together and depend on each other (Cui et al., 2019). The innate immune response provides essential signals to induce and activate adaptive immunity (Iwasaki and Medzhitov, 2010). Antigen-presenting cells (APCs) recognize antigens on cell surface receptors, such as Toll-Like Receptors and Sensing Pathogen Receptors, and then present them through Major Histocompatibility Complex (MHC) proteins (Berczi & Szentivanyi, 2003; Baumgartner & Malherbe, 2010). Complex binding with MHC activates T lymphocytes via T cell receptors (Davis et al., 2003). MHC I maintains the expression of CD8+ T cells, whereas MHC II maintains the expression of CD4+ T cells (Abbas et al., 2021). Helper T cells activate several subsets of T cells with diverse functions (Luckheeram et al., 2012).
Role of adjuvants in the recognition process of T cell receptors. Adjuvants can enhance Thelper cells, differentiate Thelper cells into Thelper 1 (Th1), and enhance the cellular immune response (Pulendran and Ahmed, 2006). Adjuvants that induce adaptive immune responses are known to exist in two ways: delivery systems and activating fractions of the immune system (Guy, 2007). Most FMD vaccines are inactivated, less immunogenic, and only induce a short-term immune response compared to natural infections, requiring booster vaccinations to maintain immunity (Pulendran & Ahmed, 2011)
Cytotoxic T cells and IgG-specific antibody responses in females and males occur through different mechanisms. This mechanism is due to differences in antigen recognition by TLR and PAMPs. Antigen recognition in females is dominated by TLR3, TLR7, and TLR9 receptors, whereas it is different in males by TLR2 and TLR4 (Taneja, 2018). Hormonal balance influences the differences in immune responses between males and females (Torcia et al., 2012). In males, antigen recognition by macrophages induces an immune response via Th1 through TLR4 and TLR2. In contrast, in females, the immune response is formed through endosomal TLRs, such as TLR8, in response to estrogen (Taneja, 2018). TLRs are classified into two groups based on their location: cell surface and intracellular (endosomes, lysosomes, or endoplasmic reticulum) (Rodríguez-Habibe et al., 2020). The receptors on the cell surface include TLR1, TLR2, TLR4, TLR5, TLR6, and TLR11, whereas the endosomal receptors include TLR3, TLR7, TLR8, and TLR9.
Helper T cells are targeted for vaccine development. These cells can induce and maintain cellular and humoral immune responses through cytokine secretion of cytokines (McHeyzer-Williams et al., 2006; Cui et al., 2019). Activating these cells to perform their functions will help by activating various cells in the innate immune system, B lymphocytes, and cytotoxic T cells, as well as suppressing immune reactions (Luckheeram et al., 2012; Cui et al., 2019; Mitoma et al., 2021). According to Carr et al. (2013), the expression of these cells can be detected seven days after the primary vaccination.
All observations in this study showed that T helper cells are significantly expressed. The expression in these cells generally showed a significant decrease until D14, followed by a constant decline from D28 until D56. Increasing on D21, memory cells generate these cells through the differentiation of naïve helper T cells after antigen recognition. The activated memory cells that emerge after antigen exposure persist significantly compared to naïve helper T cells, resulting in a faster and more efficient immune response than the primary immune response (Gasper et al., 2014). This theory aligns with this research design, which involves booster shots at 14-day intervals following primary immunization. Memory cells generate a quick and robust immune response and are characterized by efficient vaccination (Kandel et al., 2022). However, helper T cells due to vaccination are not as good as in natural infections because the number of memory cells that are formed is lower (Mitoma et al., 2021).
Cytotoxic T cells are an integral part of the adaptive immune response. These cells kill infected cells, and tumor and helper T cells influence cytotoxic T cell activation (Stockinger et al., 2006; Moore et al., 2019).
Antigen-presenting cells present peptides through cross-presentation with MHC I. These cells include dendritic cells, monocytes, B cells, neutrophils, and endothelial cells (Heit et al., 2004). These mechanisms can be influenced by various interrelated factors, such as the binding of the complex MHC I to the T cell receptor (TCR), costimulatory signals for T cell binding, and the direct factors of IFN-1 and IL-12 (Sigal, 2016; Muntjewerff et al., 2020).
Cross-presentation can occur through cytosol and vacuole pathways (Cruz et al., 2017; Embgenbroich & Burgdorf, 2018). The cytosolic pathway begins when the peptide is carried from the endosome to the cytosol to be degraded by the proteasome and then transported to the endoplasmic reticulum by the transporter associated with Antigen Processing (TAP). The peptide in the endoplasmic reticulum is then bound to MHC I or thrown back into the endosome (Embgenbroich & Burgdorf, 2018; Blander, 2016). The vacuolar pathway is the main cross-presentation that begins the peptide processing at the endosomal or lysosomal site by the protease enzyme cathepsin S and then binds to MHC I at the same site (Embgenbroich & Burgdorf, 2018; Blander, 2016).
The cytotoxic T cell activation results showed significant differences in all observations. The expression levels decreased significantly on D14 and then increased significantly on D21. A significant decrease occurred on D28 and then increased significantly on day 56. Such conditions occur because of the immune response mediated by memory cells. Memory cytotoxic T cell differentiation is a complex series of events that begins with priming, expansion, and contraction (Lauvau & Soudja, 2015). The activation of these cells is a set of long-lived cell populations that are antigen-specific and can provide better protection. These cells can survive without changing their phenotypes (Samji & Khanna, 2017). Cytotoxic T cell differentiation can be influenced by various factors, including dose, antigen binding to the receptor, costimulatory signals, presenting cell type, T helper cell activation, cytokines, and chemokines (Fousteri et al., 2011).
A study conducted by Guzman et al. (2010) showed that cattle vaccinated with a commercial inactivated FMD vaccine can induce cytotoxic T cells, but depends on various factors such as the genetic nature of the animal and the cross-reactivity of the antibodies generated. FMD-inactivated vaccines can stimulate cytotoxic T-cells through cross-presentation (Guzman et al., 2008; Carr et al., 2013).
The helper and cytotoxic T cells in this study were similar to those reported by Cui et al. (2019) and Zhang et al. (2023). Cui et al. (2019) compared the helper T cells and cytotoxic T cells in mice before and after 14 days of booster and showed no significant difference in the antigen treatment group against vaccine formulations using ISA 206 adjuvant. These results are consistent with the research of Zhang et al. (2023), which characterized T cell subsets in Chinese Frisien cattle and showed a decrease in the expression of helper T cells and cytotoxic T cells on day 14 post-immunization. Increased expression of helper and cytotoxic T cells occurs on day 7 post-vaccination and decreases until day 14 post-vaccination (Zhang et al., 2023).
The main focus of FMD vaccine development is an increase in antibodies mediated by B cells (Lee et al., 2020a). Immunoglobulin G (IgG) was observed in most antibody parameters. IgG is a specific and neutralizing antibody that appears on days 4–7 post-vaccination and peaks at three weeks post-vaccination (Golde et al., 2005). This condition is important considering that the highest viremia phase occurs on days 2-3, with severe clinical symptoms on days 3–5 post-infection (Alexandersen et al., 2003; Lee et al., 2020b). The indicator of successful vaccination is the secretion of IgG specific to serum (Zhou et al., 2022).
The activation of helper T cells is closely related to B cell activation and antibodies (Lee et al., 2020b). According to Lee et al. (2022), B cell activation occurs through two mechanisms: T-lymphocyte cell-dependent and T-lymphocyte cell-independent. T-lymphocyte cell-independent activation mechanisms are known to be type I and type II. The T lymphocyte cell-dependent activation pathway involves MHC binding to TCR and binding of CD40L to CD40. Activation of the type I pathway directly activates B cells through PAMPs that are recognized by PRR. Activation of type II pathway antigens is directly recognized by B cell receptors, such as CD21, CD19, and CD81, or recognized through activated complement through B cell epitopes, such as C3d (Bonilla & Oettgen, 2010; Kwak et al., 2019).
The results of this study showed a significant increase until day D28 and a decrease until day D56. The variation in IgG-specific antibodies post-immunization can be influenced by the dose, quality, and adjuvant chosen, and the immune response caused by post-immunization is shorter and lower (Habiela et al., 2014).
The IgG-specific antibodies used in this study were similar to those reported by Cui et al. (2019) in mice treated with different FMD vaccine formulas. Treatment with the antigen formulation showed increased IgG-specific antibodies until day 42, with the highest titer occurring on the same day. Treatment with the addition of ISA 206 adjuvant showed that IgG secretion increased until day 21 post-booster, then decreased on day 28 post-booster, then increased until day 42 post-booster with the highest titer on day 21.
The results of this study indicate that the combination of MontanideTM ISA O/W with biological FMDV titers 107 TCID50 is the optimum for immune response in BALB/C mice (Mus musculus). The limitations of this study were not tested for the challenge and safety test for potency. Further research is needed to investigate the potency of guinea pigs in animal models high level (Guinea pig).
All research methodologies, procedures, and utilization of experimental animals were approved by the Animal Care and Use Committee (ACUC) at the Faculty of Veterinary Medicine, Airlangga University, Surabaya, Indonesia (certificate number 2). KEH.101.06.2023. The inclusion criteria of the ethics board that approved the research were Dr. Nusdianto Triakoso, M.Ag., and DVM. This article reports compliance with the ARRIVE (Animal Research: Reporting of in vivo Experiments) guidelines (Percie du Sert, et al., 2020).
This study was conducted from October to December 2023. The entire research process was conducted at the Animal Development & Research Facility, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia; Laboratory of Virology, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia; Research Center for Vaccine Technology and Development (RCVTD), Institute of Tropical Disease, Universitas Airlangga, Surabaya, Indonesia; and Laboratory of Animal Structure, Anatomy, and Development, Faculty of Mathematics and Natural Sciences, Universitas Brawijaya, Malang, Indonesia.
Figshare: Evaluation of Immune Response in Mice (Mus musculus) Post-Immunization with Foot-and-Mouth Disease Virus Master Seed Candidate Vaccine. Doi: https://doi.org/10.6084/m9.figshare.28327520
This project contains following underlying data:
The data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Reporting guidelines
ARRIVE checklist for ‘Evaluation of Immune Response in Mice (Mus musculus) Post-Immunization with Foot-and-Mouth Disease Virus Master Seed Candidate Vaccine,’ Doi: https://doi.org/10.6084/m9.figshare.28327520 (Krisdiyantoro et al., 2024).
The data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The authors are thankful to the Research Center for Vaccine Technology and Development, Institute of Tropical Disease, Airlangga University, Surabaya, Indonesia for conducting the experiments.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Foot and mouth disease vaccine development, FMD serology, FMD vaccine potency in various animal models, FMD vaccine regulation
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 06 May 25 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)