Keywords
Type 1 Diabetes, Schistosoma mansoni, IPSE, Omega-1, Th1 response, Th2 response
Type 1 diabetes (T1D) is characterized by the immune-mediated destruction of pancreatic beta cells, resulting in insulin deficiency and hyperglycemia. Existing insulin therapies manage symptoms but do not fully stabilize blood glucose levels. Schistosoma mansoni infection or its eggs inhibit T1D in rodent models. This study evaluated the antidiabetic effects of Schistosoma mansoni-derived soluble egg antigen (SEA) and glycoproteins (IPSE and Omega-1) in a streptozotocin-induced diabetic rat model.
Glycoproteins were designed using in silico methods and synthesized following epitope prediction, antigenicity assessment, and molecular docking analysis. These glycoproteins were administered at doses of 50 μg and 100 μg per rat in a stratified randomized trial comprising eight treatment groups and two control groups, each containing five streptozotocin-induced diabetic rats: SEA (A-B), Omega-13 (C-D), IPSE2-3 (E-H), insulin-only (I), and PBS (J). Blood glucose, weight, IFN-γ, IL-4 levels, and pancreatic histology were assessed.
In silico analysis of IPSE and Omega-1 showed strong antigenicity (epitopes >0.60), binding energies from -7.9 to -11.9 Kcal/mol, molecular weights of 1261.40–2034.30 Da, and theoretical pI of 8.23–9.70. In vivo, IPSE and Omega-1 significantly reduced glucose (from 5.76–26.16 mmol/L to 3.94–18.92 mmol/L, p < 0.05), promoted weight gain (192.3–235 g to 231.3–250.2 g), and caused mild-moderate pancreatic infiltration.
These findings suggest that SEA, IPSE, and Omega-1 are promising candidates for novel diabetes therapies, warranting further investigations.
Type 1 Diabetes, Schistosoma mansoni, IPSE, Omega-1, Th1 response, Th2 response
Diabetes is a metabolic disease caused by insufficient insulin production or inability to utilize it.1 As of 2021, 537 million adults worldwide have diabetes, with an estimated 4 million deaths from the disease in 2017.2 Type 1 diabetes (T1D) is an autoimmune disease and a form of diabetes in which the immune system destroys pancreatic beta cells, influenced by both hereditary and environmental factors.3,4 IFN-γ is a proinflammatory cytokine that plays a significant role in the pathogenesis of diabetes. It promotes autoimmune inflammation and β-cell destruction but also participates in regulatory mechanisms that can limit disease progression.5–7 T1D affects approximately 5–10% of the diabetic population and is currently on the rise, especially in developed countries.1,8,9 According to Mobasseri et al. (2020), the incidence of T1D is 15 per 100,000, 8 per 100,000, 15 per 100,000, and 20 per 100,000 in the continental subgroups of Africa, Asia, America, and Europe, respectively.10 Additionally, these regions had global prevalence rates of 6.9 per 10,000, 3.5 per 10,000, and 12.2 per 10,000 for continental subtypes of T1D, respectively.10 The current treatment for T1D primarily focuses on managing symptoms through insulin therapy and lifestyle modifications. These strategies have several shortcomings that can affect patient outcomes and quality of life.9 However, there have been significant advances in the management of T1D, including nanomedicine, gene therapy, islet cell transplantation, stem cell therapy, and immunotherapy.9
Helminth therapy has emerged as a promising approach in immunotherapy, particularly for autoimmune diseases including T1D.11 Helminths, such as Schistosoma mansoni are the main cause of intestinal schistosomiasis, a disease that affects millions globally and is responsible for a significant number of deaths annually, with estimates ranging from 130,000 to 300,000 annually, with the majority of cases occurring in Africa.12,13 Notably, research has shown that administering schistosome eggs can reduce the severity of several autoimmune diseases, including experimental autoimmune encephalomyelitis, and prevent the onset of colitis and diabetes in non-obese diabetic (NOD) mouse models, highlighting the potential immunomodulatory effects of the parasite.14 Studies have included the use of crude Schistosoma eggs, Soluble Egg Antigen (SEA),15 and Soluble Worm Antigen (SWA).12,16,17 The therapeutic benefits are linked to immunoregulation triggered by the administration of these Schistosoma products, which include a shift from a T helper 1 (Th1) to a T helper 2 (Th2) immune response.18,19 However, ethical and safety concerns have hindered the progress of these clinical trials. Exploring the potential of S. mansoni isolated and derived products is necessary for safer and controlled clinical trials and eventually for novel treatments for T1D among other diseases.12
Products derived from S. mansoni, such as Omega-1-a ribonuclease glycoprotein secreted by S. mansoni eggs and found within SEA, modulate the host immune system, which could lead to a protective effect against T1D.20–22 Everts et al. (2009) reported that the glycoprotein can condition dendritic cells (human monocyte-derived) both in vitro and in vivo, inducing Th2 polarization with characteristics comparable to those of whole SEA.20 Both the natural and recombinant forms of Omega-1 can induce Th2 responses.20 Another glycoprotein representing 80% of Schistosome egg secretions, Interleukin-4-inducing principle (IPSE), has broad immunomodulatory functions, such as triggering the production of interleukin (IL)-13 and IL-4.23
Omega-1 and IPSE stimulate Th2 responses through distinct mechanisms. Omega-1 acts via glycosylation and RNase activity, binds to the mannose receptors of dendritic cells, and inhibits protein synthesis.24 Conversely, IPSE interacts with IgE to activate basophils, triggering the release of IL-13 and IL-4 without requiring glycosylation or RNase activity.23
The aim of this study was to evaluate the potential of S. mansoni SEA and synthetically synthesized glycoproteins (IPSE and Omega-1) to inhibit the progression of T1D by evaluating their effects on blood glucose levels, immune modulation, and pancreatic infiltration.
Male Wistar rats, aged four weeks, were purchased from Kenya Institute of Primate Research (KIPRE). The animals were group-housed in spacious cages to allow sufficient space for exercise, social interaction, and natural behaviors, such as climbing and burrowing. Cage floors were covered with wood chips to provide comfort, hygiene, and opportunities for foraging and digging. The animal room was maintained at a temperature range of 20–24°C with a relative humidity of 40–60% under a 12-hour light/dark cycle. The animals were acclimatized for 14 days prior to the experimental procedures to reduce stress and physiological variability. Routine husbandry included regular cage cleaning to maintain hygiene without causing undue disturbance and providing unlimited food and water. The humane endpoints were adapted from Silva-Reis and colleagues (2023).25 They included body weight, hair/tail appearance and grooming, skin, walking, mental status, and response to stimuli. These were assigned a score of 0-3 where a total score of 4 or 3 for some parameters was an indicator of euthanisia.
In this study, blood samples were collected from three of five rats per group based on pre-established inclusion criteria designed to minimize animal stress and blood volume loss. The rats were randomly chosen within the group after diabetes induction, prior to the experiment, to adequately represent the group. The decision to limit sampling to three rats was made a priori to comply with animal welfare guidelines restricting total blood volume removal per animal and to reduce potential confounding effects from repeated blood draws, such as stress-induced hyperglycemia. No animals were excluded from the study, and all data from the sampled rats were included in the analysis.
The sample size of five rats per group was decided based on several factors, including the use of the resource equation approach,26 including an attrition rate of 20–50%,27,28 previous similar studies in diabetic rat models,29 resource availability, and ethical guidelines.
Diabetes was induced in animals by administering streptozotocin (STZ), obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA), following a 6-8 hour fasting period. STZ was freshly dissolved in 50 mM citrate buffer (pH 4.5) immediately before use and injected intraperitoneally into Wistar rats at a single dose of 40 mg/kg30 ( Figure 2). To prevent hypoglycemic shock after STZ administration, 10% glucose solution was provided for 24 h to the treated rats.31 Rats with blood glucose levels > 5.2 mmol/L were selected for this study.
S. mansoni glycoproteins (IPSE and Omega-1) were searched and retrieved from the NCBI (https://www.ncbi.nlm.nih.gov/) and UniProt (https://www.uniprot.org/) databases using the names of proteins and GenBank accession numbers in the literature. The bioinformatic tool BepiPred 3.0 ( https://services.healthtech.dtu.dk/services/BepiPred-3.0/ ) was used to predict the linear B-cell epitopes on S. mansoni IPSE and Omega-1 protein sequences. The software leverages numerical embeddings from the protein language model ESM-2 to enhance the prediction accuracy for both linear and conformational B-cell epitopes.32 while antigenicity was assessed using Vaxijen (https://www.ddg-pharmfac.net/vaxijen/VaxiJen/VaxiJen.html). This was performed to determine the interaction sites of the proteins that are involved in triggering the Th2 response. Expasy’s ProtParam suite was used to determine the theoretical pI and molecular weight of the predicted epitopes from IPSE and Omega-1.33
Our goal was to synthesize an active segment solely responsible for directly inducing the Th2 response. Therefore, after predicting the epitopes, we assessed their antigenicity and analyzed the predicted derivatives. Molecular docking was performed using PyRx (https://pyrx.sourceforge.io/) to predict how these sequences would interact with the target molecule for each protein (IPSE with IgE and Omega-1 with Mannose receptor). The 3D structures of the sequences were predicted using the RPBS portal (https://mobyle.rpbs.univ-paris-diderot.fr/cgi-bin/portal.py#jobs::overview), and protein structure preparation for molecular docking was performed using ChimeraX (https://www.cgl.ucsf.edu/chimerax/).
The final peptide sequences were based on the binding affinity and energy from molecular docking. The sequences were then sent to GenScript (Piscataway, NJ, USA) for synthesis.
Stratified randomization was used for allocation based on the blood glucose levels of the animals after diabetes induction. Each peptide (SEA, Omega-13, IPSE2 and IPSE3) was assigned to a group of five diabetic rats at two different doses (50 and 100 μg/rat). The peptides were administered intraperitoneally daily for five days,34–36 and the control groups, insulin and PBS, were each assigned to groups of five diabetic rats. After a treatment period of five days, the rats were monitored for an additional five days. Blood samples were collected at baseline, after diabetes induction, post-treatment, and post-monitoring ( Figure 1). Following this, the rats were euthanized by introducing 100% CO2 at a rate of 30%-70% of the chamber volume per minute, at a flow rate of 1.5 L/min for 2-3 minutes, and their organs were harvested for histological examination.
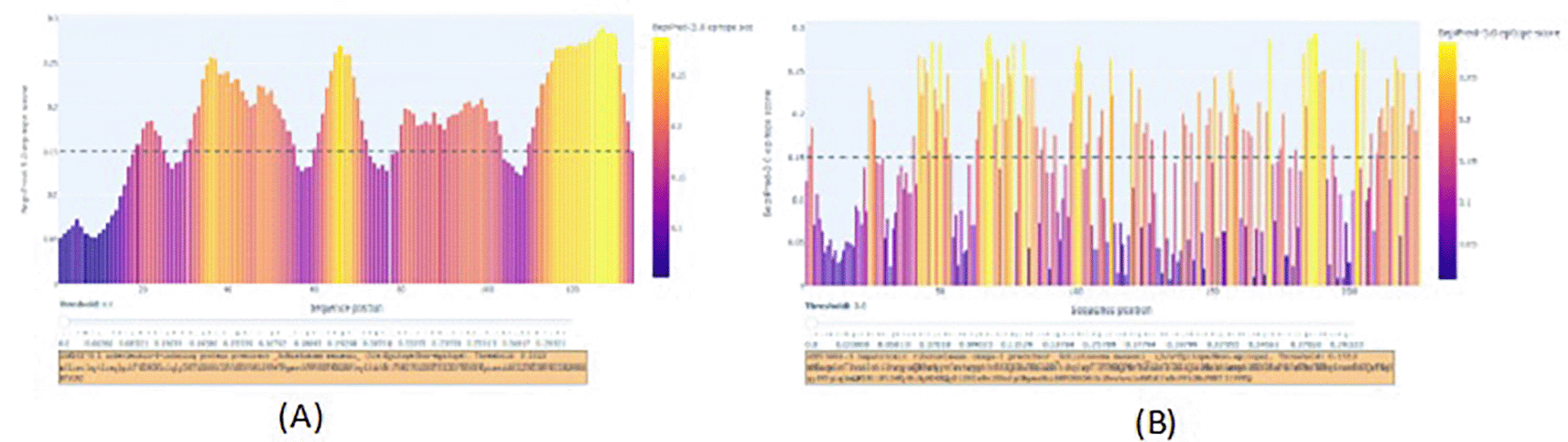
Omega-1 and IPSE’s sequences were found40 with epitopes highlighted by the yellow color. (A) Predicted IPSE epitopes. (B) Predicted epitopes in the Omega-1 sequences.
Rats were provided unlimited access to food and water, and body weight measurements were performed at four time points. Behavioral changes were also monitored.37
Blood glucose levels were measured weekly using a glucometer (On Call Plus) with blood collected from the tail bleed. Blood samples collected at different time points were processed to obtain serum and used for immunological examinations. The two main cytokines of interest in this study were IFN-γ and IL-4 (representing Th1 and Th2 cytokine profiles). ELISA kits (U-CyTech Biosciences) were used to analyze the cytokine profiles of the experimental and control groups.
The assay used a sandwich ELISA format, in which plates were coated with cytokine-specific capture antibodies, blocked with bovine serum albumin (BSA), and incubated with serum samples and recombinant standards. Biotinylated detection antibodies were then added, followed by streptavidin-horseradish peroxidase (HRP) polymer conjugates and 3,3’,5,5’-tetramethylbenzidine (TMB) substrate to provide a colorimetric signal proportional to the cytokine concentration. The reaction was terminated with a stop solution, and the absorbance was measured at 450 nm.
After sacrificing all rats by introducing 100% CO2 at a rate of 30%-70% of the chamber volume per minute, at a flow rate of 1.5 L/min for 2-3 minutes, the pancreas was harvested and stored in 10% formalin for 14 days, and examination was performed according to previously described techniques.16,37 Following fixation, the tissues were dehydrated using graded alcohol, cleared in xylene, infiltrated with paraffin wax, and embedded in paraffin blocks for thin sectioning with a microtome. Following sectioning, the tissues were mounted on slides and stained with hematoxylin and eosin (H&E) for microscopic analysis. Sections of the islets were examined, and the degree of cellular infiltration was evaluated as no infiltration (0), mild infiltration (1), moderate infiltration (2), and severe infiltration.38
GraphPad Prism (Version 8.02) was used to conduct all analyses. Data are shown as means ± SEM. Repeated measure ANOVA was used to analyze blood glucose, weight and cytokine concentration of the groups across the different time points followed by a Tukey’s comparison test. p- values < 0.05 were considered significant and calculated by GraphPad Prism.
An In-silico approach encompassing epitope prediction, antigenicity analysis, and molecular docking was used to investigate the potential immunogenicity and target binding properties of IPSE and Omega-1 to synthesize peptides from the complete sequence of glycoproteins. Protein sequences of IPSE were obtained using Protein Data Bank accession number 4AKA39 or Uniprot using Q869D4 and Omega-1 from the Protein database accession number ABB73003.1 (NCBI).24 These sequences were subjected to epitope prediction, and the antigenicity of the predicted epitopes was analyzed. One of the IPSE’s epitopes was found in the literature with derivatives39 which we incorporated in this study ( Table 1). The antigenic epitopes were then docked. Thus, the final peptides that were synthesized and used in this study were IPSE2, IPSE3 and Omega-13 ( Table 2), where IPSE2 is a derivative of IPSE3 (the predicted epitope found in the literature).
The BepiPred 3.0 scores for IPSE ( Figure 1A) and Omega-1 ( Figure 1B) were above the threshold of 0.15, indicating a high probability that the predicted IPSE and Omega-1 proteins are likely to be part of an epitope. Multiple predicted antigenic epitopes for IPSE (IPSE3 epitope, IPSE3 literature, and IPSE2 derivative) and Omega-1 (Omega-13 epitope) ( Table 1) were observed with a score range of 0.6520–1.3383. The predicted molecular weight was between 1261.40 to 2034.30 daltons while theoretical pI ranged between 8.23 to 9.70 ( Table 1). To determine their ability to bind to target molecules, IPSE derivatives (IPSE2 and IPSE3) and Omega-13 epitopes were docked. The predicted epitopes exhibited strong binding to the target molecules (IgE for IPSE derivatives and Mannose receptor for Omega-1) with IPSE3 and IPSE2 having binding energies of -11.7 kal/mol and -11.9 kal/mol respectively while Omega-13 had a binding energy of -7.9 kal/mol ( Table 2). These findings imply that specific regions within IPSE and Omega-1 possess inherent immunogenic characteristics and validated specific molecular interactions, suggesting their potential role in modulating immune response.
Omega-13 indicates the epitope predicted from the complete Omega-1 sequence, IPSE3 epitope indicates the epitope predicted from the complete IPSE sequence, IPSE3 literature indicates the part of the complete sequence of IPSE found in literature as the interaction site for IgE binding39 and IPSE2 derivative indicates the sequence derived from the interaction site (IPSE3) identified in the literature.39
Blood glucose levels in treatment and control groups
To assess the efficacy of SEA, Omega-1, and IPSE variants in reducing hyperglycemia, diabetes was induced in rats using streptozotocin (STZ), and blood glucose levels were monitored after five days of treatment ( Figure 1). STZ induction increased blood glucose levels from baseline (4.0-4.6 mmol/L) to diabetes induction (5.0-26.0 mmol/L) ( Figure 3A-J). Following treatment, SEA (50 μg, 100 μg) and Omega-13 (50 μg, 100 μg) significantly (p < 0.05) reduced blood glucose levels compared to the PBS control ( Figure 3A-D,J). IPSE3 at 100μg showed a significant blood glucose reduction ( Figure 3H) while IPSE3 at 50 μg showed no reduction ( Figure 3G). IPSE2 at 50 μg and 100 μg showed a non-significant reduction in blood glucose levels ( Figure 3 E-F). Insulin, as a positive control, also significantly reduced blood glucose ( Figure 3I). These findings imply that SEA, Omega-1, and IPSE2-3 (at higher doses) possess therapeutic potential for managing hyperglycemia in this STZ-induced diabetes model.
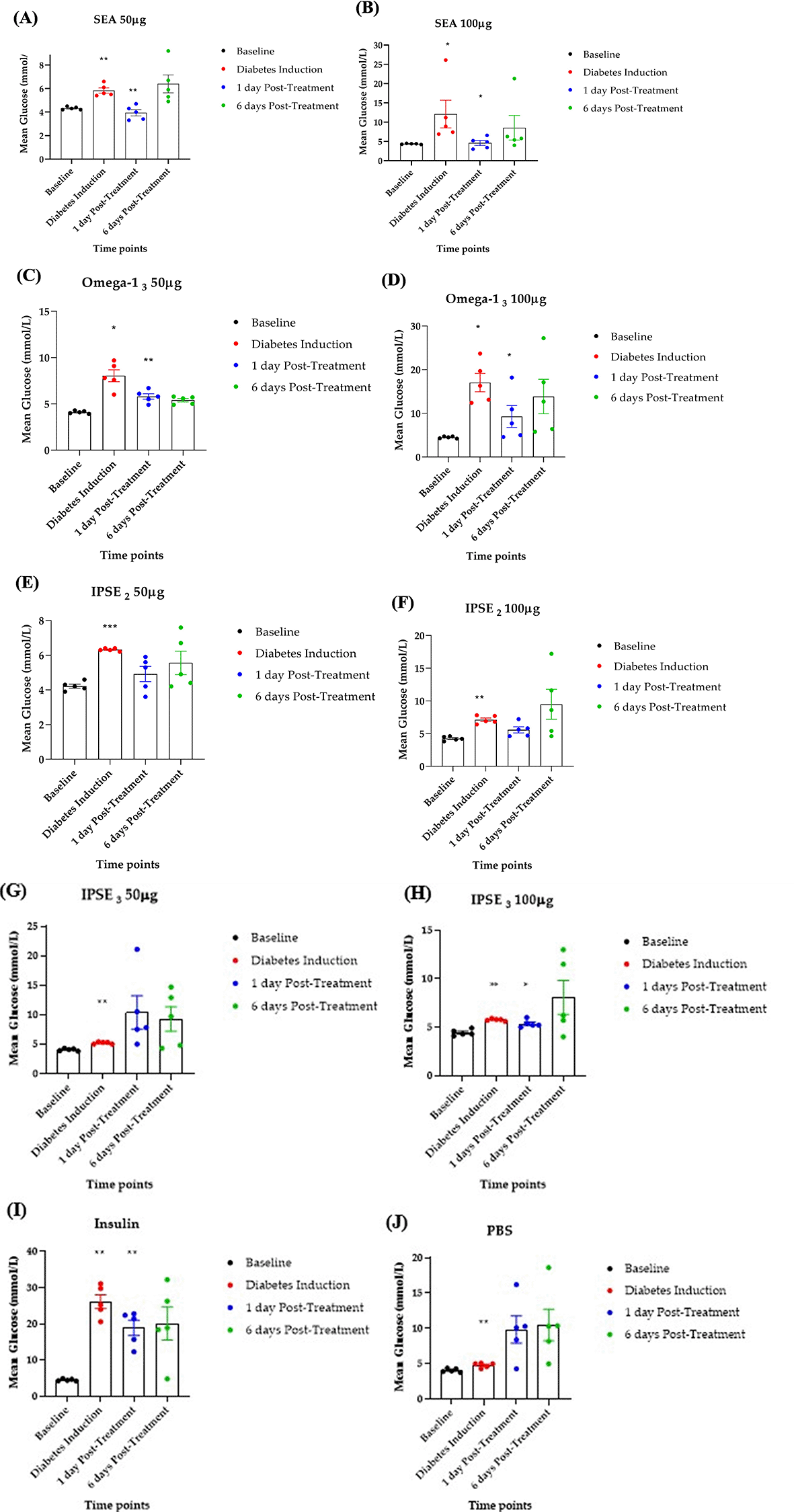
n=5 rats per group. After treatment, blood glucose levels reduced significantly for Insulin (positive control), SEA, Omega-13 and IPSE3 (p< 0.05). It also reduced for IPSE2 but this was not significant.
Mean weight
The weight of each group showed a progressive increase after diabetes induction and exponential weight gain post- treatment ( Figure 4).
Cytokine levels were assessed following treatment to examine the effects of SEA, Omega-1, and IPSE variants on Th1 (IFN-γ) and Th2 (IL-4) cytokines, major cytokines involved in Th1 and Th2 immune responses, and balance in the STZ-induced diabetes model. Induction of diabetes resulted in a significant (p < 0.05) increase in IFN-γ levels. Treatment with 50 μg and 100 μg SEA significantly suppressed IFN-γ elevation ( Figure 5A-B). Conversely, Omega-13 ( Figure 5C-D) and IPSE2-3 ( Figure 5E-F) treatments increased IL-4 levels, but this was insufficient to suppress IFN-γ levels. Insulin treatment also increased IL-4 and reduced IFN-γ levels without causing a major cytokine shift ( Figure 5I), whereas the untreated control showed continuously elevated IFN-γ levels ( Figure 5J). These findings imply that treatments with SEA, and to some extent IPSE/Omega-1, modulate the Th1/Th2 cytokine balance in the diabetic state, with SEA notably suppressing diabetes-induced Th1 elevation.
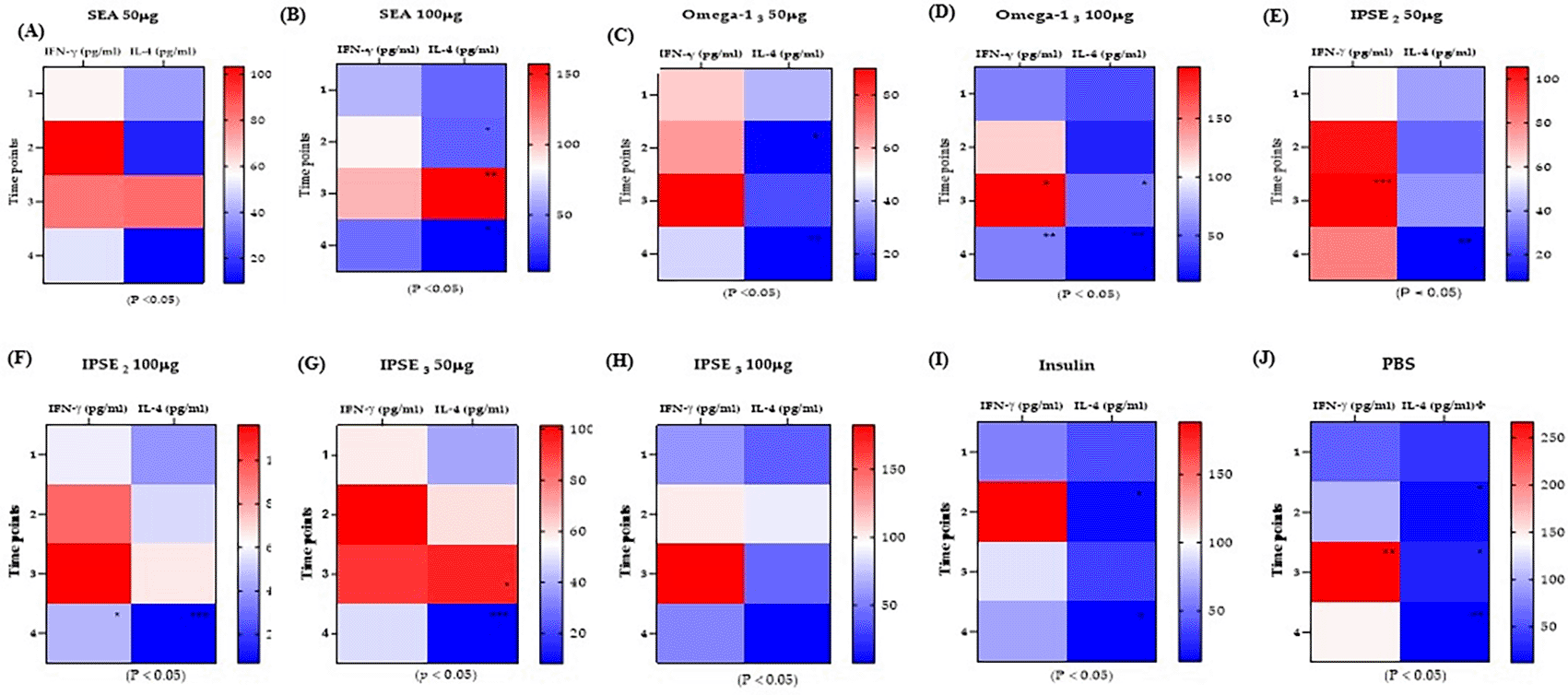
Following induction (Time point 2), IFN-γ levels generally increased while IL-4 levels decreased. (p < 0.05) but after treatment (time point 3) IL-4 is seen to also increase (p < 0.05). Both IFN-γ and IL-4 decrease at time-point 4.
To assess the relative change (%) in mean blood glucose, IFN-γ, and IL-4 levels from diabetes induction to a day post-treatment ( Figure 6), we used the following formula:
Histological examination showing leukocytic infiltration in harvested pancreas is a hallmark finding in T1D.
To assess islet pancreatic infiltration in the treated groups and the controls, the animals were euthanized, the pancreas was harvested, and histological examinations were performed after staining. Mild to moderate infiltration was observed in the treated groups compared to that in the negative control group ( Figure 7). Further observations were as follows: in a non-diabetic rat, no pancreatic islet infiltration ( Figure 7 I). In contrast, in the insulin group ( Figure 7 II), similar images were observed in STZ-diabetes-induced rats treated with insulin. Mild/reduced mononuclear leukocyte infiltration into pancreatic islets. The untreated group showed severe inflammatory cell infiltration (mononucleated leukocytes and macrophages) into the pancreatic islets and disruption of islet architecture with increased cellularity ( Figure 7 III). For Omega-13, peri-islet macrophage infiltration and moderate islet infiltration were observed ( Figure 7 IV-V). IPSE2 showed moderate mononucleated leukocyte infiltration into the pancreatic islets and mild degeneration and mononucleated leukocyte infiltration into the pancreatic islets ( Figure 7 VI-VII). IPSE3 showed mild pancreatic infiltration (with mononucleated and multilobed leukocytes) within pancreatic islets ( Figure 7 VIII). IPSE3 showed vacuolation of peri-islet cells and severe infiltration ( Figure 7 IX). SEA showed mild pancreatic infiltration and vacuolation of peri-islet cells ( Figure 7 X-XI). These findings imply that treatment with SEA, IPSE, and Omega-1 slows down the pathogenesis of the disease.
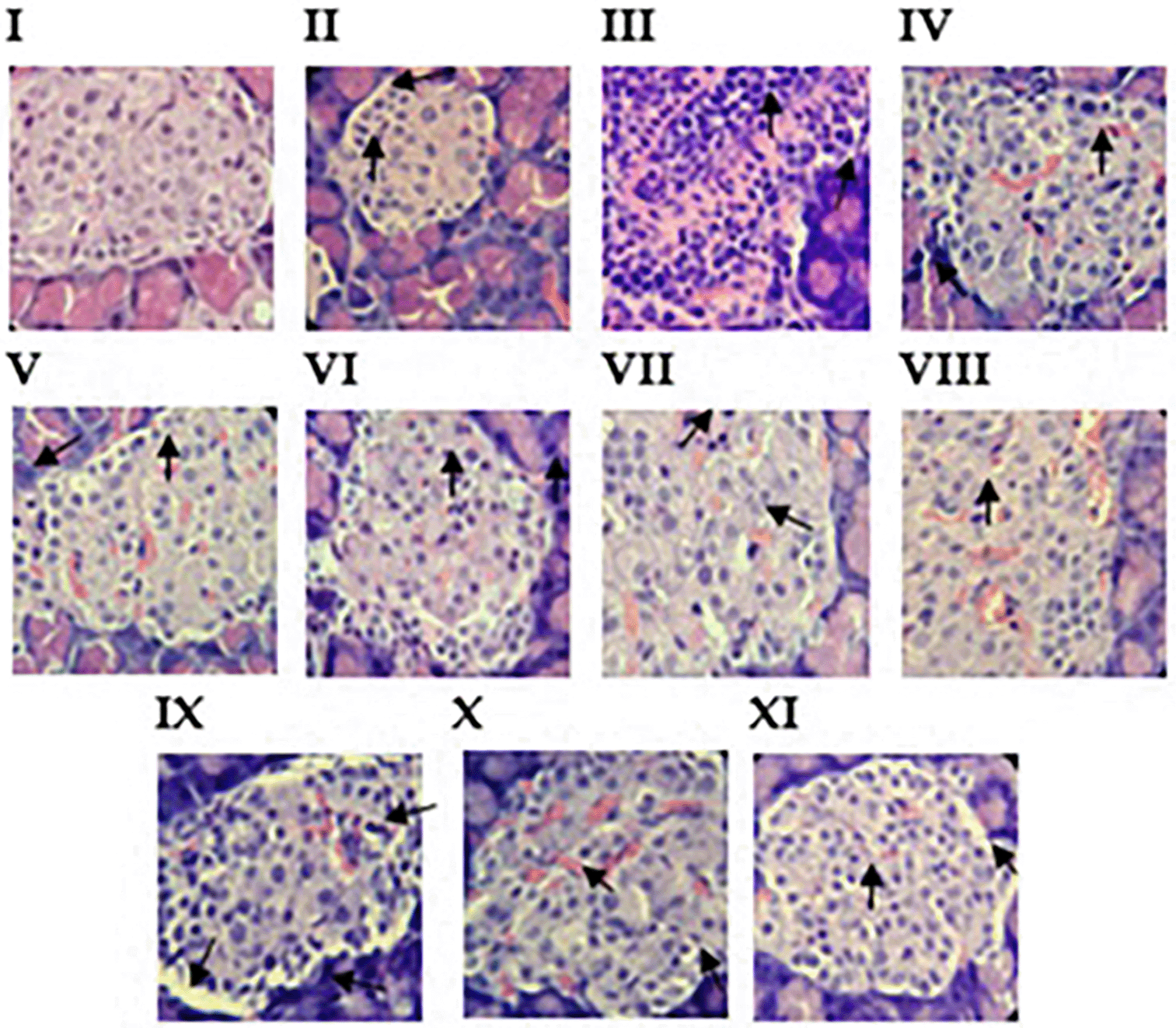
The groups shown include the following: (I) Normal group, showed normal characteristics of islet appearance in nondiabetic rat (II) Insulin group, showed mild islet infiltration, (III) PBS (untreated group), showed severe islet infiltration (IV-V) Omega-13 50 μg and 100 μg, showed moderate and mild islet infiltration, (V1-VII) IPSE2 50 μg and 100 μg, showed moderate and mild islet infiltration, (VIII-IX) IPSE3 50 μg and 100 μg showed severe, mild islet infiltration and vacuolation and (X-XI) SEA 50 μg and 100 μg showed mild islet infiltration.
Pancreatic infiltration in the treated groups and control group was graded according to how much infiltration was observed in the islet cells, and these were assigned as mild, moderate, and severe to indicate the extent of the infiltration in an increasing order and scores 0-3 ( Table 3). The treated groups were mild/moderately infiltrated, while the control group (PBS) showed severe infiltration, as previously stated ( Table 3, Figure 7).
This study demonstrated that purified Schistosoma mansoni egg proteins IPSE and Omega-1 can temporarily inhibit the progression of type 1 diabetes (T1D) in an STZ-induced diabetic Wistar rat model. Our major finding was that both IPSE and Omega-1 significantly reduced blood glucose levels and modulated immune responses, with SEA inducing a shift from a pro-inflammatory Th1 cytokine profile (IFN-γ) toward an anti-inflammatory Th2 profile (IL-4). This immunomodulatory effect aligns with previous research demonstrating the protective role of Th2 responses in autoimmune diseases, including T1D, and supports the hypothesis that helminth-derived proteins can modulate autoimmunity.12,41
The observed reduction in blood glucose levels following treatment with SEA and the two glycoproteins suggests that IPSE and Omega-1 can transiently suppress autoimmune-mediated beta cell destruction or improve residual beta cell function. Interestingly, although SEA administration clearly shifted cytokine profiles, the individual proteins IPSE and Omega-1 reduced blood glucose levels without consistently inducing a full Th2 cytokine shift. This finding implies that blood glucose regulation may be partially independent of a complete cytokine profile change, which is consistent with Hübner et al. (2012), who demonstrated helminth protection against diabetes without a strict Th2 shift.42 This also raises the possibility that the doses used or the use of protein fragments rather than full-length proteins limited the immunomodulatory capacity of IPSE and Omega-1 alone.43,44 Ideally, full-length IPSE and Omega-1 exert their immunomodulatory effects through distinct molecular mechanisms that promote Th2 responses and influence metabolic regulation. IPSE specifically binds to IgE on basophils via its antigenic epitope, triggering IL-4 release without cross-linking, which fosters Th2 differentiation and immune modulation.23,39 Conversely, Omega-1 binds strongly to the mannose receptor on dendritic cells through its glycosylated epitopes,22 enabling its RNase activity to degrade RNA and block IL-12 synthesis, thereby priming dendritic cells to activate Th2 responses.24 In silico findings supported these interactions by demonstrating strong binding affinities between IPSE2-3, IgE, Omega-13 and the mannose receptor. These mechanisms not only drive immune polarization, but may also indirectly enhance β-cell function and insulin signalling, contributing to the observed temporal therapeutic effects.19
The relative changes in blood glucose, IFN-γ, and IL-4 levels suggest that SEA can rival or surpass insulin in lowering blood glucose and beneficially modulate immune responses in diabetic rats.19,45 It also demonstrates that the effects of the peptides, particularly, IPSE3 effects may be dose-dependent and require careful optimization to maximize benefits and minimize potential risks. This agrees with previous studies that demonstrated that variants of IPSE interaction sites (IPSE2 in this study) have a higher binding affinity for IgE than the actual IPSE interaction site (IPSE3).39 This relative change also supports the concept that targeting both metabolic and immune pathways is important for comprehensive diabetes management.46,47 Additionally, it suggests that immune modulation (reducing IFN-γ and increasing IL-4) could be as important as blood glucose control in preventing diabetes progression and complications.19,45,47
The temporary nature of the therapeutic effect, with blood glucose levels and IFN-γ increasing after treatment cessation, suggests that sustained administration or higher doses may be necessary to achieve long-lasting benefits. This is supported by earlier studies showing that early exposure to S. mansoni antigens prior to diabetes onset is critical for durable protection.16 Since our model induced diabetes chemically after treatment initiation, it may not fully replicate the natural autoimmune progression, potentially limiting the ability to prevent disease onset entirely.30
Histological analysis showed mild to moderate pancreatic immune infiltration and vacuolation in the treated groups, indicating partial suppression of immune cell invasion and ongoing cellular stress. This suggests that IPSE and Omega-1 can slow autoimmune damage but do not fully prevent it, highlighting the need for optimizing dosing and treatment duration.16–18 The presence of vacuolation may also reflect early islet cell injury, which can be reversed with prolonged treatment.
T1D was induced using a 40 mg/kg STZ dose, which typically causes partial beta cell destruction and moderate hyperglycemia, as opposed to the severe insulin deficiency and weight loss observed at higher doses (e.g., 50–70 mg/kg).48,49 The progressive post-induction weight gains observed in this study likely reflect residual beta cell function, allowing limited insulin secretion to maintain metabolic homeostasis and mitigate catabolic wasting. However, the exponential weight gain post-treatment with SEA and IPSE/Omega-1 suggests that these helminth-derived components may enhance insulin sensitivity, protect residual beta cells, or directly modulate metabolic pathways to reverse diabetes-associated catabolism. This contrasts with studies using high-fat diets combined with 40 mg/kg STZ, in which diabetic rats exhibited reduced weight gain, highlighting the potential of IPSE and Omega-1 to counteract metabolic dysfunction.50 While these findings imply therapeutic efficacy, further validation of beta-cell mass, insulin secretion, and tissue-specific blood glucose uptake is critical to distinguish between STZ dosing artifacts and genuine treatment effects.48,51 If confirmed, the ability of IPSE and Omega-1 to stabilize weight in T1D models could signify a novel mechanism for managing diabetes-related metabolic stress, warranting exploration in autoimmune models such as NOD mice.48,49
The limitations of this study include the use of protein fragments rather than full-length proteins, which may have altered the biological activity, as protein conformation and epitope presentation are critical for immune modulation. The relatively short treatment and follow-up periods may have been insufficient to observe the long-term effects. Moreover, the STZ model, while convenient, does not fully mimic the autoimmune etiology of T1D, limiting the assessment of its prophylactic potential.
Future research should address these limitations by investigating the synergistic effects of combined IPSE and Omega-1 proteins to enhance immunomodulatory efficacy, and utilizing full-length IPSE and Omega-1 proteins to better replicate native antigenic structures and improve biological activity. Extending the treatment duration and exploring dose escalation may help to achieve sustained blood glucose control and immune modulation. Advanced delivery methods such as nanotechnology or microencapsulation can help improve protein stability, bioavailability, and targeted pancreatic delivery. Studies in NOD mouse models, which more closely mimic human autoimmune diabetes, evaluate prophylactic and therapeutic effects and explore detailed mechanistic studies to understand how IPSE and Omega-1 interact with immune cells and pancreatic tissues at the molecular level.
Collectively, our results provide compelling evidence that IPSE and Omega-1 exert therapeutic effects in a model of autoimmune diabetes, likely through complex immunomodulatory and metabolic mechanisms, positioning them as novel protein-based therapeutics for this disease.
The Kenya Institute of Primate Research (KIPRE) Institutional Scientific Review Committee (ISERC) reviewed and approved this study. (ISERC/06/2024) on 10th November, 2024.
Figshare: Raw underlying data for Evaluating the potential of Schistosoma mansoni Soluble egg antigen (SEA and Glycoproteins (IPSE and Omega-1) in inhibiting the progression of Type 1 Diabetes: https://doi.org/10.6084/m9.figshare.29090960.v152
The project contains the following underlying data:
Data are available under the terms of Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Figshare: ARRIVE guideline checklist for Evaluating the potential of Schistosoma mansoni Soluble egg antigen (SEA and Glycoproteins (IPSE and Omega-1) in inhibiting the progression of Type 1 Diabetes: https://doi.org/10.6084/m9.figshare.29090963.v153
Data are available under the terms of Creative Commons Attribution 4.0 International license (CC-BY 4.0).
We are sincerely grateful to the African Union for their scholarship and research funding. Mr. Thomas Adino and Dr. Benjamin Mwongela. and Madam Mary (Animal Sciences, KIPRE) for their immense help with the in-vivo and histological work, the TID (Tropical and Infectious Diseases Department, KIPRE) team Mr. Elisha Opiyo, Linda Obiero, Sam Momanyi, and staff for their help and suggestions with immunological assays.
We acknowledge the use of Perplexity for assisting with the language editing and manuscript polishing. The authors take full responsibility for the contents of this paper.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)