Keywords
Cecal ligation & puncture, inflammatory mediators, Montelukast, NF-κB signaling cascade, Sepsis associated acute kidney injury
This article is included in the Advances in Fibroblast Research collection.
Sepsis-associated acute kidney injury is ubiquitous among patients with critical conditions and contributes to high mortality rates. SA-AKI was experimentally elicited in murine models via cecal ligation and puncture.
This study aimed to determine the possible protective effects of montelukast on sepsis-induced acute kidney injury in a mouse sepsis model.
Albino male Swiss mice (n = 40) were allocated into four distinct groups: (i) normal group, (ii) CLP group, (iii) vehicle group, and (iv) Cecal Ligation and Puncture + Montelukast group (20 mg/kg one hour before Cecal Ligation and Puncture). Blood and tissue biochemical/routine indicators, renal function, Sepsis-associated acute kidney injury -related pathophysiological processes, and nuclear factor kappa B (NF-κB) p65 gene expression in septic mice were assessed by histological hematoxylin and eosin (H&E) staining, immunohistochemical (IHC) staining, quantitative reverse transcription polymerase chain reaction, and Enzyme-Linked Immunosorbent Assay.
The findings highlight that Montelukast reversed CLP-induced increases in serum blood urea nitrogen, creatinine (Cr), and kidney injury molecule levels. It also significantly inhibited elevated concentrations of interleukin (IL)-1β, tumor necrosis factor alpha (TNF-α), F2-isoprostane, and caspase-3 in renal tissues. Additionally, NF-κB protein levels were notably lower in the CLP+ montelukast group than in Cecal Ligation and Puncture group P<0.001. In addition, montelukast significantly mitigated extensive tubular damage in the murine sepsis group p<0.001.
These findings indicate that montelukast may serve as a promising therapeutic agent for sepsis-induced AKI.
Cecal ligation & puncture, inflammatory mediators, Montelukast, NF-κB signaling cascade, Sepsis associated acute kidney injury
Sepsis is a life-threatening condition caused by infection-induced organ dysfunction syndrome.1 Sepsis is a life-threatening medical condition that occurs when an infection leads to systemic impaired function of the tissues and organs in response to this infection, leading to immunosuppression.2 This is often due to an uncontrolled immune response, cytokine storm, and oxidative stress that cause multiple organ failure, eventually leading to death.3 They frequently result from microorganisms, such as bacteria, viruses, and fungi, which can lead to organ dysfunction in most cases.4 Sepsis is related to several morbidities in the heart, kidney, liver, and central nervous system.5 Acute Kidney Injury is a common outcome associated with the clinical scenario of sepsis is Acute Kidney Injury.6 The pathogenesis of acute kidney injury in sepsis is multifaceted. Among these contributing factors, oxidative stress and inflammation are pivotal etiological agents of septic Acute Kidney Injury.7 Sepsis-associated acute kidney injury originates from intricate, heterogeneous mechanisms that culminate in renal injury. These mechanisms may arise directly from the infectious agent and the corresponding host immune response or may represent indirect ramifications of sepsis or its therapeutic intervention. Various pathophysiological mechanisms may interact and participate in Acute Kidney Injury in patients with sepsis, including systemic and renal inflammation, microcirculatory anomalies, microcirculatory dysfunction, metabolic reprogramming, mitochondrial impairment, and dysregulation of the renin-angiotensin-aldosterone system (RAAS).8 Inflammation, recognized as a critical element in sepsis, appears to significantly influence the pathogenesis of Sepsis-associated acute kidney injury. Pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) can stimulate toll-like receptors (TLRs). TLR-2 and TLR-4 are expressed on the surface of tubular epithelial cells within the renal system.9 The engagement of TLR-2 and TLR-4 initiates a cascade of inflammatory responses, which is marked by the secretion of pro-inflammatory cytokines including IL-1α, IL-6, IL-8, and TNF-α.10
The current investigation was conducted using a cohort of 40 albino Swiss mice, with a weight spectrum of 25–30 g and an age range of 8–12 weeks. All euthanizing procedures were conducted under a combination of ketamine- and xylazine-based anesthesia, with diligent efforts made to mitigate individual distress. The experimental methodologies and protocols employed in this study were approved on August 29, 2024, under reference number (20553) from the ethics committee for the care and utilization of laboratory animals.
The current study was done with a total of 40 albino Swiss mice, aged 8-12 weeks and weighing 25-30 g, obtained from the College of Science, University of Kufa. These animals were housed in the animal house within their cages under 12:12 light: dark cycles with 25°C room temperature, 60-65% humidity, and free access to water and libitum. All animal procedures were conducted based on the guidelines of the Kufa University and were approved by the Institutional Animal Care and Use Committee (IACUC). The experimental protocol was reviewed and approved by the Research Ethics Committee, Faculty of Pharmacy, Kufa University under approval number (2055 on August 29, 2024). All efforts were made to minimize animal suffering, encompassing the use of appropriate anesthesia (xylazine of (20mg/ml) and ketamine of 100mg/ml), and animals were monitored closely throughout the study. Humane endpoint and euthanasia protocols were strictly followed through cardiac puncture following intraperitoneal injection of thiopental sodium (50 mg/kg) to induce deep anesthesia to ensure a painless and ethical procedure.
In the current study, Cecal Ligation and Puncture used in earlier studies, including those performed by Refs. 11, 12, was employed to induce sepsis in animals. It is introduced by making a midline incision measuring 1.5 cm while the subject is under general anesthesia, wherein xylazine of (20 mg/ml) and ketamine of 100 mg/ml are mixed (2:1) and administered.13 The cecum was ligated, situated below the ileocecal junction, and punctured twice with a cutting cannula to inflict kidney organ damage during the first 24 h of acute sepsis. Subsequently, the puncture hole was used to squeeze a tiny amount of fecal material from behind the perforation site. Thereafter, the anterior abdomen was sutured to prevent leakage. Sham mice were subjected to the same procedures, except that Cecal Ligation and Puncture was not performed.
Mice were classified into the subsequent four distinct groups (n=10):
Sham group: Evidently, mice exhibited no apparent signs of disease.
CLP cohort: Mice belonging to this cohort experienced a Cecal Ligation and Puncture surgical procedure.
Vehicle group: Murine classified within this category was administered an equivalent volumetric measurement of the solvent dimethyl sulfoxide (DMSO) intraperitoneally; Cecal Ligation and Puncture was performed after 1 h, and then the animals were euthanized after 24 h.
Montelukast group: Mice affiliated with this group received montelukast 20 mg/kg intraperitoneally14; Cecal Ligation and Puncture was performed after 1 h, and then the animals were euthanized after 24 h.
After 24 h, the mice were euthanized under anesthesia and blood was collected using the direct heart puncture method. Blood was left in a gel tube rack for approximately 20 min to allow for clot formation, after which it was centrifuged at 10,000 rpm for approximately 10 min. Then, the supernatant was retained at -20 °C for sequences of biochemical assessment.15 The right kidney tissues were fixed in 10% formaldehyde for histological investigation (H&E) and IHC analysis,16 whereas the left kidneys of different groups were collected and divided into two sections. The first section was homogenized in cold phosphate-buffered saline (PBS) (pH 7.4), and the supernatants were used for Enzyme-Linked Immunosorbent Assay (ELISA). The remainder of the tissue (1/3) was immersed in the TRIzol reagent for gene expression measurement. For analysis, the tissue homogenate was stored at -80°C.17
After fixation with 4% paraformaldehyde for 24 h, the right kidney was embedded in paraffin. H&E and periodic acid-Schiff (PAS) reagents were used to stain 4 μm thick sections cut from the renal tissue wax blocks. Tissue damage was scored as follows: 0, no harm; 1, 0–25; 2, 25–50; 3, 50–75; 4, >75%.18
BUN and Cr concentrations were measured using a Biolis colorimetric assay kit (Biolis, Tokyo, Japan).
ELISA kit instructions (Sunlong, China) were followed while processing tissue samples kept in a refrigerator at -80°C for evaluation of TNF-α, IL-1β, F2-isoprostane, and caspase-3, in addition to measurement of serum KIM-1 levels. Absorbance was measured at 450 nm using a microplate reader to create a standard curve and determine the concentration.
Paraffin-embedded blocks of the renal sections were studied after xylene deparaffinization; the rehydration step of these sections was performed by decreasing the alcohol concentration. Then, blockade of Peroxidase activity was blocked with H2O2, while protein blockers were used for non-specific binding sites, primary antibodies against NF-κB (rabbit NF-κB antibody, 1:200) were incubated at 4°C overnight, followed by the addition of a secondary antibody (biotinylated antibody) done at 37°C for 30 min. HRP was added at the same time. Finally, each slide was treated with the chromogen (100μl/slide) for 15 min. All sections were counterstained with hematoxylin. Positive immunostaining was visualized as brown granules contained in the cytoplasm.19
Total RNA was extracted using TRIzol reagent, according to the manufacturer’s instructions. We used an ABI 7500 real-time PCR machine and the Power SYBR Green PCR master mix to run real-time PCR in triplicate on cDNA produced by a reverse transcription reaction. Table 1 displays the primer sequences used, with GAPDH as the internal control. The 2^-∆∆Ct technique was used to determine the relative expression levels of target genes.
This study utilized version 9.3.1 of GraphPad Prism for statistical analysis https://www.graphpad.com/demos/. One-way analysis of variance (ANOVA) was used to examine differences across groups. Subsequently, the Bonferroni method for multiple comparisons was used to conduct post-hoc tests. All tests were deemed statistically significant when P was less than 0.05. All data are presented as the mean ± SEM.
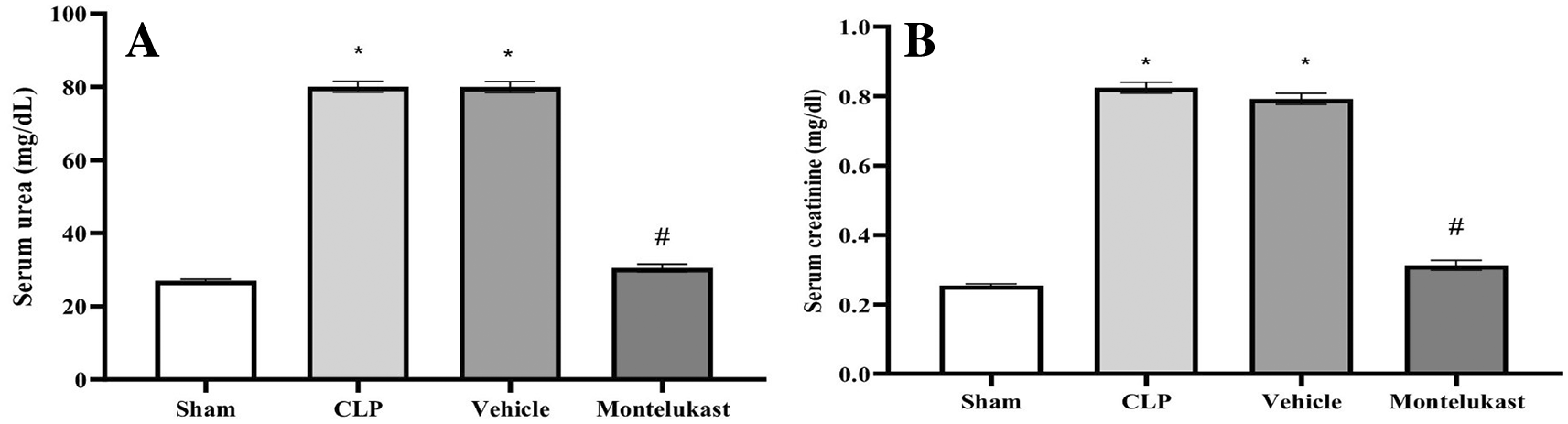
The results indicated that the CLP cohort had remarkably elevated serum BUN ( Figure 1A) and Cr ( Figure 1B) levels, in contrast to the sham cohort. Additionally, the BUN and Cr levels in the montelukast group were significantly lower than those in the Cecal Ligation and Puncture (CLP) cohort.
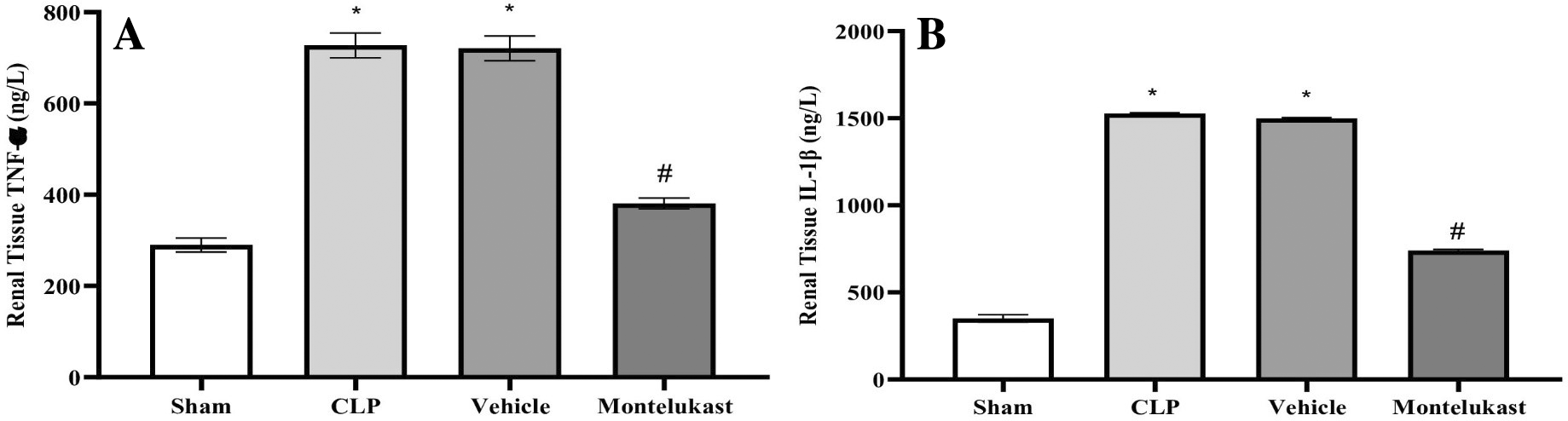
According to these findings, the Cecal Ligation and Puncture cohort exhibited a notably heightened level of TNF-α ( Figure 2A) and IL-1β ( Figure 2B), which was in stark contrast to the sham cohort. Furthermore, TNF-α plus IL-1β concentrations in the montelukast cohort were significantly lower than those in the CLP cohort.
The results indicated that caspase-3 tissue levels in the Cecal Ligation and Puncture cohort were remarkably higher than those in the sham cohort. Additionally, the montelukast group had significantly lower levels of caspase-3 than the Cecal Ligation and Puncture group Figure 3.
The results indicated that Cecal Ligation and Puncture cohort had a significantly higher concentration of F2-isoprostanes than the sham cohort. Additionally, the cohort administered montelukast exhibited significantly reduced concentrations compared to the CLP cohort Figure 4.
The results indicated that the CLP cohort had remarkably higher serum KIM-1 levels than the sham cohort. Additionally, the KIM-1 levels in the montelukast group were significantly lower than those in the Cecal Ligation and Puncture group Figure 5.
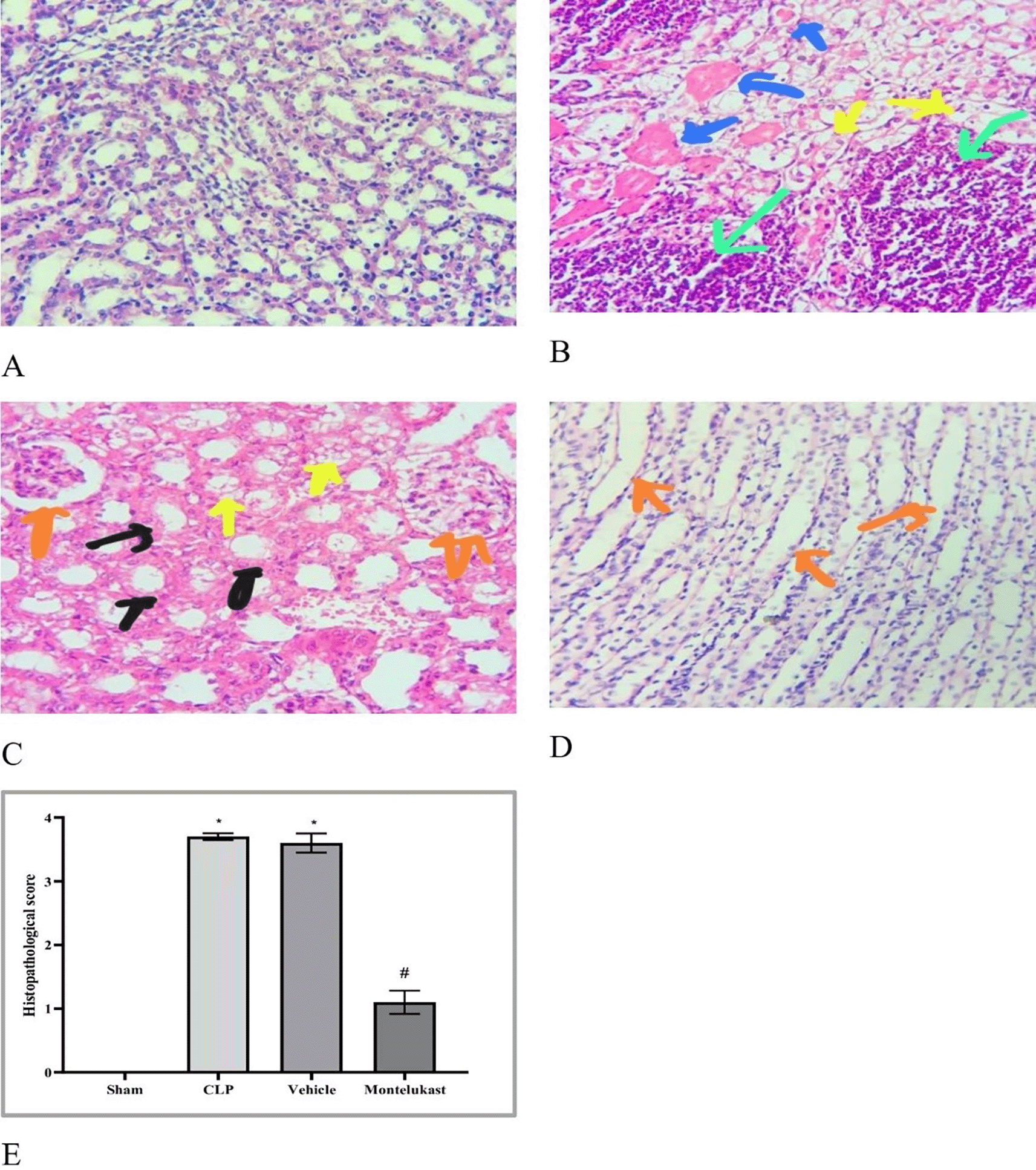
As illustrated in Figure 6, significant pathological alterations were observed in both Cecal Ligation and Puncture and vehicle cohorts. Nevertheless, the nephroprotective effects induced by Cecal Ligation and Puncture were markedly ameliorated by pretreatment with montelukast.
As shown in Figure 7, the CLP group had a lower DCT than the sham group, indicating a significant increase in the NF-κB p 65 gene mRNA expression. Additionally, there was a substantial DCT surge in the montelukast group compared to the CLP group, representing a decrease in NF-κB p 65 gene mRNA expression.
Figure 8 illustrates the intensity of positive NF-κB p 65 staining in renal tissue, in addition to negative staining, as follows:
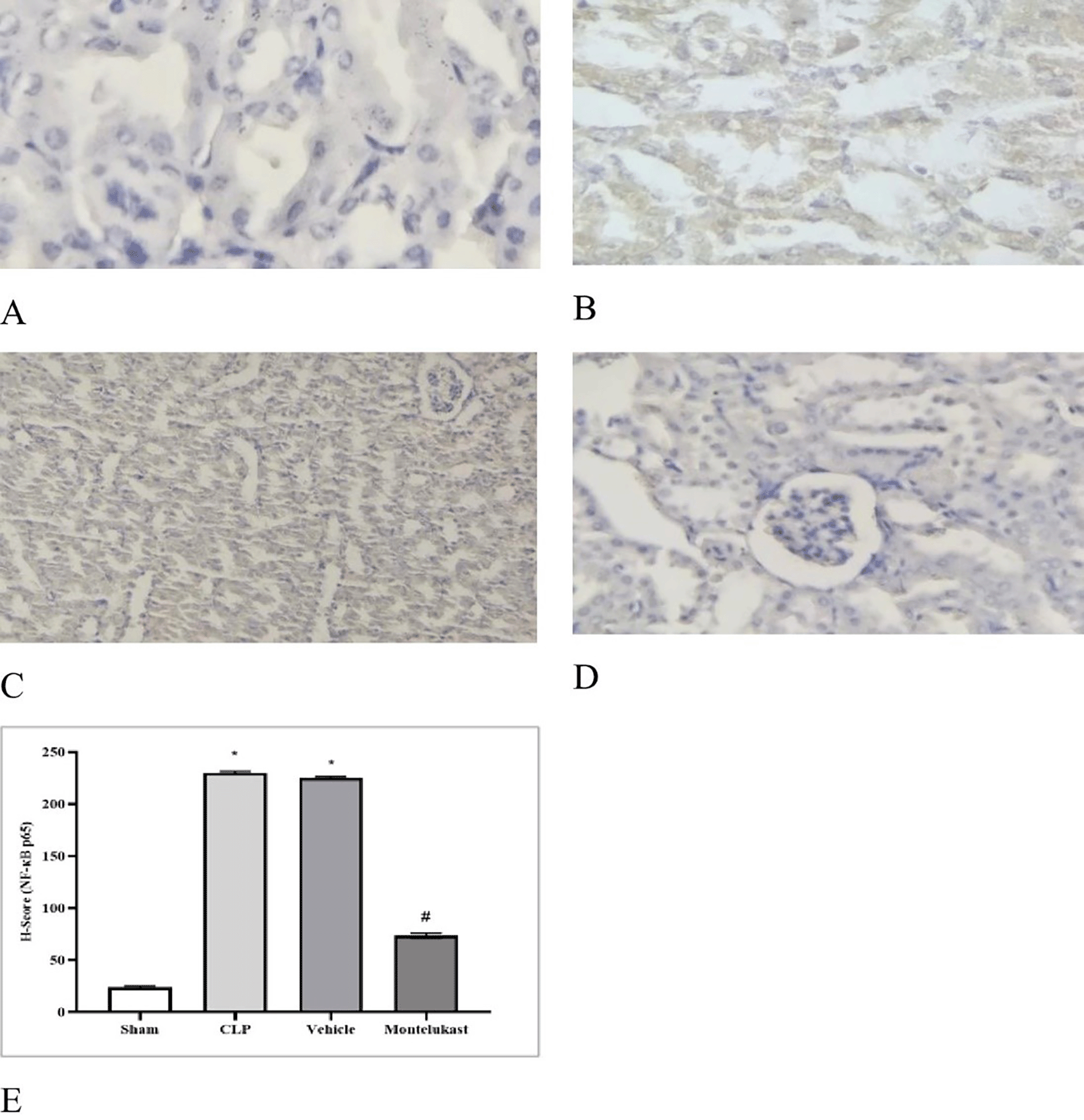
Polymicrobial sepsis is a life-threatening condition characterized by dysfunction of multiple organs resulting from an aberrant response of the body to microbial invasion.20 Several experimental and clinical investigations have shown that the immunosuppressive state induced by sepsis is typified by decreased antimicrobial effector functionalities, thereby increasing vulnerability to infections.21 Immunosuppression associated with sepsis is multifaceted and is believed to arise from compromised cytokine production and reduction in the phagocytic capabilities of myeloid cells. The present investigation revealed that the tissue concentration of the pro-inflammatory cytokine TNF-α was markedly elevated in the Cecal Ligation and Puncture cohort compared to that in the ostensibly healthy cohort. This investigation corroborates previous findings,22 which demonstrated that in sepsis models, the concentrations of proinflammatory cytokines, particularly TNF-α, were elevated in CLP mice and that increased concentrations of TNF-α correlated with mortality outcomes. In addition, this result was consistent with a previous study23 showing that renal ischemia-reperfusion injury (RIRI) is a major cause of AKI, characterized by significant inflammation that exacerbates tissue damage. Additionally, the level of tissue TNF-α was significantly lower in the montelukast group than that in the Cecal Ligation and Puncture group. Our results were confirmed by an investigation conducted by Khodir et al., which provided evidence suggesting that montelukast may confer a protective effect on the heart in the context of LPS-induced cardiac injury in rat models.24 These findings imply that the anti-inflammatory effects of montelukast may be ascribed to the attenuation of pro-inflammatory mediators.25 Furthermore, our study revealed a notable increase in the tissue levels of IL-1β in the CLP cohort compared to the sham group. This work concurs with a prior study conducted by Ibadi et al. in exploring the lung damage induced by CLP, and found that the Cecal Ligation and Puncture procedure caused a significant surge in pro-inflammatory cytokine levels, IL-1β.26 Another study investigated the effects of resveratrol on IRI in rats. They found that the level of IL-1β became altered (increased significantly) in ischemic rats.27 On the other hand, the present study, regarding the influence of montelukast on the concentration of IL-1β, demonstrates that the levels of tissue IL-1β were considerably diminished in the montelukast cohort, in contrast to CLP. Our study is in agreement with those visualized by Ref. 25 and found that compared to the LPS cohort, management with montelukast lessened the surge in serum IL-1β concentration. In addition, the current study found that the CLP and vehicle groups had significantly higher tissue levels of caspase-3 than the sham group. Similar results showed that the sepsis group had higher caspase-3 levels than the normal physiological state.28 Moreover, this work concurs with a prior study that examined renal damage induced by RIRI. They found that RIRI caused a significant surge in the kidney marker of apoptosis, caspase-3, compared to the sham group.29,30 Additionally, in the present study, concerning the effect of montelukast on the level of caspase-3, the concentration of renal caspase-3 was remarkably lowered within the montelukast cohort, contrary to the CLP group. To the best of our knowledge, no prior study has investigated the pharmacological effects of montelukast on caspase-3 during sepsis. This finding could be ascribed to the ability of montelukast to reduce the levels of NO and renal HO-1. Montelukast effectively mitigated elevated concentrations of ET-1, MCP-1, and TNF-α. These effects were associated with a reduction in caspase-3 expression in the kidneys, confirming its anti-apoptotic activity.31 Moreover, the current investigation found that the CLP and vehicle cohorts had considerably higher kidney tissue concentrations of F2-isoprostane than the sham cohort. This study supports another study that established a notably higher concentration of the oxidative stress marker (F2-Isoprostane) within the sepsis cohort than in the sham cohort.32 In the present study, regarding the impact of montelukast on the expression of F2-isoprostane, it was observed that montelukast markedly reduced the concentration of F2-isoprostane within kidney tissue compared to the Cecal Ligation and Puncture group. Additionally, our work is compatible with,33 which examined whether montelukast possesses the potential to conserve lung function during instances of polymicrobial sepsis. The results of this study showed that mice subjected to sepsis exhibited a notable increase in the level of F2-isoprostane within lung tissues, in stark contrast to the sham cohort. Compared to the sepsis cohort, the administration of montelukast resulted in a significant reduction in F2-isoprostane levels in the lung tissue. This finding can be attributed to the montelukast inhibitory effect of the NF-κB/NLRP3 pathway in which NLRP3 can induce reactive oxygen species production.34 Furthermore, the current study showed that the mRNA expression of KIM-1 was remarkably higher in the Cecal Ligation and Puncture cohort than in the sham cohort. This work agrees with a previous study that confirmed that the mRNA levels of KIM-1 in Lyn mice that underwent sepsis were remarkably higher than those in sham mice.35 In addition, the current study aligns with another research effort that established the upregulation of inflammatory marker KIM-1 in both the RIRI and vehicle groups when compared to the sham group.29–30 In the present investigation, concerning the impact of montelukast on the expression of KIM-1, it was observed that montelukast markedly reduced the concentration of KIM-1 in kidney tissue compared to the CLP group. To the best of our knowledge, this investigation represents an inaugural work that elucidates the influence of this pharmacological agent on renal KIM-1 expression within the context of the CLP model of sepsis in mice. This observation may be attributed to the ability of montelukast to inhibit the ERK signaling pathway.36 Collier and Schnellmann posited that the proposed mechanism underlying acute renal injury is initiated by the phosphorylation of STAT3 and signal transducer and activator of transcription (ERK1/2).37 In addition, the present investigation revealed that the mRNA expression levels of NF-κB p65 were notably elevated in the Cecal Ligation and Puncture CLP cohort compared to the sham cohort. This work corroborates findings from a previous study, which indicated that the phosphorylation level of p65 within the renal tissue of the Cecal Ligation and Puncture cohort was significantly increased when juxtaposed with the sham cohort.38 In the present study, concerning the impact of montelukast on the expression levels of NF-κB p 65, there was a notable reduction in NF-κB p65 expression within the renal tissue relative to the CLP cohort. This study is the first to clarify the effect of this pharmacological agent on kidney NF-κB p65 expression in a murine model of Cecal Ligation and Puncture -induced sepsis. This result may be attributed to the ability of montelukast to mitigate IL-1β-induced NF-κBp65 phosphorylation, thereby inhibiting nuclear translocation and subsequent NF-κB activity related to gene expression.39 Moreover, the Cecal Ligation and Puncture and vehicle groups showed remarkable histopathological changes compared to the sham group. The renal tissue of the sham group mice had normal architecture, whereas the kidneys obtained from the mice in the Cecal Ligation and Puncture cohort exhibited signs of hemorrhage, severe inflammation, increased cytoplasmic eosinophilia, eosinophilic casts, and cytoplasmic vacuoles, as well as loss of brush border. These observations are consistent with those obtained by19 in their study on groups of Cecal Ligation and Puncture mice. They reported that Cecal Ligation and Puncture causes inflammation, necrosis, hemorrhage, and degeneration of kidney tissue. Additionally, the Montelukast group showed a significantly lower level of renal tissue injury than did the sepsis group. Our results agree with Khodir and colleagues’ study on rats to examine the renoprotective effects of Montelukast after Cecal Ligation and Puncture.40 This finding can be attributed to their antioxidant and anti-inflammatory properties.
Montelukast safeguarded renal function in septic mice exhibiting AKI by attenuating the release of inflammatory mediators by inhibiting NF-κB-P65 expression. Additionally, it reduces the extent of oxidative stress, thereby improving kidney outcomes. Furthermore, it can inhibit the adverse consequences associated with apoptotic progression following renal injury by modulating the concentrations of apoptotic factors.
The experimental methodologies and protocols employed in this study were approved on August 29, 2024, under reference number (20553) from the ethics committee for the care and utilization of laboratory animals.
Excel data Montelukast. https://doi.org/10.6084/m9.figshare.28599605.v241
Reporting guidelines: arrive checklist. https://doi.org/10.6084/m9.figshare.2915093942
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pharmacology, clinical pharmacy, neuroscience
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 07 Jul 25 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)