Keywords
Automated UV-C Machine, Disinfection, COVID-19, IR remote control, User center design.
This article is included in the Pathogens gateway.
This article is included in the Coronavirus (COVID-19) collection.
The global outbreak of the COVID-19 pandemic in 2019 highlighted the urgent need for innovative technologies for sterilization and disinfection of various healthcare utilities. While steam sterilization is widely used in both developing and industrialized countries, it has limitations in disinfecting several critical healthcare items such as hospital rooms, beds, N-95 masks, ambulance beds, medical clothing and devices. During the pandemic, Ethiopia and other developing countries faced significant challenges in sterilization and disinfection system for these healthcare utilities in COVID-19 treatment centers.
Methodology
The development of the UV-C dry surface disinfection and sterilization device followed a structured engineering design approach, with a focus on functionality, efficiency, and safety. The methodology included key stages such as problem identification and needs analysis, concept design and circuit development, material selection with specification, prototype fabrication, product testing and validation.
The results of the sterilization and disinfection efficiency test demonstrated that the UV-C device achieved over 90% effectiveness, confirming its viability as an efficient solution for sterilization in healthcare settings and other industries.
The development of these automated UV-C sterilizers addresses a critical gap in disinfection technology, particularly in resource-limited settings, and enhances the capability to manage infectious diseases like COVID-19 and related pandemics.
Automated UV-C Machine, Disinfection, COVID-19, IR remote control, User center design.
Efficient and cost-effective disinfection technologies are essential to meet the diverse sterilization and infection prevention needs of healthcare institutions. Among these, sterilization techniques are widely recognized for their ability to eliminate all microorganisms from surfaces and fluids, thereby preventing disease transmission. The selection of appropriate sterilization technologies depends on the nature and complexity of the medical equipment or environment being treated. In recent years, ultraviolet (UV-C) technology has gained significant attention as an effective method for disinfection.1,2 Ultraviolet germicidal irradiation (UVGI), a specific application of UV-C light, utilizes short-wavelength ultraviolet radiation to inactivate microorganisms. It achieves this by damaging their nucleic acids and disrupting DNA strands, rendering the organisms incapable of performing essential cellular functions.3,4 UV-C-based dry surface disinfection is increasingly favored over traditional methods due to its effectiveness and versatility. It plays a crucial role in reducing the spread of infectious diseases and is widely used in healthcare settings, including hospital rooms, medical equipment, patient beds, COVID-19 treatment centers, ICU rooms, tuberculosis (TB) treatment areas, ambulances, and public transportation systems.5–7 Studies have shown that UV radiation is a powerful disinfectant for air, water, and dry surfaces, and when applied correctly, it can significantly reduce the risk of infection, including that caused by the COVID-19 virus. This is particularly important given that the virus can survive on plastic and steel surfaces for up to three days.8–10 Standard cleaning and disinfection methods may leave behind residual contamination, highlighting the need for a more comprehensive and effective solution such as UV-C treatment for enhanced infection control.
Ultraviolet (UV) light is a form of electromagnetic radiation with wavelengths shorter than visible light but longer than X-rays. Based on wavelength and other characteristics, UV light is classified into three main types: UV-A, UV-B, and UV-C. UV-A and a small portion of UV-B can penetrate the Earth’s atmosphere, while UV-C radiation is completely absorbed by the ozone layer and does not naturally reach the Earth’s surface. Among these, UV-C light particularly in the wavelength range of 200 to 300 nanometers is referred to as “germicidal UV radiation” due to its strong absorption by nucleic acids.11–13 This absorption disrupts the DNA and RNA of microorganisms, rendering them inactive. Germicidal UV-C light, especially at a wavelength of 253.7 nm, has been shown to be highly effective for sterilization and decontamination purposes.14,15 Because microorganisms have limited natural defenses against UV-C radiation, they cannot survive prolonged exposure, making UV-C a powerful tool in disinfection and sterilization applications.
The research team developed a UV-C-based surface sterilization and disinfection device suitable for a wide range of applications in healthcare facilities. This innovation was part of a broader effort to provide effective and affordable solutions to reduce the spread of COVID-19 and other hospital-acquired infections. The device, named the “Simbona UV Apparatus,” was developed through a collaboration between Simbona Africa Healthcare’s R&D team and researchers from Jimma University. To address the diverse disinfection needs of healthcare settings, the UV-C sterilizer was designed in three different models; i.e. large, small, and quadrilateral each tailored to specific use cases based on the size and nature of the surfaces or equipment requiring sterilization.
UV-C disinfection technology operates on the fundamental principle that ultraviolet radiation damages the DNA or RNA of microorganisms. This high-energy light induces the formation of pyrimidine dimers and other disruptions in nucleic acid structures, effectively inhibiting the ability of bacteria, fungi, and even some plant and animal cells to reproduce. Similarly, UV-C radiation irreversibly damages the RNA of viruses, leading to their inactivation.16,17 A variety of disinfection methods are used in healthcare and industrial settings, including chemical, steam, heat-based, and UV-C disinfection. Chemical disinfectants work by destroying the cellular structures of microorganisms, thereby disrupting their metabolism, synthesis, and growth processes.18 In contrast, UV-C disinfection triggers a series of photochemical reactions that alter or destroy the DNA or RNA in the pathogens’ cell walls. The resulting disintegration of nucleic acids prevents cell division and reproduction, rendering the microorganisms inactive.19,20 Special UV-C lamps serve as the primary light sources for this process, with mercury arc lamps being the most commonly used due to their efficiency. Approximately 85% of the energy emitted by mercury arc lamps is concentrated at a wavelength of 253.7 nm within the optimal germicidal range of 250–270 nm.21,22 When electrical energy excites mercury vapor inside the lamp, it emits UV-C radiation. This radiation penetrates the target organisms, destroying their genetic material and causing cell death.
According to the International Ultraviolet Association (IUVA), UV-C disinfection technologies have proven effective in reducing the transmission of viruses such as SARS-CoV-2, the virus responsible for COVID-19. Their findings are based on current disinfection data and empirical evidence.23–26 Figure 1, illustrates the mechanism and potential of UV-C radiation to inactivate bacteria, fungi, and the SARS-CoV-2 virus, including its various mutations, as discussed in the blog “Power of UVGI (UV-C) Disinfection to Inactivate Microbes.”
DNA and RNA are nucleic acids that serve as essential building blocks of life.27,28 They are composed of subunits called nucleotides, each consisting of three components: a phosphate group, a five-carbon sugar, and an organic base. The sugar in DNA is deoxyribose, while in RNA it is ribose. The organic bases in DNA include adenine (A), guanine (G), cytosine (C), and thymine (T). In RNA, thymine is replaced by uracil (U). Adenine and guanine are purines, characterized by a double-ring structure, while cytosine, thymine, and uracil are pyrimidines with a single-ring structure. Both purines and pyrimidines are composed of nitrogen and carbon atoms.29–31 In biological systems, the genetic material of viruses can consist of either DNA or RNA and may exist in single- or double-stranded forms. In UV-C disinfection, these nucleic acids are the primary targets. UV-C radiation inactivates microorganisms by inducing structural damage to their DNA or RNA, thereby disrupting their biological functions and ability to replicate. DNA and RNA absorb UV-C light most effectively at wavelengths between 200 and 300 nanometers. This absorption triggers photochemical reactions that cause biological damage, such as the formation of pyrimidine dimers, which inhibit normal cellular processes and ultimately lead to cell death. When UV light irradiates a cell, DNA functions as a chromophore, absorbing the UV photons and forming mutagenic lesions that result in irreversible damage to the genetic material. This process leads to the inactivation or destruction of individual cells and entire microbial populations.32–35
The development of the Simbona UV-C device for dry surface disinfection followed a structured engineering design approach, with a strong emphasis on functionality, efficiency, and safety. The methodology involved several key stages, including need assessment identification and requirements analysis, concept design, circuit system development, material selection, prototype assembly, product testing, and final product refinement to ensure regulatory and performance conformity. Each stage incorporated a rigorous design process, including detailed material specifications and performance considerations to optimize the final product.
Identification of need and product requirement analysis: Due to the lack of dry surface sterilizers for various applications particularly in healthcare settings, the research team identified a clear need for such a product. This need became especially apparent during and after the COVID-19 pandemic, highlighting the importance of an effective, safe, automated, and compatible sterilization solution for surfaces and selected medical utilities. At this stage, the product development team outlined several key design requirements. These included the use of UV-C lamps, a remotely controlled automated system, a stable power regulation unit, and a portable, compact, and ergonomic design. The concept design and circuit development phase involved the creation of a preliminary electronic circuit. This circuit incorporated essential components such as relays, power regulators, automatic switches, a remote control module, and a light control fuse. An AutoCAD layout of the Simbona UV-C sterilization machine was developed, emphasizing portability and feasibility across different product models, Figure 2. In this manuscript, only the layout of the smaller model is presented. Ergonomically, the Simbona UV-C device is a floor-standing, easily movable unit designed for optimal usability. A user-centered design approach was adopted throughout the development process to ensure straightforward operation and intuitive control. Overall, the product development methodology followed a structured path from initial concept to the creation of a functional prototype, with the goal of eventual commercialization.
To design the complete Simbona UV-C disinfection device, a variety of materials and electronic components were utilized. Key materials included 1.8 mm high-grade metal sheets for creating a compact and enclosed casing, along with essential electronic components such as 254 nm UV-C lamps, a timer, power switch, IR remote control module, electromagnetic relays, Bluetooth module, and power regulators. 254nm UV-C lamps were selected as the primary light source for their ability to emit germicidal electromagnetic energy targeting pathogenic organisms. A six-digit timer was integrated into the circuit to track the lamp’s operational hours and control the duration of light exposure. These UV-C lamps typically have an average lifespan of approximately 8,000 hours. To ensure user safety and prevent direct exposure to harmful UV-C rays, an infrared (IR) remote control was incorporated, allowing users to switch the device on and off remotely. This feature eliminates the need for manual handling of the power switch, representing one of the machine’s intelligent safety enhancements. 12V DC electromagnetic relay module was placed between the UV-C lamps and the main power supply to manage the electrical load effectively. Additional smart features such as a Bluetooth module and a lamp lifespan counter circuit were also included to enhance the device’s functionality and monitoring capabilities. The complete circuit layout and component connectivity architecture are illustrated in Figure 3. The outer casing of the device was fabricated using 1.8 mm high-grade metal sheets. Three different models of the UV-C device quadrilateral design, and both small and large vertical stand versions have been developed and successfully commercialized. The structural design and physical appearance of these three models are shown in Figure 4.
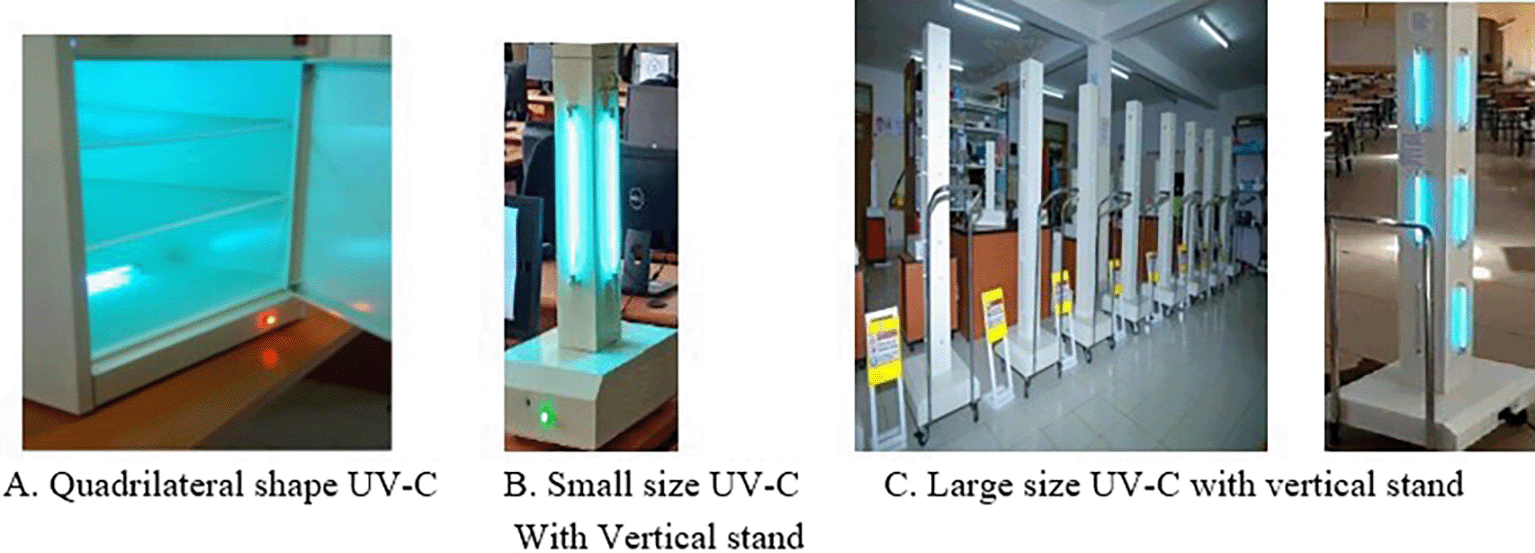
A functional prototype was developed using the selected components. The UV-C lamps, relay modules, and control circuitry were enclosed within a well-protected and sealed casing to ensure operational safety. All circuit connections were properly insulated to prevent electrical hazards. Three models of the functional prototype are shown in Figure 4 (A, B, and C) separately. Those different models are designed to provide specific application based on their ergonomic structure and no of UV-C lamps integrated on them. As clearly illustrated, the UV-C lamp tubes are strategically positioned on all sides of the machine to ensure comprehensive and efficient disinfection of the targeted surface area. This arrangement was specifically designed to enhance coverage and maximize the contact area for effective sterilization. However, some design limitations were noted. For instance, IR remote controllers are not integrated into all models for managing the automatic switching mechanism. In particular, the quadrilateral-shaped device does not include remote control functionality. This omission is intentional, as the UV-C light in this model is fully enclosed within the inner surfaces of the machine, significantly minimizing the risk of exposure to human eyes and reducing the necessity for remote operation.
The Simbona UV-C disinfection device was tested under controlled laboratory conditions to evaluate its effectiveness in microbial reduction. Bacterial cultures were prepared by microbiologists, exposed to the UV-C machine, and analyzed to determine the level of microbial reduction over varying exposure times. This phase also included validation of the device’s operational efficiency and safety compliance. Key features such as the protective enclosure and the automatic shutdown mechanism were assessed to ensure adherence to UV safety guidelines. The disinfection capacity of the Simbona UV-C device was evaluated at the Microbiology Laboratory of Jimma University Medical Center by a team of microbiologists. All test procedures and results are summarized in Table 1. For performance and validation testing, the larger model of the UV-C device equipped with 12 UV-C lamps arranged in a 3x4 series-parallel configuration was used. This model is specifically designed for disinfecting hospital rooms to prevent healthcare-associated infections. The average exposure duration was set between 15 and 30 minutes, with a minimum effective sterilization area of approximately 6 square meters. In our validation experiment, we selected an exposure time of 20 minutes and tested the disinfection effectiveness at distances of 3 meters, 2 meters, and 1 meter. Based on this setup, four different bacterial samples were prepared for testing and analysis. Trained microbiology and parasitology professionals conducted the validation test, following the experimental protocol outlined in the table below.
| Experiment | Samples | Total Colonies count before UV-C exposure | Exposure time | UV-C distance from the sample and (surface area covered by UV light) | Colonies count after UV-C exposure on the sample | Effectiveness |
|---|---|---|---|---|---|---|
| Sample A | 80 colonies | 20 minutes | 3 meters (9m2) | 21 | 73.75% | |
| 2 meters (4m2) | 11 | 86.25% | ||||
| 1 meter (1m2) | 01 | 98.75% | ||||
| Sample B | 87 colonies | 20 minutes | 3 meters (9m2) | 17 | 87.50% | |
| 2 meters (4m2) | 09 | 89.65% | ||||
| 1 meter (1m2) | 03 | 98.85% | ||||
| Sample C | 90 colonies | 20 minutes | 3 meters (9m2) | 22 | 75.56% | |
| 2 meters (9m2) | 13 | 85.56% | ||||
| 1 meter (4m2) | 02 | 97.77% | ||||
| Sample D | 96 colonies | 20 minutes | 3 meter (1m2) | 22 | 77.08% | |
| 2 meter | 08 | 91.66% | ||||
| 1 meter | 03 | 96.87% | ||||
| Result | The number of colonies counted & compared to each other (before and after UV exposure) | |||||
| Final Result | The Simbona UV-C machine demonstrates a disinfection and surface sterilization efficiency exceeding 96% within a 1-meter radius after 20 minutes of exposure. Across all tested distances (from 1 to 3 meters), the device achieves an average effectiveness of 88.18%. As clearly demonstrated in the results, the device performs more effectively at shorter distances, with efficiency gradually decreasing as the distance increases. Overall, the Simbona UV-C machine offers a highly promising solution for dry surface sterilization and infection control in various hospital settings. As illustrated in Figure 5, complete elimination of bacterial colonies from sample dish was observed following exposure to the UV-C machine, in accordance with the experimental protocol. Currently, all three models of the device are in active commercial use, serving a variety of disinfection needs in healthcare environments ( Figure 6). | |||||
The effective use of technology for sterilization and disinfection is a critical pillar in delivering infection-free clinical services. Many healthcare tools, devices, and utilities cannot be effectively sterilized using conventional methods such as steam autoclaves or chemical disinfectants. In this context, the application of UV-C radiation as a disinfectant technology has shown significant and promising results, particularly for dry surface sterilization of equipment that cannot withstand high-temperature or chemical-based sterilization. To support sustainable infection prevention practices, it is essential to develop innovative technologies that address these limitations. In response to this analysis, the research team at Jimma University has developed a multi-functional, user-centered UV-C-based dry surface sterilizer and disinfection device. Key features of the device include uniformly arranged UV-C lamps for consistent light distribution, an infrared (IR) remote control, a six-digit timer, a lamp age counter, and an ergonomic design. The device is available in three models with each offering large surface area coverage and enhanced usability. Its compact and portable design makes it ideal for large-scale application in diverse environments.
The Simbona UV-C dry surface sterilizer offers a practical, efficient, and locally developed solution to address critical disinfection challenges in healthcare environments, especially in resource-limited settings. With demonstrated effectiveness exceeding 90%, the device strengthens infection prevention efforts and supports pandemic preparedness by providing safe and reliable dry surface sterilization for items that cannot be treated with conventional methods. Its development marks a significant advancement in indigenous medical technology innovation, with potential for broad application across healthcare and related sectors.
Due to the lack of national software procurement agreements in Ethiopia, this research relied primarily on freely available (locally accessible software tools). (https://getintopc.com/softwares/3d-cad/autocad-2018-free-download-7477962/, https://www.autodesk.com/education/edu-software/overview). The Mechanical component designs were carried out using AutoCAD 2018, which is freely accessible within the country for academic and non-commercial purposes. Although AutoCAD is a proprietary tool, open-source alternatives are used to develop this product free of cost.
All product development data used in this article are included within the text. Additional data are available from the corresponding author upon reasonable request.
The authors would like to acknowledge IDRC for the financial support. This research work was supported through the R&D Impact Voucher program funded by the International Development Research Centre (IDRC) and implemented by Villgro Africa. We also would like to forward our appreciation to Jimma University and Ministry of Innovation and Technology, Ethiopia, for providing technical and regulatory support.
Furthermore, the authors would like to acknowledge that a preliminary version of this work was made available as a pre-print on Research Square (DOI: 10.21203/rs.3.rs-4378248/v1)36
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Molecular microbiology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 21 May 25 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)