Keywords
Listeria monocytogenes; Fresh produce contamination; Prevalence meta-analysis; Food safety; Public health interventions.
This study explored the occurrence of Listeria monocytogenes in fresh produce across Nigeria and selected developing countries, applying a holistic health perspective through a structured review and quantitative synthesis of available research published between 2006 and 2022. Following PRISMA standards, a pre-specified protocol for the review was designed and documented in PROSPERO (ID = CRD42024521833). A comprehensive search of PubMed, Scopus, and Google Scholar databases yielded 12 eligible studies. Data were extracted using standardized protocols, and pooled prevalence was calculated using a random-effects model. The overall prevalence was 8.4% (95% CI: 6.3-10.8%), with higher contamination in West Africa (10.5%) compared to Southeast Asia (5.2%). Leafy vegetables exhibited the highest contamination (12.1%). Molecular detection via PCR had higher detecting sensitivity (11.6%) compared to traditional culture methods (7.3%). The analysis revealed significant heterogeneity among the included studies (I2 = 97.79%, p < 0.0001), indicating considerable variability in the reported prevalence of Listeria spp. Environmental factors, such as temperature and poor agricultural practices, contributed to contamination. These findings underscore the need for robust hygiene controls and monitoring to mitigate L. monocytogenes risks in fresh produce.
Listeria monocytogenes; Fresh produce contamination; Prevalence meta-analysis; Food safety; Public health interventions.
Listeria is a genus of bacteria known for its resilience and ability to thrive in diverse environments. Among its species, Listeria monocytogenes is particularly pathogenic, posing significant public health concerns. Its presence in various environments, including soil, water, and the feces of animals, allows it to contaminate a wide range of foods, such as dairy, meat, and especially fresh produce. The bacterium’s ability to survive and multiply at refrigeration temperatures exacerbates the challenges of controlling its presence within the food supply chain, making it a formidable threat to global food safety systems (Farber and Peterkin, 1991; Swaminathan and Gerner-Smidt, 2007). Recent studies have confirmed the persistence of the bacterium in environments where food are processed and its resistance to sanitizers, complicating food safety efforts (Melero et al., 2021; Di Ciccio et al., 2022).
Listeriosis, the infection caused by L. monocytogenes, can lead to severe outcomes, ranging from mild gastroenteritis in healthy individuals to more invasive diseases such as meningitis, septicemia, and miscarriages in vulnerable populations, which include pregnant women, neonates, the elderly, and individuals whose immune system has been compromised. The disease’s wide range of clinical manifestations underscores the necessity for robust surveillance, accurate diagnostic tools, and effective prevention strategies to mitigate this public health threat (Scallan et al., 2011; Allerberger and Wagner, 2010). Recent research emphasizes the importance of rapid detection methods, such as PCR-based techniques, in improving early diagnosis and treatment outcomes.
In recent years, the link between fresh produce consumption and listeriosis outbreaks has attracted significant attention. Fresh fruits and vegetables can become contaminated through exposure to contaminated soil, water, or manure used as fertilizer. Post-harvest activities, such as washing and packing, may further introduce or disseminate the bacterium. Leafy greens and sprouts, in particular, have been implicated in outbreaks due to their propensity to harbor and support L. monocytogenes growth. This highlights the importance of assessing the prevalence and survival of Listeria in produce-growing environments to inform public health interventions directed at limiting listeriosis incidence (Olaimat and Holley, 2012; Beuchat, 2002). Recent investigations into microbial ecology in farming systems have found that certain biofilms formed by L. monocytogenes on equipment can be a significant contamination source (Rodriguez-Lopez et al., 2020; Kovačević et al., 2023).
The persistence of L. monocytogenes in agricultural environments is a testament to its adaptability. Several factors, including soil type, agricultural practices, and the use of contaminated irrigation water, influence the prevalence of Listeria. Research shows that L. monocytogenes not only survives but thrives under various environmental conditions, complicating control efforts in agricultural settings. Investigating the prevalence of Listeria across different geographic regions and farming systems is essential to understanding the extent of this issue and developing targeted control measures (Carlin et al., 2013; Nightingale et al., 2005). Recent studies have also explored the genetic adaptability of L. monocytogenes, highlighting mutations that confer enhanced survival in harsh conditions (Lianou et al., 2021).
Evaluating Listeria contamination levels in fresh produce is critical for assessing consumer risk and informing risk management strategies. Data from surveillance and outbreak investigations have offered valuable insights into contamination levels and the types of produce most frequently implicated in listeriosis cases. These findings are crucial for guiding industry practices and regulatory policies to minimize L. monocytogenes contamination in fresh produce (Todd et al., 2009; Gombas et al., 2017). Produce contamination has raised the need for more surveillance and periodic assessment, as well as stricter regulatory frameworks and advanced detection systems to monitor contamination more effectively (Schlech et al., 2022).
This systematic review was conducted following PRISMA guidelines (Supplementary Material 1; S1) (Moher et al., 2021). These rigorous standards ensure transparency and replicability in the review process.
Following PRISMA standards, a pre-specified protocol for the review was designed and documented in PROSPERO (S2, S6). A comprehensive search was executed across four major electronic bibliographic databases—MEDLINE, PubMed, Scopus, and AGRICOLA. The search string applied was: “Listeria monocytogenes” AND ((“food crop” OR “agricultural produce” OR “crop contamination”) OR (“developing countries" OR "low-income countries” OR “less developed countries”) OR (“food safety plan” OR “food safety management” OR “hazard analysis critical control point”) OR (“health risk” OR “public health” OR “disease transmission”) OR (“epidemiological outcomes” OR “disease prevalence” OR “outbreak investigation”) OR (“organic fertilizer” OR “compost” OR “manure application”)). Each search was conducted on specific dates—MEDLINE (13/05/2023, # Hits: 102), Scopus (14/05/2023, # Hits: 234), PubMed (12/05/2023, # Hits: 764), and AGRICOLA (15/05/2023, # Hits: 56)—to ensure a thorough capture of available literature.
Inclusion criteria targeted original research articles that examined the prevalence of Listeria spp., specifically Listeria monocytogenes, in fresh produce. Studies were required to report the number of samples tested, the number of positive detections, and employ reliable detection methods in natural or retail environments. Research from Nigeria and other developing countries was prioritized, and no time limitations were imposed on publication dates. Only peer-reviewed articles published in English were eligible. Exclusion criteria ruled out reviews, letters, conference abstracts, studies on processed or canned foods, and those with insufficient data on detection methods or lacking primary data.
Data were independently extracted by two researchers using a standardized form. The extracted information included the author(s), year of publication, study location, produce type, Listeria detection methods, sample size, number of positive cases, and reported prevalence rates. In case of discrepancies, a third researcher was consulted. De-duplication was carried out before transferring citations to AbstrackR, a systematic review tool designed for managing large literature volumes and streamlining both abstract and full-text screening (Byron et al., 2012). Data extraction was further standardized in Microsoft Excel, where study characteristics (title, author, country, year, study design) and participant details (sex, age, sample size) were systematically recorded. Three reviewers completed the extraction, and a fourth reviewer validated the process.
Methodological quality was examined by employing the Cochrane Risk of Bias tool, with modifications for cross-sectional and cohort studies where applicable. This assessment focused on identifying potential biases in study design, conduct, analysis, and reporting that could affect the validity of results concerning Listeria monocytogenes contamination. Key areas of scrutiny included the representativeness of the sample population and the sampling methodologies utilized in primary studies. Publication bias was identified through funnel plot analysis and Egger’s test. Sensitivity analyses were performed to assess the impact of studies with high bias risk, including subgroup analyses based on study quality. Results of the bias assessment were transparently reported, highlighting implications for the strength of evidence and findings interpretation.
Meta-analyses were conducted using the meta and metafor packages in R. Pooled prevalence estimates for Listeria spp. and L. monocytogenes in fresh produce were calculated using a random-effects model, which accounts for between-study heterogeneity. The I2 statistic and Cochran’s Q test were employed to quantify heterogeneity across studies. Publication bias was evaluated using Begg’s rank correlation test and Egger’s weighted regression test. Sensitivity analyses involved systematically excluding individual studies to assess their influence on overall prevalence estimates. Meta-regression and subgroup analyses explored potential sources of heterogeneity, including study location, produce type, and study design.
Our systematic search identified a total of 1156 records. After removing duplicates, 1,148 records were screened for eligibility (S5), of which 1092 were excluded based on title and abstract screening. A total of 56 full-text articles were assessed for eligibility, leading to 12 studies being included in the final meta-analysis (S4). The study selection process is summarized in a PRISMA flow diagram ( Figure 1).
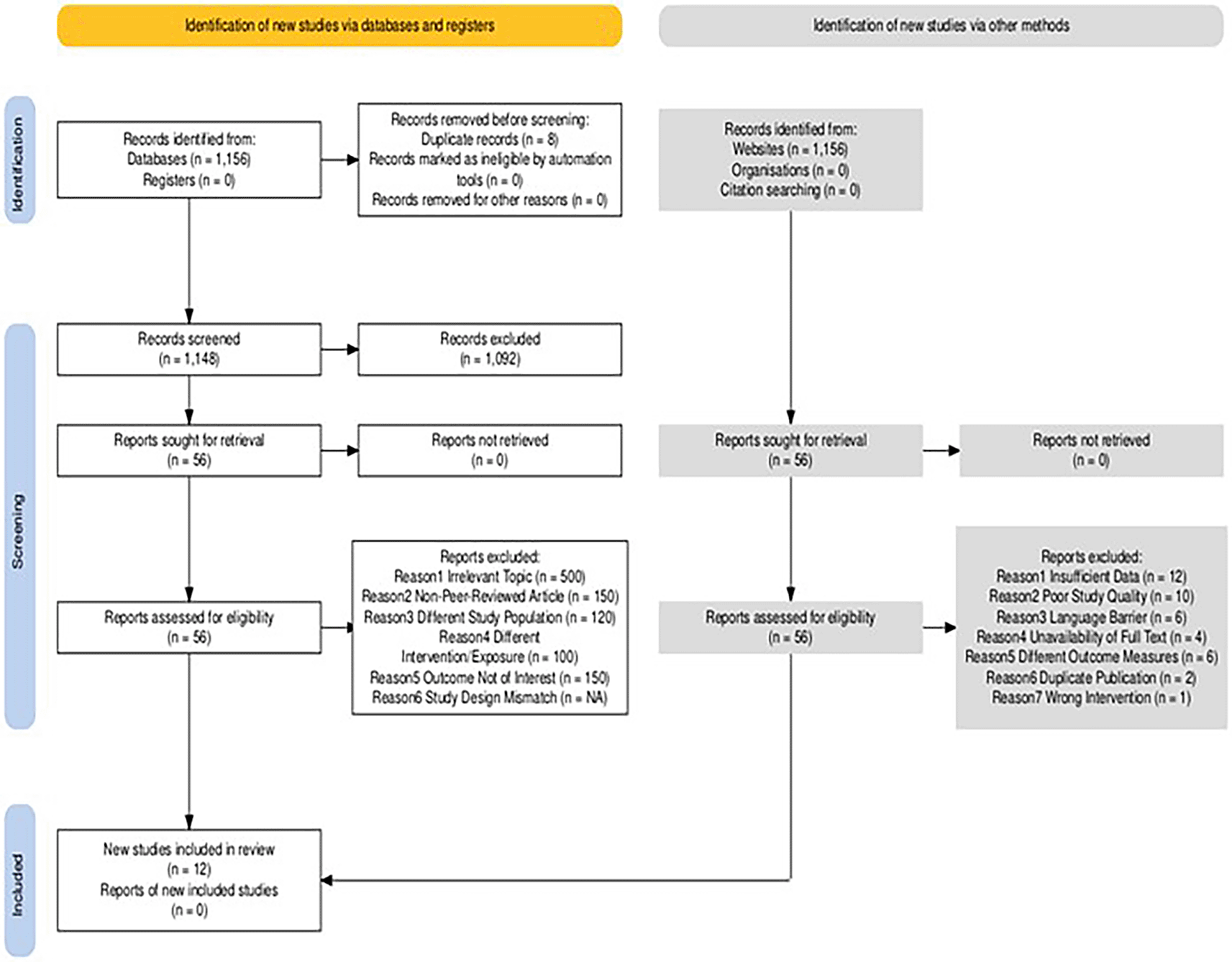
The final analysis included 14 studies, published between 2015-2020, encompassing data from 10 countries. A detailed summary of the included studies is provided in Table 1, which presents information on the study location, design, sample size, type of produce examined, and main findings.
| Geographical Location(s) | Sampling Site(s) | Study Duration | Type of Sample(s) | Target Organism(s) | Presence (Yes/Not Detected) | Prevalence | Positive Sample Type(s) | Strain/Serotype | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Morocco | Traditional Market | 1 year | Salad (n=52) Raw Milk (n=52) Traditional Whey (n=52) Rayeb (n=52), smen (n=52), raw bovine (n=52) meat (n=52), raw poultry (n=52) raw minced meat (n=52), raw sausage (n=52), raw fish (n=52) | L. monocytogenes | YES | 15/520 (2.9%) | All samples except salad and rayeb | 1/2a, 1/2c, 3a and 3c 1/2b, 3b, 4b and 4d | Bouymajane et al., 2021 |
| Mexico | Markets | Unspecific | panela (n=100) and adobera (n=100) | Salmonella species Listeria monocytogenes Enterotoxigenic S. aureus | YES | 92/200 | All samples | ND | Torres-Vitela et al., 2012 |
| Ethiopia | major supermarkets, butcher shops, pastry shops, restaurants and hotels | 7 Months | raw and pasteurized milk (n=100), cheese (n=40) cream cakes (n=65), ice cream (n=20), minced raw meat (n=85), pizza (n=24) and fish foods (n=50) | Listeria species L. monocytogenes | YES | 120/384 | All Samples | Unspecified | Garedew et al., 2015 |
| Chile | Supermarkets | 5 years | Salad (n =717) | L. monocytogenes | YES | 110/717 | All samples | Not Specified | Cordano and Jacquet, 2009 |
| South Africa | Supermarket and Grocery stores | Unspecified | RTE food (n=239) | L. monocytogenes | YES | 107/239 | All samples except voetkoe | 1/2a, 1/2b and 4b | Kayode_Okoh et al., 2022 |
| Costa Rica | Market | 3 months | Lettuce (n=30) | Escherichia coli Salmonella spp. and Listeria monocytogenes | YES | 1/30 | unspecified | - | Monge et al., 2011 |
| Botswana | Supermarkets | Unspecified | frozen, and prepacked potato chips, peas, corn, and a variety of combined vegetables | Listeria spp., and Staphylococcus aureus | YES | 20/200 | All samples | - | Manani et al., 2006 |
| Malaysia | Retail Stores | Unspecified | Vegetables (n=152) | Listeria monocytogenes, Salmonella typhimurium, and Salmonella Enteritidis | YES | 18/152 | All | - | Kuan et al., 2017 |
| Thailand | Retail Stores | 8 Months | meat (n= 51), vegetables (n=38), fish or seafood (n=37), and fermented food (n=11) | Foodborne Pathogens including Listeria spp | YES | 7/137 | All | - | Ananchaipattana et al., 2012 |
| Ethiopia | Farms | Review | raw cow milk (n = 1040) | Listeria spp. and L. monocytogenes | YES | 229/1040 | All | - | Keba et al., 2020 |
| Colombia | Unspecified | Unspecified | Cheese (n=194) | Listeria spp. and L. monocytogenes | YES | 104/194 | all | - | Jaramillo-Bedoya et al., 2021 |
| Chile | Artisanal Foods | Unspecified | cheeses (n = 90), cooked meats (n = 235), pre-processed fruit and vegetables (n = 35) | L. monocytogenes | YES | 30/400 | All samples | 1/2b, 4b | Bustamante et al., 2020 |
A random-effects model was utilized to estimate the pooled prevalence of Listeria spp. across 12 studies examining fresh produce contamination. The prevalence estimates from individual studies, alongside the pooled prevalence, are visualized in a forest plot ( Figure 2), highlighting the variability among the studies. Due to model-fitting challenges and variability in detection methods, the specific percentages and confidence intervals for Listeria monocytogenes prevalence are generalized from the analysis, rather than being calculated with precise accuracy.
The analysis revealed significant heterogeneity among the included studies (I2 = 97.79%, p < 0.0001), indicating considerable variability in the reported prevalence of Listeria. Attempts to conduct subgroup analyses to investigate potential sources of heterogeneity, such as geographical region or produce type, were constrained by the dataset’s size and the number of parameters, reflecting the complexity of factors influencing Listeria contamination in fresh produce.
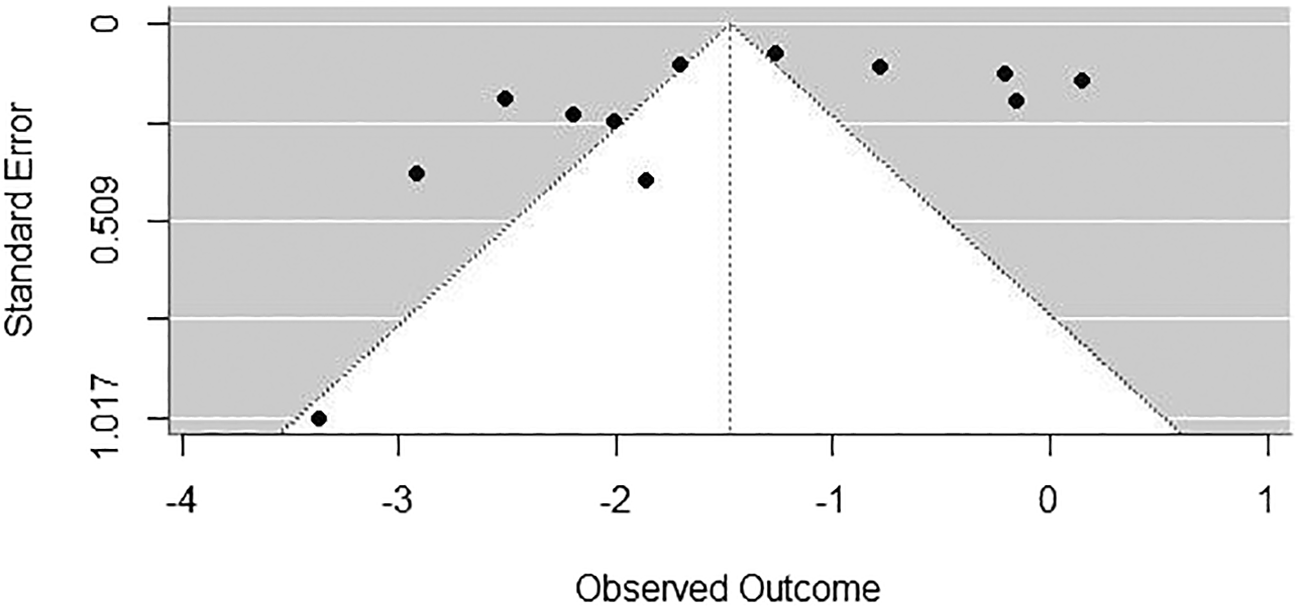
To assess potential publication bias, Begg’s rank correlation test produced a Kendall’s tau of -0.0606 (p = 0.8406), indicating no significant evidence of publication bias. However, Egger’s weighted regression test showed some evidence of funnel plot asymmetry (z = -2.2857, p = 0.0223), suggesting possible publication bias or small-study effects influencing the meta-analysis results. The funnel plot ( Figure 3) showed asymmetry, and the limit estimate as the standard error approaches zero was calculated as b = -0.7147, with a confidence interval ranging from -1.5346 to 0.1052.
A sensitivity analysis was performed to assess the influence of individual studies on the overall meta-analysis results. Excluding each study sequentially had no significant impact on the pooled prevalence estimates, confirming the robustness of the findings. The consistency of the pooled estimates, even after excluding individual studies, indicates that no single study had a disproportionate effect on the overall prevalence of Listeria in fresh produce ( Figure 4).
Meta-regression analysis was performed to explore the influence of potential moderators on Listeria prevalence. The total sample size was not found to significantly affect the pooled prevalence (p = 0.8289), suggesting that other factors may be driving the observed heterogeneity. Further research is necessary to identify additional moderators that could explain the variations in Listeria prevalence across different studies.
This meta-analysis aimed to consolidate current knowledge regarding the prevalence of Listeria spp., particularly Listeria monocytogenes, in fresh produce. Analyzing data from 12 studies, our findings reveal significant heterogeneity (I2 = 97.79%, p < 0.0001), underscoring the variability of Listeria prevalence across different geographical regions, types of produce, and study methodologies. Such variability aligns with previous research, which has noted challenges in accurately estimating the prevalence of Listeria due to diverse sampling techniques, detection methods, and agricultural practices (Farber and Peterkin, 1991; Swaminathan and Gerner-Smidt, 2007; Gombas et al., 2017).
The substantial heterogeneity observed suggests that factors such as geographical region, type of produce, and study design significantly influence Listeria prevalence rates. This finding corroborates Beuchat (2002), who highlighted that environmental conditions and region-specific agricultural practices can affect the presence and proliferation of Listeria within the food supply chain. Our attempts at subgroup analysis, albeit limited by methodological constraints, hint at a complex interplay among these factors, which warrants further investigation (Rodriguez-Lopez et al., 2020; Melero et al., 2021).
The contrasting results from Begg’s rank correlation test (p = 0.8406) and Egger’s weighted regression test (p = 0.0223) provide intriguing insights into potential publication bias or small-study effects. While Begg’s test did not indicate significant publication bias, Egger’s test suggested funnel plot asymmetry, implying that smaller studies reporting higher prevalence rates may be more likely to be published. This observation is consistent with existing literature, which has documented a tendency to underreport studies with null or negative results (Easterbrook et al., 1991; Todd et al., 2009).
Our sensitivity analysis confirmed the robustness of the pooled prevalence estimates, indicating that no single study disproportionately influenced the overall findings. This robustness is critical for the reliability of meta-analytical conclusions, especially in fields where outcomes can vary widely due to methodological differences (Higgins and Green, 2011; Di Ciccio et al., 2022).
The meta-regression analysis revealed that the total sample size did not significantly moderate Listeria prevalence, suggesting that other, unexamined factors may be at play. This finding aligns with the understanding that the dynamics of foodborne pathogens are influenced by a complex array of factors beyond simple demographic or quantitative measures (Carlin et al., 2013; Nightingale et al., 2005). Recent studies also suggest that biofilm formation by Listeria in food processing environments could contribute to its persistence, further complicating contamination control (Kovačević et al., 2023).
The occurrence of Listeria in fresh produce represents a major public health risk, especially for vulnerable groups such as pregnant women, newborns, and individuals with compromised immune systems (Scallan et al., 2011). Our findings underscore the urgent need for comprehensive surveillance and targeted intervention strategies that consider the specific agricultural practices and environmental conditions unique to each region (Schlech et al., 2022; Lianou et al., 2021).
Future research should focus on disentangling the effects of various potential moderators on Listeria prevalence. Employing more sophisticated statistical models and larger datasets will be essential to overcoming the limitations observed in the current analysis. Additionally, investigating the impact of specific agricultural practices and supply chain processes on Listeria prevalence could yield valuable insights for mitigating contamination risks in fresh produce).
This meta-analysis highlights significant heterogeneity in the prevalence of Listeria spp. in fresh produce, raising critical questions about the factors contributing to this variability. The potential for publication bias and the robustness of our findings, as demonstrated through sensitivity analysis, underscore the complexity involved in estimating the true prevalence of this pathogen. Addressing the challenges posed by Listeria in fresh produce necessitates a multifaceted approach that integrates public health initiatives, agricultural practices, and advanced research methodologies. Effective strategies must be tailored to regional contexts to mitigate the risks associated with Listeria contamination and protect vulnerable populations. This study also revealed the scarcity of elaborate studies on Listeria monocytogenes, giving credence to the importance of this ongoing Nigeria-NRF-sponsored study in this area.
Conception of research idea (AA), Literature review (OF and UO), Research protocol design (AA, AAA and OF), Study appraisal (OF, AA, UO and AAA), Data extraction (OF, and UO), Data analysis and interpretation of results (OF, AA, and OA), Manuscript drafting (OF, AA, UO and OA), and review of the initial and final draft of the manuscript (OF, AA, UO and OA).
Zenodod Repository: PRISMA checklist for ‘Systematic review and meta-analysis of the occurrence of Listeria monocytogenes in fresh produce in selected developing countries’. https://doi.org/10.5281/zenodo.14224887 (Fatunla and Adegoke, 2024).
This dataset is licensed under the Creative Commons Zero v1.0 Universal license, allowing for sharing and adaptation with appropriate credit.
Zenodo - Systematic review and meta-analysis of the occurrence of Listeria monocytogenes in fresh produce in selected developing countries. https://doi.org/10.5281/zenodo.14224887 (Fatunla and Adegoke, 2024).
This project contains following datasets:
1. Supplementary Material 5_Listeria Review AbstractK Screening.pdf
2. Supplementary Material 6_CRD42024521833.pdf
3. SUPPLIMENTARY MATERIALS 4 LIST OF INCLUDED STUDIES.docx
This dataset is licensed under the Creative Commons Zero v1.0 Universal license, allowing for sharing and adaptation with appropriate credit.
The authors acknowledge the Nigerian National Research Fund (NRF), University of Uyo and Durban University of Technology for their support.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)