Keywords
Clinical trials, registries, research transparency, research data accessibility, world health assembly resolution.
This article is included in the Global Public Health gateway.
This article is included in the All trials matter collection.
In 2022, WHO’s World Health Assembly adopted resolution WHA75.8, emphasizing the critical role of clinical trials in generating high-quality evidence and promoting equitable access to health interventions globally. In response, rapid landscape reviews were conducted to assess global clinical trial regulations, capacities, and funding distribution.
The analysis synthesized regulatory frameworks from 94 countries, institutional capacity data from the WHO International Clinical Trial Registry Platform (ICTRP), and funding data from World RePORT for trials registered between 2018-2022. Gaps in data availability and quality were assessed.
Most countries reference international ethical guidelines, with universal requirements for ethics approval and informed consent. However, only 66% mandate public trial registration, and 40% require results reporting, with stark disparities between high- and low-income countries. High-income countries host over half of global trials; low-income countries contribute less than 1% despite high disease burden. Clinical trials sponsored by non-commercial entities are particularly scarce in low- and middle-income countries. Funding remains concentrated in the Americas and European regions, primarily driven by major funders such as the National Institutes of Health in the United States of America and European Commission. Significant data accessibility challenges persist due to incomplete registry records, inconsistent standards, lack of harmonized identifiers, and limited bulk data access.
Urgent actions include reinforcing international standards for trial registries, harmonizing data fields, improving registry interoperability, leveraging unique identifiers, enhancing multilingual accessibility, auditing data quality, pooling analytical resources, promoting open data policies, and investing in registry infrastructure and trained personnel.
Addressing data gaps and inequities in clinical trial ecosystems requires concerted action by global stakeholders. Improved data transparency and interoperability are essential to guide equitable research investments, foster coordination, and strengthen clinical trial capacity worldwide.
Clinical trials, registries, research transparency, research data accessibility, world health assembly resolution.
In May 2022, recognizing the indispensable role of clinical trials in advancing knowledge about health interventions, and the importance of promoting equity in clinical trial capabilities globally, the 75th World Health Assembly adopted resolution WHA75.8: Strengthening clinical trials to provide high-quality evidence on health interventions and to improve research quality and coordination.1 The resolution underscores the importance of prioritizing resources to address health needs and ensuring the rigorous scientific and ethical conduct of clinical trials. It also emphasizes the necessity for trials to be conducted across diverse settings, particularly among historically under-represented and under-served populations, in order to advance equitable access to health interventions with a favourable risk-benefit profile. The resolution advocates for enhanced collaboration and coordination among public and private entities to align clinical trial efforts with public health priorities, especially in addressing the health needs of lower income countries. It also calls for the development of robust national clinical trial capabilities, adherence to international standards, and promotion of transparency through the prospective registration and timely reporting of trial results.
In response to this resolution, rapid landscape reviews were conducted in 2023 to better understand the current global clinical trial regulatory frameworks, who is funding trials and where, and current capacities. Building on these reviews, this policy brief explores international priorities in the space and provides actionable recommendations for future progress in strengthening clinical trial ecosystems.
Our analysis ( Table 1) of clinical trial regulations identified from 94 countries observed that, despite regional variation, 75 of 94 countries’ national regulatory frameworks cover 9 or more of 11 key components of clinical trial conduct and governance. International guidelines, such as Declaration of Helsinki and The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), are commonly referenced, and research ethics committee approval and informed consent from trial participants are universally required by national guidelines.
However, the review revealed a lack of governmental requirements for public registration of clinical trials and reporting of trial results in many countries. Two-thirds (66%) of the 94 countries require prospectively registering all clinical trials in a public registry prior to enrolling participants, while 40% have mandates for public reporting of trial findings—two critical mechanisms to promote transparency and increase the value of trials. Furthermore, disparities in governmental guidance are seen between high-income countries (N = 44) and low-income countries (N = 12), in particular on explicit privacy protections (100% vs. 58%), trial registration (80% vs. 25%), and reporting of trial results in registries (66% vs. 0%).
Between 2018 and 2022, about half of the 340,397 trials in WHO International Clinical Trial Registry Platform (ICTRP), were recruiting participants in high-income countries (HICs), with the highest number of recruitments in the United States ( Figure 1). China and India saw fast-growing clinical trial activity, being 2nd and 3rd among the top 10 countries by the total number of trials registered, the others being HICs in North America, Europe, and Japan ( Figure 2). Low-income countries accounted for less than 1% of total clinical trials worldwide, which is disproportionate to their high disease burden. The numbers of clinical trials, sponsored by entities other than commercial companies or individuals [1], are disproportionately lower in WHO’s African region and South-eastern Asian region, compared to other regions ( Figure 3). This indicates that clinical trials for non-commercial purposes face particular challenges in low- and middle-income regions (LMIC).
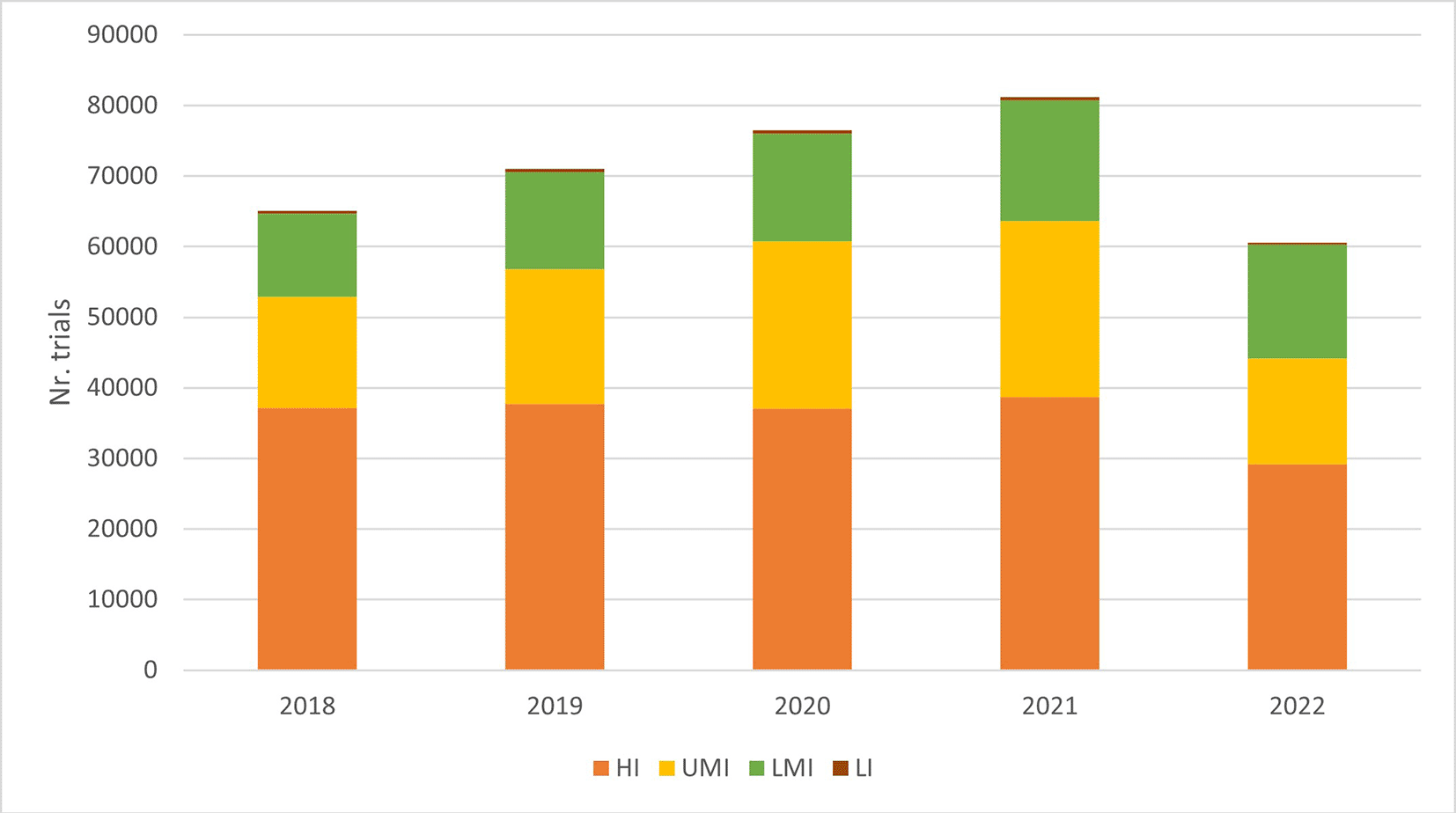
Based on data of 4829 trials (approximately 1%) registered between January 2018 and December 2022, whose funding information could be traced in World RePORT,3 the WHO Americas region accounts for 77% of clinical trial funding, reflecting a stark imbalance in the global distribution of clinical research funding relative to the population size and gross domestic product (GDP). The National Institutes of Health in the United States of America, European Commission, European and Developing Countries Clinical Trials Partnership, Wellcome Trust and Bill and Melinda Gates Foundation were the largest funders of clinical trials.3 Furthermore, the lack of transparency in funding sources - reflected by the very low percentage of registered trials that could be linked to a grant number in the World RePORT, reveals a gap in clinical trial oversight and public reporting, that could hinder funding coordination and exacerbate inequities in clinical trial capability and infrastructure.
The rapid reviews highlight significant gaps in the availability and accessibility of information about the global clinical trial landscape. Better completeness and quality of data would facilitate a better understanding of regional disparities and areas for improvement.
A substantial proportion of government documents, particularly in low- and middle-income countries, were either unavailable online or accessible only in local languages—not only limiting their utility in informing global mapping initiatives, but also hindering cross-border knowledge-sharing and opportunities for increased international collaboration and investment.
Clinical trial registry records also often lacked consistency and completeness in data quality as well as a lack of harmonization in the way data are collected both between and within registries. This makes aggregation challenging. Additionally, some registries do not support requests for bulk data access or do not support automated data extraction, further constraining the potential for summary analyses. These limitations are compounded by inconsistencies in regulatory requirements regarding the public registration of trials before they begin and the reporting of results after trial completion.
Furthermore, it is difficult to analyse fragmented datasets that include free-text data fields, different names for the same sponsor, and incomplete reporting on data components such as recruitment sites and funding sources. Limited curation and oversight of data entry processes further compromise data quality. For instance, deduplication of trials registered across multiple platforms is hindered by inconsistencies in trial identifiers, sponsor names and other data inconsistencies. Similarly, noncompliance with WHO’s international standards for trial registration and results reporting reduces the completeness and accuracy of data available for global analyses of the trial landscape.4–6
Such barriers undermine efforts to establish equitable representation of trial activity, particularly in underrepresented regions and resource-constrained settings. These issues not only obscure insights into the true scale and distribution of clinical trials, but also hinder efforts to improve transparency, accountability, and ethical standards in clinical research worldwide.
To improve transparency and accountability for a strengthened and equitable global clinical trial ecosystem, urgent actions to enhance accessibility and quality of information about clinical trial are of paramount importance. We recommend the following actions:
• Reinforce and promote adherence to the WHO International Standards for Clinical Trial Registries, which covers registrations and results reporting standards, to ensure uniformity and interoperability across registries.7 Implement standardized fields across all clinical trial registries, covering essential data such as sponsor names, recruitment locations, and funding sources. Wherever feasible, use structured formats rather than free-text entries to increase reliability and facilitate quality control and analyses.
• Enhance functions of existing global platforms, such as the WHO ICTRP, for harmonizing trial data, including mechanisms to address cross-registry duplication. This could leverage unique identifiers such as the Universal Trial Number (UTN) and Research Organization Registry (ROR) IDs, to ensure consistent identification of sponsors, funders, and recruitment sites across registries and facilitate accurate linkages to facilitate and expedite analyses.
• Leverage advanced technology, cross-country partnerships, and funding resources for curation of critical datasets informing evidence generation and research prioritization and provide multilingual interfaces and resources to increase accessibility.
• Regularly audit registry data for accuracy and completeness against established standards, coupling with training and support to sponsors and investigators to enhance compliance with registration and reporting standards.8
• Pool resources and capacity to enhance ongoing data analysis and visualization, such as the WHO Global Observatory of Health R&D, to make clinical trial data accessible and digestible to stakeholders, such as policymakers and researchers.
• Establish multi-stakeholder initiatives involving governments, international organizations, and sponsors to streamline data input and facilitate data transfer between trial registries, particularly for multinational trials.
• Advocate for open data policies and infrastructure investment that encourage the inclusion of bulk download and access in all clinical trial registries, enabling automated data collection and integration. Establish performance metrics to monitor progress with data accessibility, quality, and completeness.
Implementation and periodic monitoring of progress on the recommended actions can help to address the global disparities and data gaps in trial regulation, capacity, and funding identified in our rapid landscape reviews. A critical next step is to invest in increasing accessibility and quality of data about clinical trials. National and global health stakeholders, including research funders, policymakers, oversight agencies, sponsors and researchers, must take concerted action to comply with international standards and ensure sufficient financial support for the data infrastructure of trial registries as well as their highly trained personnel. Better data about clinical trials will enable transparency, prioritization, and coordination to develop equitable clinical trial capabilities and health outcomes globally.
Methodologies of the rapid landscape review and dataset are accessible via https://osf.io/j6nkq/?view_only=ff77ceefd169452ebf877b80ac770ebc. DOI:10.17605/OSF.IO/J6NKQ.9
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Authors are grateful for the contributions from Dr Paula Fearon and Dr Heather Eshleman in the data analysis of clinical trial funding.
1 The types of organizations are based on a controlled list of categories defined by The Research Organization Registry (ROR) – a global, community-led, curated registry of open persistent identifiers for research organizations.2
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Does the paper provide a comprehensive overview of the policy and the context of its implementation in a way which is accessible to a general reader?
No
Is the discussion on the implications clearly and accurately presented and does it cite the current literature?
Yes
Are the recommendations made clear, balanced, and justified on the basis of the presented arguments?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Cardiovascular Clinical Trials and Epidemiology, acute coronary syndromes, heart failure
Does the paper provide a comprehensive overview of the policy and the context of its implementation in a way which is accessible to a general reader?
Yes
Is the discussion on the implications clearly and accurately presented and does it cite the current literature?
Yes
Are the recommendations made clear, balanced, and justified on the basis of the presented arguments?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Clinical trials; Host-microbial interactions;
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 26 Jun 25 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)