Keywords
Lactiplantibacillus plantarum, antibacterial activity, lactic acid bacteria, anti-adhesion, whole genome sequencing
Lactiplantibacillus plantarum is a Gram-positive lactic acid bacterium known for its probiotic benefits, commonly found in fermented foods and mammalian gastrointestinal tracts. L. plantarum strain L47-2 was selected for its in vitro probiotic characteristics.
We investigated its antibacterial activity against pathogenic bacteria using the dual culture overlay method and assessed survival in a mimicked gastrointestinal tract by subjecting the bacteria to pH levels of 1.0–4.0 and bile salt concentrations of 0.1–0.5%. Adhesion to Caco-2 cells was also evaluated. Safety evaluations included phenotypic tests for antibiotic resistance and hemolytic activity, genotypic screening for virulence genes, and whole genome sequencing.
After 4 hours, L47-2 survived well at pH 2.0 (57.0±3.6%) and 3.0 (66.3±2.5%), and maintained 35.0±3.0% survival in 0.4% bile salt. The L47-2 strain demonstrated antibacterial activity against gastrointestinal pathogens and exhibited safety as a probiotic strain. Notably, the L47-2 strain had high autoaggregation (76.3±3.2%) and coaggregation with specific pathogens ranging from 33.1 to 46.7%. Additionally, when compared to the assessed pathogens, the L47-2 strain showed the highest surface hydrophobicity, 68.4±0.4%. This strain exhibited potential adhesion to Caco-2 cells and inhibited the adhesion of all tested pathogens. It was most effective at inhibiting pathogenic bacterial strains rather than competing and displacing them. Pearson correlation analysis revealed significant positive relationships between autoaggregation and coaggregation (P=0.037), autoaggregation and adhesion to Caco-2 cells (P=0.020), and hydrophobicity and adhesion to Caco-2 cells (P=0.016).
The findings shed light on this strain’s probiotic potential and safety, as well as its already established functional capabilities, together with its potential applications as a biopreservative in the food industry and for the prevention and treatment of infectious diseases.
Lactiplantibacillus plantarum, antibacterial activity, lactic acid bacteria, anti-adhesion, whole genome sequencing
Lactic acid bacteria (LAB) are typical microbes in several fermented dairy products. Of these, Lactobacillus is a well-known genus that serves as probiotic and non-pathogenic bacteria in the intestine, inhibiting pathogenic bacteria and stimulating immune cell response.1–3 LAB encompasses various genera, including the revised genus Lactobacillus, as well as Lactiplantibacillus, Limosilactobacillus, Lacticaseibacillus, Leuconostoc, Pediococcus, Streptococcus, and Weissella.4 The potent probiotic qualities balance the microbial ecology in the intestine, preventing diarrheal diseases by defending against harmful intestinal bacteria.5,6 Therefore, the probiotic bacterial properties should include being generally recognized as safe (GRAS), resistance to acidic and bile salt conditions, adherence to intestinal epithelial cell surfaces, antimicrobial activity against enteric bacterial pathogens, and no hemolytic activity or antibiotic resistance.7,8
Recently, LAB have played a role as a bio-preservative in food products. LAB produces bacteriocins-antimicrobial peptides that inhibit Listeria monocytogenes and various strains of Salmonella.9,10 These natural preservatives are becoming more important due to rising concerns over antibiotic resistance and consumer preference for foods with fewer chemical additives.11 Studies have shown that LAB can effectively combat both Gram-positive and Gram-negative bacteria, suggesting that they have potential as safe alternatives in food preservation.9,10 LAB also exhibit immunomodulatory effects, which can enhance human health by regulating immune responses. Research indicates that LAB-fermented products can activate innate immune responses and modulate adaptive immunity, making them beneficial for individuals with compromised immune systems.12 Furthermore, LAB have been shown to inhibit viral infections, such as noroviruses and coronaviruses, through mechanisms that may involve competitive inhibition and modulation of the host’s immune response.13 The therapeutic applications of LAB are expanding beyond traditional uses. Recent advancements include the development of genetically modified LAB strains that can serve as live delivery vectors for therapeutic agents.11 This innovation is particularly relevant in the context of synthetic biology, where LAB are being engineered to produce bioactive compounds with medical applications.11 Integration of artificial intelligence in this field is expected to enhance the efficiency and safety of these microbial systems, leading to novel therapeutic options.
Lactiplantibacillus plantarum, previously known as Lactobacillus plantarum, is a prominent LAB species recognized for its diverse applications in food production and probiotic health benefits.14 This Gram-positive, rod-shaped bacterium is commonly found in fermented foods, the gastrointestinal tracts of mammals, and various environmental settings, including plants and insects.15 Its capability to adapt to different ecological niches is attributed to its robust metabolic versatility and genetic plasticity. L. plantarum cells typically measure 0.9–1.2 μm in width and 3–8 μm in length, often appearing singly, in pairs, or in short chains.15 This species thrives across a broad range of temperatures (15 to 45°C) and pH levels (as low as 3.2), demonstrating significant acid and bile salt tolerance, which facilitates its survival in the human digestive system.15 L. plantarum is also widely acknowledged for its probiotic potential. It exhibits several beneficial traits, including the capability to adhere to intestinal epithelial cells, produce antimicrobial compounds such as bacteriocins, and enhance gut health by modulating the microbiota composition.16–18 The strain’s capacity to withstand low pH environments and bile salts further supports its role as a functional probiotic in various dietary applications.17,18 In the food industry, L. plantarum serves as a starter culture for the fermentation of dairy products, vegetables, and meats.19 It contributes to flavor development, texture improvement, and preservation by producing organic acids and bioactive compounds.19 Additionally, its bacteriocin-producing strains are considered promising natural preservatives because of their capability to inhibit pathogenic bacteria.20 Due to its unique probiotic characteristics, L. plantarum has been recognized as a versatile and important LAB. It can tolerate acidic and bile environments and has antagonistic action against gut infections.19,21 In our previous study,22 L. plantarum strain L47-2 isolated from dairy effluent exhibited anti-Candida albicans activity and colonization, leading to its hypothesized probiotic use. The objective of this study was to evaluate the probiotic characteristics of L. plantarum strain L47-2 in relation to its safe probiotic use in gastrointestinal tract conditions.
Lactiplantibacillus plantarum strain L47-2 was isolated from dairy effluent in our previous study.22 The bacterium was kept at -80 °C in de Man Rogose Sharpe (MRS) broth (Difco Laboratories, Detroit) plus 20% (v/v) glycerol. The L. plantarum strain L47-2 was cultured in MRS medium under 5% CO2 at 37°C, while Bacillus cereus BCC 6386, Salmonella Typhimurium DMST 562, Escherichia coli ATCC 8739, Enterococcus faecalis TISTR 379, Staphylococcus aureus ATCC 6538, and Listeria monocytogenes DMST 17303 were cultured in tryptic soy broth (Difco™, USA) at 37°C.
Antibacterial activity assay
L. plantarum strain L47-2 was investigated for its antibacterial activity against gastrointestinal pathogenic bacteria using the dual culture overlay method, as previously described.23 The bacterial pathogens were B. cereus BCC 6386, E. coli ATCC 8739, S. aureus ATCC 6538, S. Typhimurium DMST 562, E. faecalis TISTR 379, and L. monocytogenes DMST 17303. First, 10 μL of an 18-h culture (1.0 × 105 CFU/mL) of the L47-2 strain was dropped onto the MRS agar plates and incubated in a 5-10% CO2 atmosphere at 37°C for 48 h to allow colonies to develop. One mL of an 18-h culture in tryptic soy broth (TSB) (Difco™, USA) incubated at 37°C (1.0 × 108 CFU/mL) of each pathogenic bacterium was mixed well into 5 mL of soft-tryptic soy agar (0.75% agar) and poured into a plate. The plate was incubated under aerobic conditions at 37°C for 24 h. The diameter of the clear zone was equal to the inhibition zone. This experiment was performed in triplicate.
This assay was slightly modified from a previous study.24 The L47-2 strain (1.0 × 108 CFU/mL) was inoculated into MRS broth with a pH of 2.0–5.0, adjusted with 1 N HCl. Unadjusted pH 6.5 MRS broth was used as a control. The cultures were incubated at 37°C for 0, 1, 2, 3, and 4 h. Colony-forming bacteria were subsequently enumerated on MRS agar. The percent survival was computed as the CFU/ml in MRS broth adjusted for pH at various times in MRS broth at pH 6.5.
This assay was slightly modified from a previous study.25 1.0 × 109 CFU/mL in PBS of the L47-2 strain was prepared, and 0.5 mL of the bacterial suspension was pipetted into 4.5 mL of MRS broth containing 0.1–3.0% (w/v) of oxgall (Sigma-Aldrich, USA) to a final cell density of 1.0 × 108 CFU/mL. MRS medium with no oxgall was used as a control. The inoculum was incubated at 37°C for 0, 1, 2, 3, and 4 h. Bacterial survival was enumerated as the CFU/ml representative of live bacteria. The percentage of survival was calculated by CFU/ml in MRS broth containing oxgall at a specific time/CFU/ml in MRS broth compared to a medium with no oxgall at the same time × 100.
This assay was performed by measuring the adhesion of bacteria to xylene as in a previous study.26 Three mL of each bacterial suspension (1.0 × 108 CFU/mL) in PBS was combined with 1 mL of xylene, and the mixture was incubated for 1 h without shaking. The optical density (OD) at 600 nm at the initial stage was spectrophotometrically measured (Thermo Scientific™, USA) as the absorbance (A0). One mL of the aqueous phase was gently pipetted to measure OD for absorbance (A1). Hydrophobicity was expressed as a percentage using the formula: (1- A1/A0) × 100.
The same procedures as from the earlier research were followed for these assays.27 For the autoaggregation assay, 4 mL of suspended bacterial cells (1.0×108 CFU/mL) in PBS were vortexed for 10 seconds and then allowed to stand at room temperature for 0, 1, 2, 3, 4, 5, 16, and 24 h. Absorbance (A) was measured at 600 nm after transferring 0.1 mL of the top suspension to 3.9 mL of sterile PBS in a separate tube. The formula used to calculate the percentage of autoaggregation was: 1-(At/A0) ×100, where At is the absorbance at a specific time (1, 2, 4, 6, 8, 10, 20, and 24 h) and A0 is the absorbance at time zero.
For coaggregation, equal volumes (1 mL) of L. plantarum strain L47-2 and each cell suspension of pathogens, including L. monocytogenes DMST 17303, E. coli ATCC 8739, E. faecalis TISTR 379, S. Typhimurium DMST 562, S. aureus ATCC 6538, and B. cereus BCC 6386, were mixed in pairs by vortexing for 10 seconds. Coaggregation was measured after incubation at 0, 1, 2, 3, 4, 5, 16, and 24 h. After transferring 0.1 mL of the upper suspension into a 3.9 mL tube containing sterile PBS, the absorbance (A) at 600 nm was measured. At the same time, 4 mL of each bacterial suspension was used as a control. The percentage of coaggregation was computed as [(AL47-2 + Apathogen)/2 - (Amix)/(AL47-2 + Apathogen)/2 × 100].28
The adherence capacity of L. plantarum strain L47-2 and gastrointestinal pathogenic bacteria to Caco-2 cells was performed as in a previous study, with modifications.29,30 The Caco-2 cell line (ATCC® Cat. No. HTB-37™) used in this study was obtained from the American Type Culture Collection (ATCC). Caco-2 cells were cultured in Dulbecco’s Modified Eagle Medium (Gibco BRL, USA) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS) (HyClone, USA) (complete DMEM; cDMEM) and incubated in a 5% CO2 atmosphere at 37°C. 100 μL of a 1.5 × 104 cell suspension/well in a 96-well plate was prepared and incubated overnight at 37°C for the following assays. All assays were conducted in triplicate, and each experiment was performed independently as follows.
Adhesion assay: Caco-2 cells were infected with each bacterium at a Multiplicity of Infection (MOI) of 10:1 (bacteria: cells) and incubated at 37°C under a 5% CO2 atmosphere for 1 h. Non-adherent bacteria were gently washed out 5 times with sterile PBS (Gibco BRL, USA), and the attached bacteria were dislodged by incubating them with 0.1% Triton X-100 (Sigma-Aldrich, USA) for 5 min. All bacteria were counted as colony-forming units (CFU) by serial dilution and colony counting on MRS agar for the L47-2 strain and tryptic soy agar for each bacterial pathogen, respectively, after incubating them at 37°C for 24 h. Bacterial adhesion was computed from %Adhesion = [Adhered bacteria/Total of added bacteria] × 100.
Inhibition assay: Caco-2 cells were first incubated with an MOI of 10:1 of the L47-2 strain for 1 h. Then, each bacterial pathogen at an MOI of 10:1 was subsequently added into the wells and further incubated for 1 h. Non-attached bacteria were removed by washing 5 times with sterile PBS. The bacteria were counted according to the adhesion assay protocol. Inhibition was computed using the formula, %Inhibition of adhesion = (1 - T1/T2) × 100, where T1 and T2 are the percentages of adhesion by pathogens with or without the strain L47-2, respectively.
Competition assay: Caco-2 cells were infected with an equal amount of the L47-2 strain and each pathogen at an MOI of 10:1 and incubated for 1 h. All unbound bacteria were removed, and the attached bacteria were dislodged and counted as performed in the adhesion assay. The percentages of competition were calculated from the adhesion of bacterial pathogens added together with the L47-2 strain relative to adhered bacteria without the L47-2 strain (control).
Displacement assay: Caco-2 cells were infected with each pathogen and incubated for 1 h, after which the L47-2 strain was added and the cells were incubated for another 1 h. Bacterial enumeration was performed as described in the adhesion assay. The percentage of displacement was calculated from adhered bacterial pathogens with or without the L47-2 strain, as described above.
Hemolytic activity assay
A streak plate technique was used to determine hemolytic activity. L. plantarum strain L47-2 was streaked onto sheep blood agar (5% (v/v) of sheep blood) and incubated at 37°C for 2 days in a 5–10% CO2 atmosphere. A zone of hemolysis was observed around the colonies. The β-hemolysis control strain was Staphylococcus aureus ATCC 6538.7
Antibiotic susceptibility profile
This method was performed as previously described.31 An overnight culture of L. plantarum strain L47-2 was adjusted to a cell density of 1.0 × 108 CFU/mL in sterile PBS. Then, 0.1 mL of this adjusted culture was inoculated into 0.9 mL of MRS broth containing several antibiotics (Oxoid, USA), including ampicillin, vancomycin, chloramphenicol, gentamycin, streptomycin, kanamycin, and tetracycline. The antibiotics were in concentrations of 2, 4, 8, 16, 32, 64, 128, 256, 512, and 1024 μg/ml and were incubated at 37°C for 24 h. The CFU/mL count of the bacteria was enumerated by a plate count technique on MRS agar. A bacterial culture in MRS broth without antibiotics was used as a control. The minimal inhibitory concentration (MIC) was the lowest antibiotic concentration that fully suppressed observable growth compared to MRS broth with no antibiotic. The minimal bactericidal concentration (MBC) was determined by transferring an aliquot from each tube of the MIC test to be spread on an MRS agar plate and incubated at 37°C, under 5–10% CO2 for 24 h.
Molecular screening of virulence genes
L. plantarum strain L47-2 was investigated for the presence of thirteen virulent genes as described in a previous study.9 The DNA of the L47-2 strain was extracted with a DNeasy® UltraClean® Microbial Kit (Qiagen, Germany). The primer sets used for gene amplification included cylA, cylB, cylM, cylLS, cylLL, ccf, cob, cpd, efaAfm, efaAfs, esp, gelE, and agg as previously described.32 E. faecalis TISTR 379 was used as a positive control. The PCR reaction was performed using a ThermoCycler (Bio-Rad, USA) in 0.2-ml PCR tubes containing 5.0 μL of 10× PCR buffer, 0.4 μL of 25 mM dNTPs, 0.2 μL of Taq DNA polymerase, 3.0 μL of 25 mM MgCl2, 1.0 μL each of 10 μM forward and reverse primers, 1 μL of 100 ng DNA template and DNase-free water to a 50 μL final volume. PCR amplifications were started at 94°C for 1 min, followed by 35 cycles of denaturation, annealing, and extension (at 94°C for 1 min, 55°C for 1 min, and 72°C for 2 min, respectively) and a final extension at 72°C for 7 min, before being stored at 4°C. The PCR products were electrophoresed at 100 V for 30 min and detected with a gel documentation system (Bio-Rad, USA).
Whole genome sequencing analysis
Genomic DNA was extracted from the L47-2 strain using a QIAamp DNA Mini Kit (Qiagen, Germany) and then subjected to 150-base-read library preparation and sequencing using the Illumina HiSeq PE150 system at Biomarker Technologies. The de novo assembly of short-read data was performed using SPAdes v3.13.1.33 The number of contigs and N50 were assessed by QUAST v.5.0.2 (https://github.com/ablab/quast). Genome completeness and contamination were evaluated using CheckM v.1.2.2.34 The number of contigs, N50, completeness, and contamination of the L47-2 strain assembled genome were 641 contigs, 254,624 bp, 99.38%, and 3.47%, respectively. Bacterial species were identified using the Type (Strain) Genome Server (TYGS) (https://tygs.dsmz.de/ and https://pathogen.watch/). A CARD Resistance Gene Identifier v. 1.2.1 (cite: DOI: 10.1093/nar/gkac920) was used to detect antibiotic resistance genes in the L47-2 genome.
All assays were conducted in triplicate. Cell culture assays were performed in 3 independent experiments, with all results shown as mean ± standard deviation values. The Pearson correlation coefficient was used for the correlation analysis. A student’s t-test was used to compare samples in one group, and one-way ANOVA was employed for more than two groups. The GraphPad Prism software version 6.0 was used for statistical analysis. P < 0.05 was considered statistically significant.
L. plantarum strain L47-2 significantly inhibited the growth of pathogenic bacteria. The inhibitory activity of this strain was ranked based on the size of inhibition zones against the pathogens listed in Table 1.
L. plantarum strain L47-2 was selected for its ability to survive under the acidic and bile salt conditions found in the gastrointestinal tract. The results showed that the L47-2 strain could survive in a pH range of 2.0–5.0 (Figure 1A). The total viable counts at pH values of 2.0 and 3.0 were 57.0 ± 3.6 and 66.3 ± 2.5%, respectively, after 4 h of exposure. The number of viable cells decreased gradually with lower pH values and longer times. Additionally, the L47-2 strain could survive at 0.1–0.4% concentrations of bile salt, showing rates of 58.3 ± 3.5, 47.6 ± 1.5, 44.0 ± 2.0, and 35.0 ± 3.0% (Figure 1B), indicating that the L47-2 strain could survive in a 0.4% bile salt concentration. These findings suggest that the L47-2 strain could survive in the small intestine.
L. plantarum strain L47-2 had the highest hydrophobicity followed by E. coli ATCC 8739, L. monocytogenes DMST 17303, S. Typhimurium DMST 562, S. aureus ATCC 6538, B. cereus BCC 6386, and E. faecalis TISTR 379, with values of 68.4 ± 0.4, 37.0 ± 1.9, 33.7 ± 1.8, 17.6 ± 0.5, 15.0 ± 2.7, 1.8 ± 0.8, and 1.7 ± 0.4%, respectively (Figure 2).
Auto- and co-aggregation assays were used to determine the capabilities of L. plantarum strain L47-2 to aggregate and co-aggregate with individual pathogen strains. As a result, autoaggregation of individual bacterial strains was increased during incubation, rising significantly after 2 h at 37°C and then gradually until 24 h (Figure 3A). Interestingly, the L47-2 strain showed higher autoaggregation, 76.3 ± 3.2%, than all pathogen strains after 24 h, except for L. monocytogenes DMST 17303 and E. coli ATCC 8739. They showed values of 85.2 ± 3.3 and 82.8 ± 5.7%, respectively. B. cereus BCC 6386 exhibited the lowest autoaggregation, 50.3 ± 2.1%.
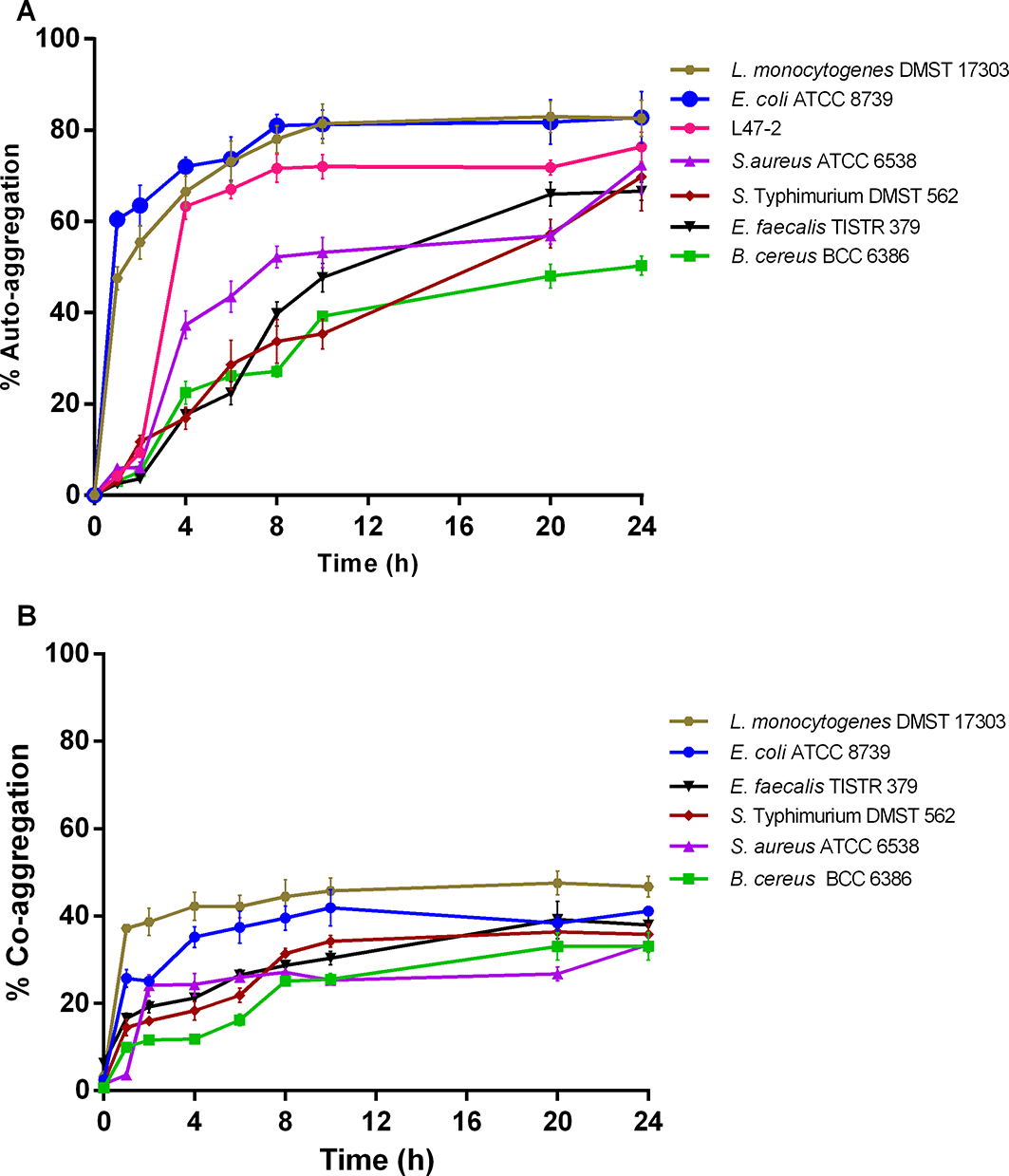
A) Shows autoaggregation of bacterial strains at 37°C and various incubation times. B) Shows coaggregation between the strain L47-2 and individual pathogen strains at 37°C and various incubation times. The data represent the mean ± standard deviation of tests performed in triplicate.
Coaggregation was determined by the capability of the L47-2 strain to coaggregate with individual pathogen strains after 24 h of incubation at 37°C. The results, shown in Figure 3B, indicated that the highest percentage of coaggregation occurred between the L47-2 strain and L. monocytogenes DMST 17303 followed by E. coli ATCC 8739, with rates of 46.7 ± 2.4 and 41.2 ± 1.0%, respectively. The lowest coaggregation occurred between the L47-2 strain and B. cereus BCC 6386, 33.1 ± 3.2%, even though coaggregation increased considerably after 2 h. This result also suggests that the L47-2 strain could coaggregate with the other individual pathogens at levels of 33.1–46.7%. This was dependent on the specific pathogen strain and incubation time. These results also suggest a high percentage of autoaggregation linked to high coaggregation activities, which influenced the L47-2 strain to coaggregate with gastrointestinal bacterial pathogens.
Lactiplantibacillus plantarum L47-2 exhibited the highest adhesion (33.1 ± 3.9%), significantly greater than all tested pathogens ( Figure 4A). Pathogenic bacteria such as B. cereus BCC 6386, E. coli ATCC 8739, S. aureus ATCC 6538, S. Typhimurium DMST 562, E. faecalis TISTR 379, and L. monocytogenes DMST 17303 showed lower adhesion, ranging between 15.6 ± 1 – 28.3 ± 0.8%. P-values indicated that L. plantarum L47-2 adheres significantly better than the pathogens. The L47-2 strain most effectively inhibited the adhesion of B. cereus BCC 6386 (up to ~80%) under the inhibition condition ( Figure 4B). Inhibition is generally higher than competition or displacement across all pathogens ( Figure 4B). The effectiveness of the L47-2 strain in reducing pathogen adhesion varied depending on the pathogen and the test conditions. This suggests its strong potential as a probiotic to prevent pathogen colonization.
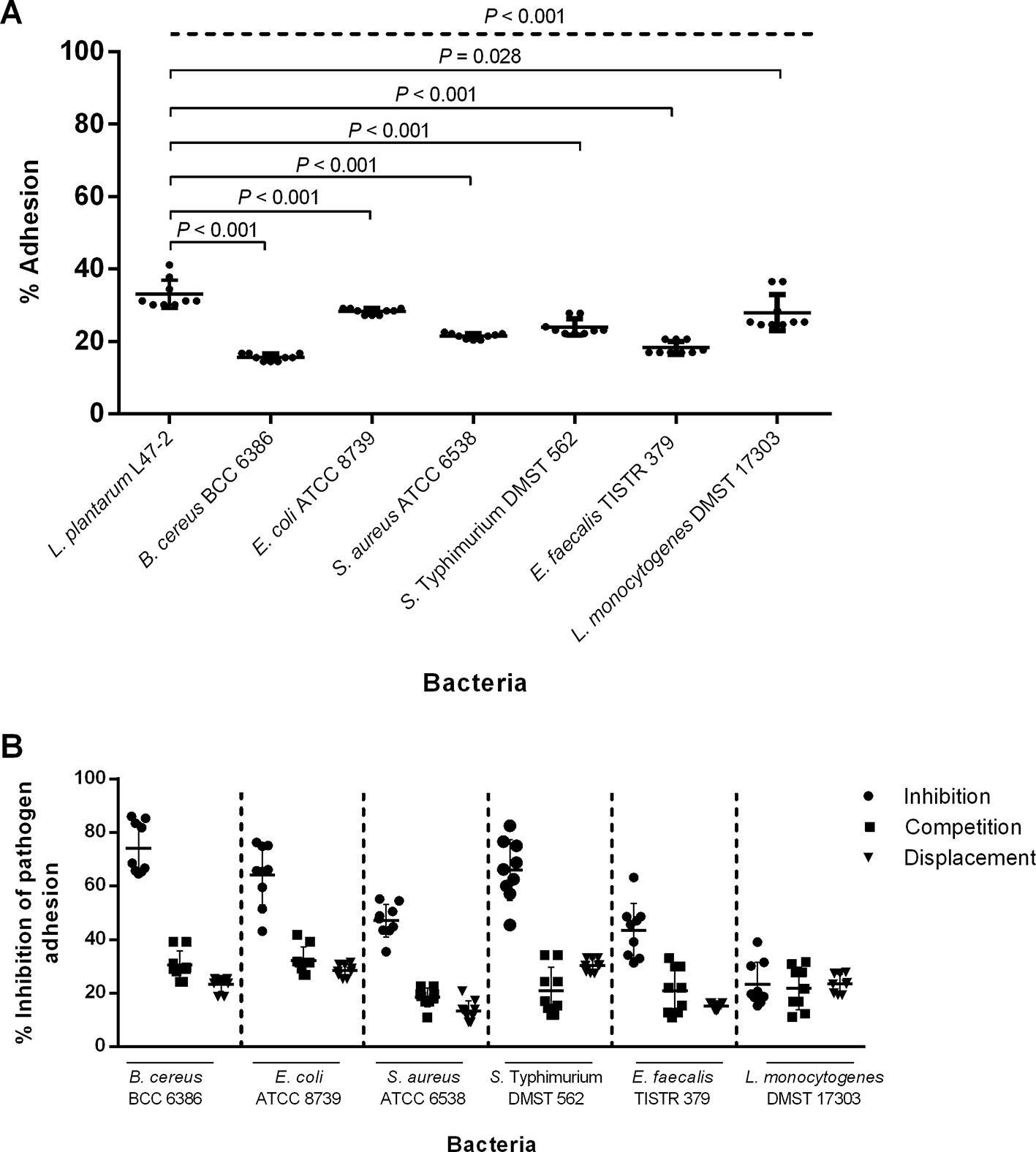
A) Shows bacterial adherence to Caco-2 cells, with the dotted line representing the P-values of the ANOVA test and the solid lines representing the P-values of t-tests. B) Shows the anti-adhesion of the L47-2 strain against pathogens, including inhibition, competition, and displacement. Individual scatter plots were displayed with mean ± standard deviation from three independent experiments, each carried out in triplicate.
Pearson correlation coefficient analysis showed that autoaggregation and coaggregation between the L47-2 strain and each tested pathogen were positively correlated (P = 0.037) ( Table 2). Auto- or co-aggregation and adhesion capabilities were positively correlated (P = 0.020 and P = 0.080, respectively). Furthermore, this study also demonstrated that hydrophobicity and adherence to Caco-2 cells were significantly correlated (P = 0.016). This finding indicates that the capabilities of the L47-2 strain to autoaggregate, coaggregate, and adhere were significantly correlated.
| Assay | Autoaggregation | Hydrophobicity | Coaggregation | Adhesion |
|---|---|---|---|---|
| Autoaggregation | - | 0.676 (0.096) | 0.837 (0.037) | 0.832 (0.020) |
| Hydrophobicity | - | - | - | 0.846 (0.016) |
| Co-aggregation | - | 0.741 (0.092) | - | 0.758 (0.080) |
Hemolytic activity assay
Hemolytic activity of L. plantarum strain L47-2 was tested to confirm the nonpathogenic character of this strain. The results revealed no hemolytic activity of the L47-2 strain with no inhibition zones surrounding colonies on sheep blood agar plates (Figure 5).
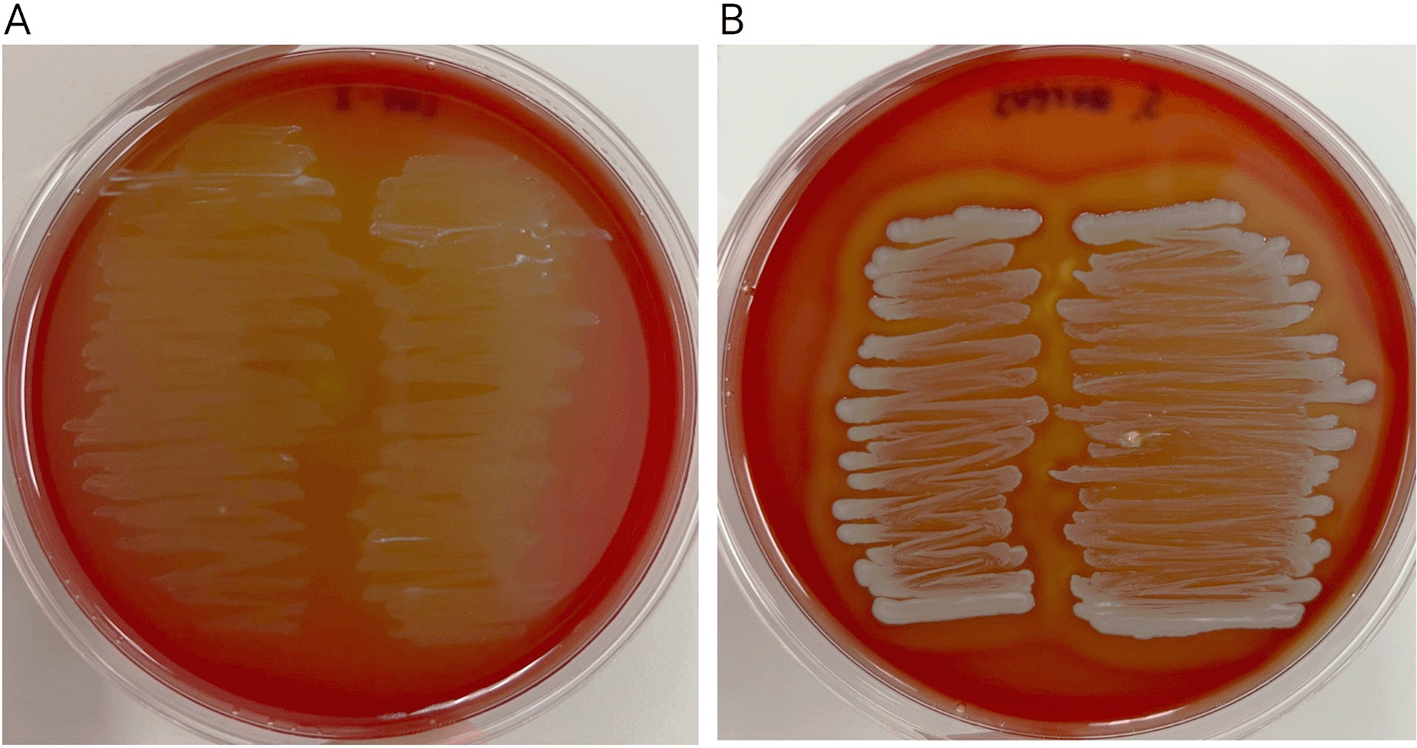
A) Shows no reaction (γ-hemolysis) of the L47-2 strain, and B) Shows a clear zone of β-hemolysis surrounding the S. aureus ATCC 6538 colony.
Antibiotic susceptibility profile and the presence of virulence genes
An antibiotic susceptibility test was applied, and virulence gene contents were determined for the L. plantarum L47-2 strain as a probiotic ( Tables 3 and 4). The results showed that the L47-2 strain was susceptible to ampicillin, tetracycline, chloramphenicol, and gentamycin, whereas it resisted vancomycin, streptomycin, and kanamycin ( Table 3). Antibiotic resistance was considered according to MIC EFSA breakpoints.35 Furthermore, the L47-2 strain was negative for all virulence genes examined, suggesting that it can be safely utilized as a probiotic strain ( Table 4).
The assembly of the raw reads produced bacterial chromosomes, each measuring approximately the same size as those previously documented for sequenced L. plantarum isolates, from 3 to 3.6 Mbp.17 The genome-based taxonomy was identified by the Type (Strain) Genome Server (TYGS) (https://tygs.dsmz.de/ and https://pathogen.watch/). The type strain of L47-2 was Lactiplantibacillus plantarum. The ANI value for L. plantarum L47-2 and L. plantarum ATCC 14917 was calculated, yielding an ANI value between the two strains of 98.8548%, indicating their strong relationship (Figure 6). We detected vancomycin resistance genes (vanH and vanY), which is consistent with the MIC results.
This study’s findings reveal that L. plantarum strain L47-2 has the potential for probiotic use due to its anti-pathogenic bacterial capabilities, survivability in acidic and bile salt conditions, favorable adhesion and anti-adhesion, lack of hemolysis, antibiotic susceptibility profile, and negative results for virulence genes. These factors confirm its safety for host consumption and promising potential for probiotic use.
Antimicrobial activity is a crucial criterion for selecting probiotics as they can inhibit gastrointestinal pathogens. In this study, the L47-2 strain exhibited antagonistic activity against all tested pathogens, in agreement with previous findings.7,26,27,36–42 Furthermore, data from our previous work and other studies showed that LAB could inhibit fungal growth, particularly opportunistic infections in AIDS patients.22,43,44 L. plantarum is classified into the LAB group as it produces secondary metabolites, including lactic acid, and can affect the vital cell functions of other microorganisms.45 Several studies have reported that the antagonistic activity of LAB was dependent on bacteriocin or bacteriocin-like substances, organic acids, hydrogen peroxide, ethanol, 1,3-propanediol, short-chain fatty acids, carbon dioxide, lactic acid, acetic acid, and phenyllactic acid.31,46–48 LAB gains the advantageous capability to prevent infections with these antimicrobial compounds. According to our recent findings and previous work,22 the L47-2 strain has antibacterial and antifungal activities.
Probiotics must survive in acidic and bile salt environments, which is a requirement for their use. The L47-2 strain survived following exposure to pH 2.0 for 4 h, suggesting it may be a good probiotic candidate. These findings are consistent with previous reports7,24,49 which reported that Lactobacillus spp. could survive under acidic and bile salt conditions for 4 h or more, indicating survival while passing through the stomach and reaching the entry of the small intestine, where the pH is 2.0-3.0 and the concentration of bile salt is 0.3%.50,51 The capability of strain L47-2 to survive under bile salt conditions was due to modifications of carbohydrate and glycosidase activity,52 production of exopolysaccharide,53,54 the configuration of proteins and fatty acids on the cell membrane,55 and enhanced adhesion to host mucus membranes.56,57
In this study, the L47-2 strain exhibited the highest percentage of cell surface hydrophobicity, followed by E. coli ATCC 8739 and L. monocytogenes DMST 17303, which is related to the autoaggregation results. The L47-2 strain could coaggregate with each tested pathogen, depending on the bacterial-specific strain and incubation time. Surprisingly, the highest coaggregation activity (46.7 ± 2.4%) was observed between the L47-2 strain and L. monocytogenes DMST 17303, followed by E. coli ATCC 8739 (41.2 ± 1.0%) due to the high autoaggregation activity of these bacteria. The results of this study concur with the premise that autoaggregation and coaggregation capacities are correlated, as reported in previous studies.31,42,58,59 The coaggregation capability of the L47-2 strain beneficially interferes with gastrointestinal pathogen colonization of host intestinal epithelial cells. Additionally, it could inhibit bacterial pathogens by producing inhibitory substances.30,60 The coaggregation results of our work agree with previous studies, which illustrated that coaggregation is strain-specific (probiotic and pathogen) and incubation time dependent.28,58,61
In the current study, the probiotic bacteria’s colonization of the gastrointestinal tract was shown by an adhesion assay. However, this capability is also characteristic of gastrointestinal pathogenic bacteria. The current study suggests that the L47-2 strain has the most significant adhesion capability at the surface of Caco-2 cells compared to the tested pathogens. Bacterial adhesion is a complicated mechanism where the bacterial cell membrane communicates with the contacting surfaces.62 However, all bacterial pathogens could adhere to the surfaces of Caco-2 cells, indicating their capability to penetrate the human intestinal mucosa. Factors affecting the Lactobacilli’s adhesion to Caco-2 cells were their proteinaceous components, carbohydrate moieties, and surface proteins.63–66 The obtained results in this study show that the L47-2 strain could protect Caco-2 cells from adhesion by all tested pathogenic bacteria, with inhibition as the most effective mechanism rather than competition and displacement. This suggests the potential use of the L47-2 strain in preventing bacterial infection in the gastrointestinal tract.
The correlation coefficient revealed that autoaggregation is significantly correlated with coaggregation (P = 0.037) and adhesion to Caco-2 cells (P = 0.020). This implies that autoaggregation and adhesion capabilities are linked to bacterial attachment to intestinal cells.67,68 Hydrophobic contacts are powerful non-covalent interactions and are regarded as critical factors in facilitating microbial adherence to host epithelial cells.58,59 As expected, higher hydrophobicity was found to be associated with increased autoaggregation, indicating high adhesion to the cell surface as described in a previous study of L. plantarum strains.69 Nonetheless, bacterial adhesion to the host cell surface depends on multifactor interactions and other factors, for instance, exopolysaccharides, S-layer proteins, mucus-binding proteins, lipoteichoic acid, and mannose-specific adhesions, which contribute to adhesion to host epithelial cells.30,70
The safety of the L47-2 strain for probiotic use was determined using antibiotic susceptibility profiles, hemolysis, as well as the presence of virulence genes. In this study, the L47-2 strain was susceptible to ampicillin, tetracycline, gentamycin, and chloramphenicol, which concurs with previous research.48,54 Kwon et al. (2021) reported that Lactiplantibacillus plantarum Q180 is sensitive to gentamycin, tetracycline, ampicillin, clindamycin, erythromycin, kanamycin, and chloramphenicol.71 However, an intrinsic resistance of Lactobacillus sp. to vancomycin has also been reported.72
The L47-2 strain has none of the 13 virulence genes according to PCR results, indicating its safety for probiotic use. The agg gene encodes an aggregation protein for adherence to eukaryotic cells; gelE encodes an extracellular metalloendopeptidase that involves hydrolysis of hemoglobin, collagen, gelatin, and other bioactive substances; and the esp is a cell wall-associated protein connected with immunological evasion.73 efaAfs and efaAfm are cell wall adhesion molecules.74 ccf, cob, and cpd encode for chemotactic attraction to human leukocytes and enable conjugation.9 The cylLL, cylLS, cylLM, cylB, and cylA genes are cytolysin (hemolysin-bacteriocin) precursors that express the formation of active cytolysin, which is highly poisonous to human and bacterial cells.73 Further research into in vivo anti-adhesion and immunomodulatory effects of the L47-2 strain would be beneficial in advancing our understanding of this strain’s potential as a promising probiotic for humans.
The commercialization prospects for new probiotics, particularly next-generation probiotics (NGPs), are promising due to several key factors influencing the market dynamics. This growth is driven by rising consumer awareness regarding gut health, increased demand for functional foods, and a shift towards preventive healthcare. Next-generation probiotics are designed not only for traditional dietary uses but also for therapeutic applications. They show promise in treating various chronic ailments and are being tailored for personalized therapies.75 This innovation aligns with trends in the healthcare sector focusing on individualized medicine, which could enhance their market acceptance and commercial viability.
Despite the positive outlook, there are challenges to commercialization of NGPs, including strain selection, survivability, and regulatory hurdles.75 Overall, Lactiplantibacillus plantarum represents a highly adaptable and beneficial microorganism with significant implications for food science and human health. Its extensive use in fermentation processes and probiotic formulations underscores its importance in both traditional and modern food systems. Further research into its genomic diversity and functional properties reveals new potential applications for this versatile bacterium.16–19
These findings show that L. plantarum L47-2 may be deemed safe as a probiotic strain. More trials are necessary to determine the acute and subacute toxicity, immunotoxicity, embryotoxicity, and other properties of these probiotic bacteria in preclinical investigations for medicinal applications.
These findings indicate that L. plantarum strain L47-2 has potential for probiotic use due to its relevant antimicrobial activities, survival in acidic and bile salt environments, as well as its anti-adhesion to the host cells of all pathogens studied, and the absence of harmful characteristics. This study conducted a safety assessment of L47-2 utilizing phenotypic and genomic analyses. Moreover, the L47-2 strain exhibited an antipathogenic effect against six pathogenic bacteria. Based on our thorough safety evaluation, we have determined that the L. plantarum strain L47-2 can be used as a probiotic food supplement. However, additional comprehensive functional studies are needed to clarify the health benefits associated with this strain.
The raw sequence data of L. plantarum strain L47-2 has been deposited in the European Nucleotide Archive (ENA) under the accession number PRJNA1220260 (http://identifiers.org/ena.embl:PRJNA1220260). The sample accession number is SAMN46715021 (http://identifiers.org/ena.embl:SAMN46715021).
We are grateful to the staff at the Department of Microbiology, Faculty of Science, Kasetsart University.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Probiotics, Food microbiology, Whole-genome sequenceing
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Lactic acid bacteria
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 07 Jul 25 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)