Keywords
Dietary monitoring technology, Wearable cameras, Non-communicable Diseases, Cardiovascular Disease
A major obstacle in understanding the relationship between diet and non-communicable diseases (NCDs) lies in the subjectivity and the bias in traditional dietary intake assessment methods. Food diaries, 24-hour food recall interviews, and food frequency questionnaires (FFQ) result in under- and over-reporting of nutrient and energy intake, compromising the accuracy of studies linking diet to NCDs. The emergence of image classification technology has facilitated a new approach to dietary intake assessment which addresses the subjective limitations associated with traditional self-reporting methods. Furthermore, a need to integrate multi-omics and advanced health analyses to comprehensively characterise NCD risk presents an opportunity to combine a range of technologies to better understand diet-induced NCD.
This observational study will adopt an enhanced surveillance method, whereby we will utilise wearable cameras and activity monitors to record dietary intake, physical activity, and sleep. Participants will wear these for 3 separate one-week periods at home (with a 2 and 3-week break in between each period). At the end of each monitoring period, participants will attend a study visit at the clinical research facility, where they will undergo body composition assessments, as well as cardiovascular disease (CVD) risk and autonomic nervous system health analysis using state-of-the-art technologies that measure Advanced Glycation End products (AGE) and accelerated photoplethysmography (APG). At the end of the first and last monitoring period, participants will also provide blood, urine, stool, and breath samples, and an in-depth interview will also be conducted during the final visit to assess participants’ perceptions towards the novel technologies. The study parameters will be integrated to advance our insights into how diet intricately influences the mechanisms underlying NCDs.
https://www.isrctn.com/ISRCTN11564218
Dietary monitoring technology, Wearable cameras, Non-communicable Diseases, Cardiovascular Disease
In 2023, the World Health Organization (WHO) revealed that non-communicable diseases (NCDs) accounted for 74% of global deaths, a notable increase from 61% in 2000 (World Health Organization, 2023). This number is expected to rise to 86% by 2048 (World Health Organization, 2023), and despite a reduction in NCD mortality rates since 2000, current trends suggest the sustainable development goal target (SDG3.4) of a one-third reduction by 2030 (NCD Countdown 2030 Collaborators, 2018) is unlikely to be met. This deceleration in reducing mortality rates and the growing prevalence of NCDs underscores the urgent need to better understand the risk factors driving NCD progression. Research into lifestyle risk factors such as diet, physical activity, and smoking, and their complex relationship with physiological complications such as hypertension, insulin resistance, dyslipidaemia, and inflammation (Kirk & Klein, 2009), is crucial for developing more effective prevention and intervention strategies.
Diets low in saturated fats (SFA) and simple sugars, and those high in dietary fibre such as plant-based diets, have been shown to reduce NCD risk, particularly cardiovascular disease (CVD) (Petersen et al., 2017). Similar benefits are observed with Mediterranean diets in lowering incidents of myocardial infarction and ischemic stroke (Delgado-Lista et al., 2022). However, the mechanistic influences of dietary components and their relative influences on NCD risk are less clear, and uncertainties in nutrition research persist, such as the complex relationship between SFA and CVD risk (Harrison, Couture & Lamarche, 2020; Maki, Dicklin & Kirkpatrick, 2021). Pathophysiological characteristics indicative of NCD, such as chronic low-grade inflammation, dyslipidaemia, and insulin resistance (Ference et al., 2018; Kosmas et al., 2023; Liang et al., 2022), and their relation to dietary intake, have allowed for the development of dietary indices to assess the inflammatory potential of dietary patterns such as the Dietary Inflammatory Index (DII) (Hariharan et al., 2022), and the Dietary Approaches to Stop Hypertension (DASH) index (AlEssa et al., 2017) which evaluates diet quality in terms of hypertension. However, these metrics were developed using self-reported dietary intake data which have inherent biases (Kipnis et al., 2002), not to mention, were developed to combat isolated systems.
Self-reporting methods rely heavily on individuals’ subjective judgment of food and portion sizes (Castro-Quezada et al., 2015). Consequently, under and over reporting of food intake presents a significant challenge in nutrition research. A systematic review found that 46% of studies observed an average underestimation of energy intake by 15%, consistent across 24-hour recalls, food frequency questionnaires, and weighed food records (Poslusna et al., 2009). Similar studies estimate misreporting is prevalent in 30-88% of individuals, whereby 25-29% of energy intake goes underreported (Rennie, Coward & Jebb, 2007; Poslusna et al., 2009). Misreporting of macronutrient and micronutrient intake also show similar results (Poslusna et al., 2009). Demographic characteristics like gender, age, weight, and Body Mass Index (BMI) (Castro-Quezada et al., 2015), as well as psychological and behavioural effects such as judgement for dietary choices (Maurer et al., 2006; Poslusna et al., 2009), are contributing factors to misreporting. Self-reporting also comes with a burden, which may influence these behavioural changes in eating. For instance, using weighed food records, a method that although is objective, may alter eating behaviour of the volunteers in a study when they required to weigh their food by choosing to eat less. Thus, the need for an objective as well as a passive approach to assessing dietary habits is warranted.
The shift towards more objective methods of dietary intake assessment is an ongoing field of research. Existing methods, such as the use of double-labelled water to measure energy intake (Westerterp, 2017), are commonly used as a reference method to quantify misreporting. However, as this method is limited to quantifying energy intake, a need for objective methods of measuring food and nutrient intake is in demand. The rising use of NMR spectroscopy for metabolomics and for objectively assessing dietary patterns (Garcia-Perez et al., 2017) presents a significant advancement in nutritional research, reducing reliance on self-reported dietary intake data. Additionally, innovative developments in image-based classification of complex meals and image-based portion-size estimation introduce promising tools in nutritional epidemiology (Lo et al., 2020, Jia et al., 2019). The technology involves training computers to identify categories, shapes, and features within images by employing deep-learning models. Advancements in image AI have notably enhanced the accuracy of image classification, making its application in dietary monitoring a promising development.
The study aims to utilise wearable cameras as a means of objective dietary assessment to address the limitations associated with conventional self-reporting methods. Currently, systems such as genomics, metabolomics, epigenomics, stool microbiology, and inflammation are often examined in isolation, leaving a gap in our understanding of their interactions and relative impacts on NCDs. Thus, by integrating multi-omics techniques and utilising non-invasive CVD health analysis technologies, the study significantly advances our insight into how diet intricately influences the mechanisms underlying NCDs.
The combined approach of integrating multiple methods of assessing dietary intake (wearable camera technology and online 24hr food recall), coupled with the collection and analysis of a wide range of biofluids (blood, urine, faeces, and breath), and the utilisation of non-invasive technologies such as bioimpedance analysis, photoplethysmography and Advanced Glycation End products assessment to measure body composition and CVD health, can help better understand the relationship between dietary intake and risk of non-communicable diseases.
Primary outcome measures
1. Dietary Assessment - The effectiveness of the wearable-camera technology and associated AI methods to determine the volunteers’ food and drink intake. Utility thereof as a reference method in comparison to an online 24-hour food recall.
2. NCD Risk - Determination of volunteers’ comprehensive non-communicable disease risk by measuring:
2.1. Metabolites found in the volunteers’ stool, fasted urine, fasted blood, breath samples and stool microbial composition.
2.2. Dietary habits (meal pattern + nutritional composition)
2.3. Physical activity and sleep pattern
2.4. Body composition in 5 sectors by Bioelectrical Impedance Analysis (BIA)
2.5. Cardiovascular risk prediction by measuring AGEs (Advanced Glycated End products) by autofluorescence in skin and artery hardening (Prasad et al., 2017) and heart rate variability and autonomic nervous system function by Accelerated Plethysmography (APG) (Elgendi et al., 2019).
Secondary outcome measures
1. Assess the volunteers’ acceptance of the developed technologies (wearable-camera, online 24hr food recalls, physical activity and sleep monitor, samples donation and non-invasive body measurements) at the end of the clinical trial using questionnaires and in-depth interview method.
2. Collect training data from the wearable-cameras for the training of AI methods.
3. Postprandial glucose, insulin, free fatty acids, and triglycerides will be measured at different time points across 6 hours after volunteers drink a mixed meal (a fasted blood sample will also taken). This will only be conducted at the Imperial College London site.
This is a multi-centre observational clinical trial that will have one health screening visit and three study visits that will take place in London, UK; Thessaloniki, Greece; Cork, Ireland, Bilbao and Valencia, Spain ( Figure 1). Eligible volunteers will need to wear a small camera attached to glasses frames ( Figure 2) and a sleep and activity wristband for three one-week periods at home with a two and three-week break in between them. The study will last for approximately eight weeks. Participants will also need to complete an online 24-hour food recall daily. At the end of the first and third study periods, the participants will need to attend study visits during which they will provide urine, stool, breath and blood samples and they will have their body composition and risk for cardiovascular disease assessed. At the end of the second period, the volunteers will attend a short study visit during which they will have their body composition and risk for cardiovascular disease assessed. When all study visits have been completed, the volunteers will be asked to answer some questions about their experience of participating in this study.
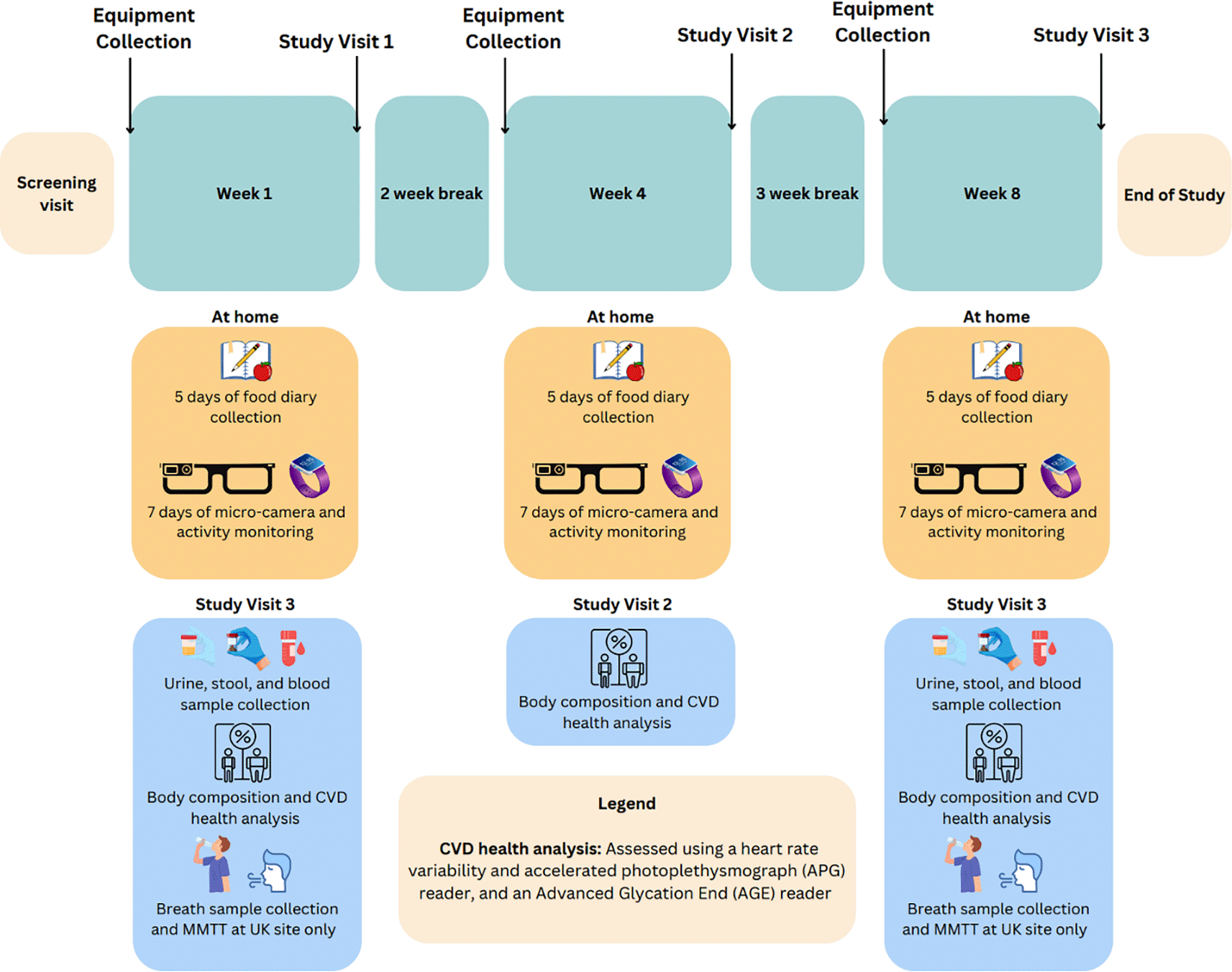
Abbreviations MMTT, Mixed Meal Tolerance Test, CVD, Cardiovascular Disease Risk. Figure created using Biorender.
The study will be recruiting adults at risk of developing NCD. Participants eligible for this study must be aged between 18 and 65 years old (inclusive) and have a Body Mass Index (BMI) greater than or equal to 25 kg/m2. Additionally, they must meet at least one of the following five criteria:
• Triglycerides levels of ≥ 150 mg/dL (1.7 mmol/L)
• HDL cholesterol levels less than 40 mg/dL (1.03 mmol/L) for males or less than 50 mg/dL (1.29 mmol/L) for females
• blood pressure with a systolic reading of ≥ 130 mmHg or a diastolic reading of ≥ 85 mmHg
• fasting plasma glucose levels of ≥ 90 mg/dL (5.0 mmol/L)
• being a current smoker
Individuals with controlled hypertension or dyslipidaemia with medication will be included in the trial. Moreover, potential participants must demonstrate a willingness and ability to sign the informed consent form, along with an understanding and compliance with the study participation requirements. Conversely, individuals will be excluded from the study if they have type 2 diabetes, chronic gastrointestinal conditions such as Crohn’s disease, irritable bowel syndrome, or ulcerative colitis, acute infectious diseases, cancer, cardiovascular diseases, autoimmune conditions, have undergone antibiotic treatment in the 12 weeks prior to enrolment, are pregnant or currently breastfeeding, are participating in another clinical trial, have participated in another clinical trial within the past 12 weeks, or are undergoing any medical intervention during the study period.
The exploratory nature of the study in assessing novel dietary monitoring technology meant power calculations were not conducted. The total sample size of this multi-centre clinical trial is 200 volunteers; 50 volunteers will be recruited at Imperial College London (United Kingdom site), 50 volunteers will be recruited at Aristotle University, Thessaloniki (Greece site), 50 volunteers will be recruited at Atlantia Clinical Trials, Cork (Ireland site), 25 volunteers will be recruited at University of Valencia-CIBEROBN (Spain site) and 25 volunteers will be recruited at CIC bioGUNE, Precision and Metabolism Lab, Derio, Bizkaia (Spain site).
Volunteers will be recruited through posters in public places, healthy volunteer databases, GP practices and social media. Interested individuals will be provided with a Participant Information Sheet (PIS) to read and they will complete an online pre-screening questionnaire to initially assess their eligibility. Following this, the volunteers will attend a health screening visit at the respective clinical research facility of each recruitment site. During this visit, the volunteers will be walked through the study protocol and shown how to use the study equipment and the online 24hr food recall tool. Once they are satisfied with the answers they have received and are still willing to participate in the study, the volunteers will sign an informed consent form. Following this, the volunteers will have their body weight and height measured, their blood pressure checked, have an ECG (Electrocardiogram) done and provide a blood sample to check their eligibility for the study. The blood sample will be used to measure HbA1c, fasting glucose, lipids, liver and renal function, full blood count, and cholesterol. In addition, all women of childbearing age will have a pregnancy test. A member of the research team will also ask the volunteer questions about general health and ask them to fill in a questionnaire on their demographics. The volunteers who pass the eligibility criteria will be contacted by the research team to schedule the day of the first study visit and the collection of the camera, wristband activity monitor and urine and stool collection kits.
Seven days before study visits 1, 2 and 3, eligible volunteers will collect the wearable-cameras and activity monitors which they will need to wear daily for seven days to monitor their dietary habits, physical activity and sleeping patterns, respectively. Five days before study visits 1 and 3, the volunteers will complete daily for five days their dietary intake on Intake24.
Study Visits 1 & 3
The evening before study visits 1 and 3 the volunteers will need to be fasted from 9 pm, however they will still be able to drink water. Upon arrival at the clinical research facility at 9 am, the volunteers will return the wearable-cameras and activity monitors. Volunteers will need to provide a fasted urine sample in the urine kits offered by the study team (more details on urine sample collection can be found below) and a stool sample in the kit provided by the research team (more details on stool sample collection can be found below). Alternatively, the stool sample can be provided during the study visit. A breath sample (more details on breath sample collection can be found below), and a fasted blood sample will be collected. Women of childbearing age will have a pregnancy test. Only during study visit 1, the volunteers will fill in a questionnaire that contains questions related to health, demographics, physical activity, and dietary habits. Next, volunteers will have their body composition measured using a Body Composition Analyser (InBody970, InBody Co., Ltd, South Korea), and the risk for cardiovascular disease and the health of their autonomic nervous system assessed using a heart rate variability and accelerated photoplethysmography analyser, called SA3000P (MEDICORE Co., Ltd, South Korea), as well as an Advanced Glycation End (AGE) reader (DiagnOptics Technologies B.V., The Netherlands). Following these measurements, the volunteers will be able to go home.
Exclusively at the Imperial College Research facility (United Kingdom site), participants will also undergo a mixed meal tolerance test (MMTT). A study doctor will insert a cannula in the arm of the volunteer which will be used for blood drawing as part of the MMTT and will stay in place for six hours. Following this, a standardised mixed meal drink (2 x ENSURE® PLUS VANILLA, ENSURE, United Kingdom) will be given to the volunteers to consume. Postprandial blood samples will be taken at 15, 30, 60, 90, 120, 180-, 240-, 300-, and 360 minutes post-drink to examine changes in plasma glucose, serum insulin, free fatty acids, and triglycerides. At the end of the study visit, the cannula will be removed, the volunteers will be offered a meal of their choice and then they will be able to go home.
Study Visit 2
The volunteers will arrive fasted on study visit 2 during which they will return the wearable-cameras and activity monitors. This visit will only include body composition (InBody970) and CVD health analysis (AGE reader and SA300P). No human samples will be collected.
Following the last study visit, volunteers will be asked to complete some short questionnaires on how they felt participating in the study, specifically how they found the wearable-camera, the wristband activity, the online 24 hr food recalls, and the body composition and CVD health analysers. In addition, ten volunteers from each study site will be randomly selected for a one-hour in-depth interview and to complete a questionnaire about their experiences throughout the study.
Stool samples. Stool samples will be collected at all sites on study visits 1 and 3. The volunteers will collect the stool samples themselves either at their homes or during the study visits using the kits provided by the study teams. The stool samples will be collected in two separate tubes; one will be kept frozen at -80 degrees for downstream metabolomics analysis while the second tube which contains a stabilizing buffer will be kept at room temperature for downstream metagenomic sequencing.
Blood samples. Blood samples will be collected at all sites on study visits 1 and 3. All sites will collect fasted blood samples from the volunteers with the aim of conducting downstream metabolomics analysis, blood biochemistry analysis, genetic and epigenetic analysis, as well as blood lipids (dry blood spot analysis), saccharides, and amino acids analysis. All blood samples will be kept frozen at -80 degrees until downstream analysis except the dry blood spot samples which will be kept at room temperature.
Urine samples. Fasted urine samples will be collected at all sites on study visits 1 and 3. The volunteers will collect the urine samples at their homes in the kits provided by the study teams on the morning of study visits 1 and 3 and keep them at room temperature until their arrival at the clinical research facility. The samples will be aliquoted in separate microcentrifuge tubes for downstream analyses and frozen immediately at -80 degrees. Analyses will include metabolomics, dietary profiling and saccharides and amino acids quantification.
Breath samples (at UK site only). Breath samples will be collected only at the UK site, Imperial College London. While fasted, the volunteers will provide two breath samples in kits provided by the study team which will be used to quantify their cancer risk (Markar et al., 2018).
Postprandial responses (at UK site only). The Imperial College London site will conduct a MMTT over a period of six hours whereby the volunteers will be offered a standardised breakfast drink (2 x ENSURE® PLUS VANILLA, ENSURE, United Kingdom) to examine changes in fasted and postprandial glucose, insulin, triglycerides, and free fatty acids. Blood samples will be taken blood samples at 0, 15, 30, 60, 90, 120, 180-, 240-, 300-, and 360-minutes post-drink. All blood samples will be kept frozen at -80 degrees until downstream analysis.
Wearable-camera . The volunteers will wear a wearable-camera attached to their glasses or, if they do not wear glasses, they will be given lens-free glasses frames to attach the camera onto. They will wear the wearable-camera during waking hours, except when using the toilets, having a shower, changing clothes or sleeping. Full instructions on how to wear and activate the wearable-camera will be given as well as instructions on how to plug it into the chargers at night. The size of the camera is that of a USB stick and therefore will be lightweight. The volunteers will need to return it on the day they come for the study visits, and they will be able to pick up the wearable-camera the day before they are meant to start wearing it again. The camera technology will capture everything in its vision, which includes food, drink, and potentially other people. Importantly, the camera will not be able to record any sounds. To ensure the anonymity of individuals, all footage captured by wearable cameras will undergo preprocessing prior to analysis. Initially, AI will be employed to generate tags for each image (Zhang et al., 2023). Only images that contain food-related tags (e.g., food, dining table, fork, bowl, etc.) will be retained to prevent the exposure of scenes unrelated to eating episodes. Furthermore, for the retained images, an additional layer of protection will be implemented by blurring the faces of the participants and other individuals residing with them, as well as any visible phone and computer screens, to prevent the inadvertent disclosure of identities and personal information (Qiu et al., 2023). Only after this preprocessing will the images be subjected to further analysis. Nutritional content of food and drink items identified from all four countries will be determined using a custom software (Lo et al., 2024) that utilises food composition databases (McCance and Widdowson’s 7th Edition Composition of Foods UK Nutritional Dataset (UKN), to generate a detailed nutritional report).
Physical Activity and Sleep Wristband (GENEActiv, Activinsights, United Kingdom)
The volunteers will wear continuously (day and night) a physical activity and sleep monitor wristband on their non-dominant hand for three seven-day periods with a break of two or three weeks in between each period. Full instructions on how to wear and charge the wristband will be given to the participants. The wristbands will be returned by the volunteers on the day they come for the study visits, and they will be able to pick them up the day before they are meant to start wearing them again.
Online 24hr Food Recall (Intake24, United Kingdom)
Intake24 is an online 24-hour multi-pass dietary recall platform. The tool guides users through the process that starts by listing what they ate/drank the previous day, then asking how much they had using portion size photos as well as recording extra details (e.g., if you had a cup of tea, did you add milk and, if so, what type?). This takes around 15 minutes to complete. The volunteers will be sent reminders by email to record what they have been eating and drinking during the five days prior to coming for a study visit. The Greece and Spain sites will use paper versions of food diaries which will be translated into English before being inputted into intake24.
Once the volunteers are enrolled in the study, they will be assigned a unique subject ID which will ensure the anonymity of the participant. This subject ID will be used throughout the study visits and data analysis. All personal data of the volunteers will be kept in locked filing cabinets in the respective research groups of each site of this multi-centre clinical trial. Only authorised research team members will have access to these documents and the final datasets. The Principal Investigator and the study team will have access to the final trial dataset. The Principal Investigator will act as custodian of the data.
Data and all appropriate documentation will be stored for a minimum of 10 years after the completion of the study, including the follow-up period. A formal data analysis plan will be drawn up following the completion of the study. A statistical significance of P<0.05 will be considered for all statistical analyses, where the evaluation of significance is applicable. We will aim to use descriptive statistics to list participant demographics, baseline features, and primary outcomes, focusing on means, standard deviations, and interquartile ranges. We will compare wearable-camera technology and online 24-hour food recalls via paired t-tests or Wilcoxon signed-rank tests depending on normality.
We will test a variety of methods for causal learning to investigate the intricate relationships between non-communicable disease risks and a variety of predictors including metabolites in stool, urine, blood, and breath samples, dietary habits, physical activity, sleep patterns, and stool microbial composition. To deal with the unknown dimension of the hidden state, we envision the use of operator-valued methods (Quan Zhou, 2024; Wei Niu et al., 2024), which have been implemented in Matlab, Python, and Julia. Data processing of the images collected using the camera technology will be processed using Python.
The results of this study will be published in relevant high-impact journals and at nutrition-related conferences and meetings. The volunteers will be sent a copy of the publication if they wish to receive it.
The study is the first of its kind to utilise dietary monitoring technology in free-living participants across multiple European countries to research NCD risk. The use of wearable cameras for passive dietary assessment is an emerging approach to objectively determine food and nutrient intake; their functionality, performance, and acceptability were previously validated to deploy in low- and middle-income countries to investigate the contribution of household food on the malnutrition burden in Ghana and Uganda (Jobarteh et al., 2020). The testing involved evaluating acceptability and accuracy whereby eyeglass cameras demonstrated strong usability and accuracy, compared to ear-fitted, hat-worn, or button-fitted devices (Jobarteh et al., 2020). The concept of wearable cameras valuably considers varying eating habits not addressed by self-reporting methods. For example, the device can be used to quantify food intake during shared eating, a common eating behaviour across various cultures and backgrounds. The idea of passive monitoring also limits burden on participants compared to self-reporting, and as previously mentioned, could minimise behavioural biases. Thus, the combination of this technology with physical activity monitoring, the utilisation of state-of-the-art CVD health assessment technologies, and the collection of a range of bifluids offers a holistic and thorough approach to investigating lifestyle-induced NCD risk. Lastly, the study will collect crucial qualitative data through interviews to evaluate the usability of wearable-camera technology to explore usability and acceptability in this demographic. This will help assess the technology’s practicality in providing insights into whether such devices can indeed serve as feasible means of objective dietary intake assessment.
Camera based dietary monitoring: As with any lifestyle monitoring technique a limitation is behaviour change induced by the monitoring itself. It is well known that self-monitoring techniques induce behaviour change. However, our data demonstrated the technique improves the accuracy of dietary monitoring. In addition, the CoDiet study will assess long term markers of dietary intake through red blood cell lipids analysis, and this assessment will be triangulated with the estimation from the camera assessment.
In the UK (the Imperial College London site), this study has received ethical approval by London Surrey Research Ethics Committee (REC) and Health Research Authority (HRA), REC reference number: 23/PR/1109. In Ireland, this study has received ethical approval by the Cork Research Ethics Committee, reference number: ECM 3 (III) 24/10/2023. In Greece, the study has received ethical approval by Committee for Ethics in Research, Aristotle University Thessaloniki (Aristotle University, Thessaloniki), reference number: 20023/2023. In Spain, the Valencia study site received ethical approval by Ethics Committee on Human Research at the University of Valencia, reference number 2023-MED-2857718 and the Derio study site received ethical approval by Ethics Committee for Research with Medicinal Products in the Basque Country (Department of Health of the Basque Government, reference number: PI2023134.
This study will be conducted in accordance with the recommendations for physicians involved in research on human subjects adopted by the 18th World Medical Assembly, Helsinki 1964, and later revisions. Any amendments (both minor and substantial) to the study protocol, participant information sheet, informed written consent form and questionnaires were sent to the relevant ethics committees of each site of this multi-centre clinical trial to be reviewed. All documentation were approved by the relevant ethics committees. All these amendments need to be consistent across all the sites. Once these amendments have been approved, they will be explained to the volunteers and be implemented in the study with the volunteers’ consent.
When consenting to participate in the study, the volunteers will have the option to agree to have the samples and data that are collected in this study to be used in future ethically approved studies. Volunteers were compensated for their time and participation in the study.
AD, MH and CY wrote the article with feedback and contribution from all the other authors. The same authors together with BL were responsible for running and collection of study samples at the Imperial College site. GF, SAM and IT are grant holders and project leaders that developed the protocol with contribution from all authors. COA and RFC are responsible for data collection and processing at Valencia. ARS, PWL and GM developed the camera technology, processing of the data collected by the camera and nutritional analysis using the camera data. GT and DR are responsible for data collection and processing at Greece. The same authors were responsible for untargeted lipidomics, acyl carnitines, saccharides, tyrosine, and tryptophan pathway analysis. IGP and NE are responsible for the metabolomic analysis of urine, stool and plasma samples. NE was also responsible for data collection at Bilbao. JM and JMP are responsible to modelling and data anlysis. NZ was responsible of the provision and running of Microcaya equipment. OO was responsible for the trial in Ireland and running of microbiota analysis from stool samples. SAM and IT were also responsible for dry blood spot and red blood cell lipidomic analysis.
Imperial College London is the main research Sponsor for this study. For further information regarding the sponsorship conditions, please contact the Head of Regulatory Compliance at:
Research Governance and Integrity Team
Imperial College London and Imperial College Healthcare NHS Trust
Room 215, Level 2, Medical School Building
Norfolk Place
London, W2 1PG
Tel: 0207 594 1862
Imperial College - Research Governance and Integrity Team (RGIT) Website
No data are associated with this article.
Mendeley Data: Using wearable camera dietary monitoring technology to explore diet-related non-communicable disease in volunteers at risk of cardiovascular disease - the CoDiet study protocol, 10.17632/yvhphdz48x.2 (Dagbasi et al., 2024).
This project contains the following underlying data:
1. CoDiet Consortium.docx
2. CODIET_General questionnaire v1.0 11012024.docx
3. CoDiet_Protocol_v1.1 20022024.docx
4. Eligibility questionnaire_CoDiet - Copy.docx
5. Equipment explanation Sheet_Volunteers v1.0 28072023.docx
6. ICF-for-Adults-with-Capacity_v1.4 24022024 (1).docx
7. PIS_CoDiet v1.3 20022024 (4).docx
8. Pre-screening questionnaire CoDiet v1.0 28072023 (6).docx
9. Screening Visit v1.0 23012024.docx
10. SPIRIT-Checklist-CoDiet Study.docx
11. Study Booklet CoDiet v1.0 03042024.docx
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the rationale for, and objectives of, the study clearly described?
No
Is the study design appropriate for the research question?
Partly
Are sufficient details of the methods provided to allow replication by others?
No
Are the datasets clearly presented in a useable and accessible format?
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: nutritional epidemiology, global nutrition, obesity and diabetes prevention and treatment
Is the rationale for, and objectives of, the study clearly described?
No
Is the study design appropriate for the research question?
Yes
Are sufficient details of the methods provided to allow replication by others?
No
Are the datasets clearly presented in a useable and accessible format?
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Diet, physical activity, sleep, screen time measurement and interventions.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 08 Jan 25 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)