Keywords
Animal Feed, Black Soldier Fly, Circular Economy, Drying Methods, Sustainability.
Black Soldier Fly (BSF) larvae is a promising alternative source for animal feed due to their ability to convert organic waste into high-quality biomass rich in essential amino acids and beneficial fatty acids. However, the post-harvest processing method is crucial affecting nutritional value. This study evaluated the effects of five processing methods (air-drying, sun-drying, oven-drying, microwave-drying, and roasting) on the amino acid and fatty acid profiles of BSF larvae reared on rice bran, milk waste, and slaughterhouse waste combined substrate.
BSF larvae were reared for seven days on a combined substrate consisting of rice bran, milk waste, and slaughterhouse waste. After harvesting, the larvae underwent five different drying treatments until a constant weight was achieved. The dried larvae were ground into powder and analyzed for amino acid and fatty acid profiles.
BSF larvae meal contained substantial amounts of essential amino acids, particularly lysine (1.76–2.41%), leucine (1.46–2.11%), and valine (1.41–1.77%). Fatty acid profiles of BSF larvae meal were predominantly composed of lauric acid (22.07–24.88%), palmitoleic acid (22.84–27.89%), and linolelaidic acid (20.95–23.86%). Microwave drying retained the highest levels of essential amino acids and fatty acids. Oven drying provided balanced retention, while roasting effectively preserved key fatty acids but posed a risk of amino acid degradation. Sun drying and air-drying maintained monounsaturated fatty acids and key amino acids, offering a cost-effective alternative despite the prolonged drying period.
Microwave drying is the most effective method for preserving the nutritional quality of BSF larvae meal, but other methods offer practical advantages depending on resource availability and processing goals. Future research should explore hybrid drying techniques, optimization of processing conditions, and long-term stability assessments to enhance the efficiency and applicability of BSF larvae meal as a sustainable feed ingredient.
Animal Feed, Black Soldier Fly, Circular Economy, Drying Methods, Sustainability.
The accumulation of organic waste from agriculture and livestock industries has become a critical environmental issue, contributing to pollution, greenhouse gas emissions, and inefficient resource utilization (Doyeni et al., 2022; Koul et al., 2022). Large amounts of organic waste are generated daily, often leading to waste management challenges and environmental contamination if not properly handled (Shi et al., 2024; Siddiqui et al., 2024). Developing sustainable waste conversion strategies is essential to reducing the environmental impact of organic waste while creating value-added products that support circular economy principles.
Black Soldier Fly (BSF) larvae have emerged as a highly sustainable and nutrient-rich feed ingredient, offering a promising alternative to conventional protein sources in animal nutrition. Their ability to convert organic waste into valuable biomass makes them particularly attractive for the feed industry (Barragán-Fonseca et al., 2024). Compared to traditional protein sources such as fishmeal and soybean meal, BSF larvae provide a more environmentally friendly solution by utilizing organic waste streams, reducing reliance on finite resources, and minimizing waste accumulation (Beyers et al., 2023; Wiyoso et al., 2023). Additionally, their nutritional composition, including a well-balanced amino acid profile and beneficial fatty acids, makes them suitable for various livestock, aquaculture, and pet food applications (Lu et al., 2022).
The rearing medium plays a crucial role in shaping the nutrient composition of BSF larvae, directly influencing their protein content, lipid profile, and overall biomass yield. Research has shown that BSF larvae can efficiently convert various organic substrates, including agriculture livestock waste, into high-quality biomass, making them a valuable resource for sustainable feed production (Amrul et al., 2022; Isnaini et al., 2023; Hosseindoust et al., 2024). Rice bran, a by-product of rice production, contains a substantial amount of carbohydrates, proteins, and lipids, making it an excellent substrate that enhances the nutritional profile of BSF larvae (Angeles et al., 2024; Laksanawimol et al., 2024). Milk waste, consisting of discarded low-quality milk, is an effective liquid medium due to its high moisture content and residual nutrients, which can support microbial activity and larval development (Apriani et al., 2025). Slaughterhouse waste, which was generated from beef production, is a nutrient-dense substrate that provides essential proteins, fibers, and microbial communities, which may enhance larval growth and feed conversion efficiency (Naser El Deen et al., 2023; Fasha et al., 2024). By combining rice bran, milk waste, and slaughterhouse waste as a growing substrate, BSF larvae can benefit from a more balanced nutrient supply, improving protein and lipid accumulation, and ultimately enhancing their value as a sustainable feed ingredient.
Various post-harvest processing methods further impact the final nutrient profile of BSF larvae meal by affecting moisture removal, protein stability, and lipid oxidation. Air-drying and sun-drying are widely used due to their cost-effectiveness and accessibility, but they require longer drying times and may pose risks of microbial contamination (Hernández et al., 2024). Oven drying provides a controlled heat source, helping to preserve protein quality while reducing microbial load (Saucier et al., 2022). Microwave drying is highly efficient in reducing drying time and retaining more nutrients due to its rapid and uniform heating, though its high energy consumption and equipment cost may limit its widespread adoption (Khodifad and Dhamsaniya, 2020; Son et al., 2023). Roasting, known for enhancing crude protein concentration by reducing moisture content, may also lead to lipid oxidation and a decrease in ether extract levels (Muthee et al., 2024).
Since different processing methods offer trade-offs between cost, efficiency, and nutrient preservation, identifying an optimal drying method is essential to maximizing the nutritional quality of BSF larvae meal. While previous studies have explored general nutrient retention under various processing methods, limited research has focused on how different processing methods influence the amino acid and fatty acid profiles of BSF larvae meal reared on rice bran, milk waste, and slaughterhouse waste combined substrate. Therefore, this study evaluated the effects of five processing methods (air-drying, sun-drying, oven-drying, microwave-drying, and roasting) on the amino acid and fatty acid profiles of BSF larvae reared on rice bran, milk waste, and slaughterhouse waste combined substrate.
Five kilograms of slaughterhouse waste was collected from a local slaughterhouse unit and placed in a sealed plastic container for fermentation. A microbial starter solution was prepared by mixing 30 mL of EM-4 starter, 90 mL of molasses, and four liters of tap water. The solution was then evenly distributed throughout the slaughterhouse waste to ensure uniform fermentation. The container was tightly sealed and left to ferment at room temperature for seven days.
One gram of BSF eggs was hatched at room temperature for seven days on a hatching substrate consisting of 400 grams of commercial catfish feed mixed with 400 mL of milk waste. The milk waste used in this study was obtained from a local dairy cooperative unit and consisted of low-quality milk that did not meet commercial standards. After hatching, the larvae were reared on a combined substrate consisting of 3 kg of rice bran + 3 L of milk waste + 1 kg of fermented slaughterhouse waste. The larvae were reared on this formulated substrate for seven days, after which they were separated from the residual substrate.
To prepare the larvae for further processing, they were rinsed with hot water, drained, and subjected to different processing methods. The study included five different processing methods, as presented in Table 1. The processing durations for each method were determined based on the time required to achieve a constant weight. Each processing method was replicated in three independent batches. The final sample for nutrient analysis was obtained by combining equal portions from the three independent batches into one composite sample. The dried larvae were ground into powder using a laboratory blender and subsequently analyzed for their nutrient profiles.
Amino acid profiling was carried out using high-performance liquid chromatography (HPLC) equipped with an XBridge C18 column (4.6 × 250 mm) and post-column derivatization using o-phthalaldehyde (OPA) followed by fluorescence detection. Sample preparation involved accurately weighing the sample, adding 2 mL of 6 N HCl, vortexing, and hydrolyzing at 110°C for 12 hours. After cooling to room temperature, the hydrolysate was neutralized with 6 N NaOH, clarified with 5 mL of 40% lead acetate and 2 mL of 15% oxalic acid, and then diluted to 10 mL using distilled water. Approximately 3 mL of the solution was filtered through a 0.45 μm Millex membrane and placed into the autosampler. The autosampler was programmed to mix 10% of the sample volume with OPA reagent, allow the reaction to proceed for 3 minutes, and then inject 20 μL into the HPLC system.
The HPLC system used in this analysis consisted of a Knauer Smartline 1000 pump, a Knauer Smartline 3950 autosampler, and a Knauer Smartline 5000 interface module. Detection was performed using a fluorescence detector. The mobile phases consisted of eluent A (0.01 M sodium acetate buffer, pH 5.9) and eluent B (a mixture of methanol: 0.01 M sodium acetate buffer: tetrahydrofuran in a ratio of 80:15:5). The gradient program started with 30% eluent B from 0 to 3 minutes and increased to 100% by minute 25, at a constant flow rate of 1.5 mL per minute. Detection was carried out at an excitation wavelength of 340 nm and an emission wavelength of 450 nm.
Standard amino acid solutions were prepared by dissolving 10 mg of each standard in 10 mL of 0.1 N HCl to obtain a 1,000 ppm concentration. The reagents included borate buffer pH 9.1 (15.5 g boric acid in 500 mL water, pH adjusted with 0.1 N NaOH), OPA reagent (0.1 g o-phthalaldehyde dissolved in 10 mL methanol with 100 μL 2-mercaptoethanol, diluted to 50 mL with borate buffer), and 0.01 M sodium acetate buffer pH 5.9. Quantification of amino acids was based on calibration curves generated from external standards analyzed under identical conditions.
Fatty acid analysis of saturated and unsaturated components was conducted through hydrolysis, methylation, and gas chromatography. A total of 5 grams of the sample was weighed into a large test tube and mixed with 10 mL of concentrated hydrochloric acid. The mixture was heated in a water bath at 80°C and continued until boiling for 3 hours. After cooling to room temperature, fatty acids were extracted using a 1:1 mixture of diethyl ether and petroleum ether (10 mL total), followed by vortexing and phase separation. The upper lipid layer was collected and evaporated in a water bath under a stream of nitrogen gas (N2) to obtain the oil phase.
For methylation, 0.5 mL of the extracted oil was transferred into a capped small test tube. A total of 1.5 mL of sodium methoxide solution was added, then the mixture was sealed and heated at 60°C for 5–10 minutes with intermittent shaking. After cooling, 2 mL of boron trifluoride methanol complex was added, followed by reheating at 60°C for another 5–10 minutes. After cooling again, the methylated fatty acids were extracted using 1 mL of heptane and 1 mL of saturated sodium chloride (NaCl) solution. The upper organic layer was collected and transferred into a gas chromatography (GC) vial for injection.
Fatty acid methyl esters were analyzed using an Agilent Technologies 7890B GC system equipped with a flame ionization detector (FID). One microliter of the sample was injected into the system with an injector temperature of 240°C, a pressure of 48 psi, total flow of 22 mL/min, column flow of 1.8 mL/min, purge flow of 3 mL/min, and split ratio of 10:1 (split flow 18 mL/min). The detector was maintained at 240°C, using helium as the carrier gas and nitrogen as the makeup gas at a flow rate of 30 mL/min, along with hydrogen at 40 mL/min and air at 400 mL/min. Separation was performed using an HP-88 capillary column (100 m × 0.3 mm ID × 0.2 μm film thickness). The oven temperature was programmed to start at 100°C with a 5-minute hold, followed by a temperature ramp of 4°C/min up to 240°C, which was then held for 10 minutes. The total run time was 50 minutes.
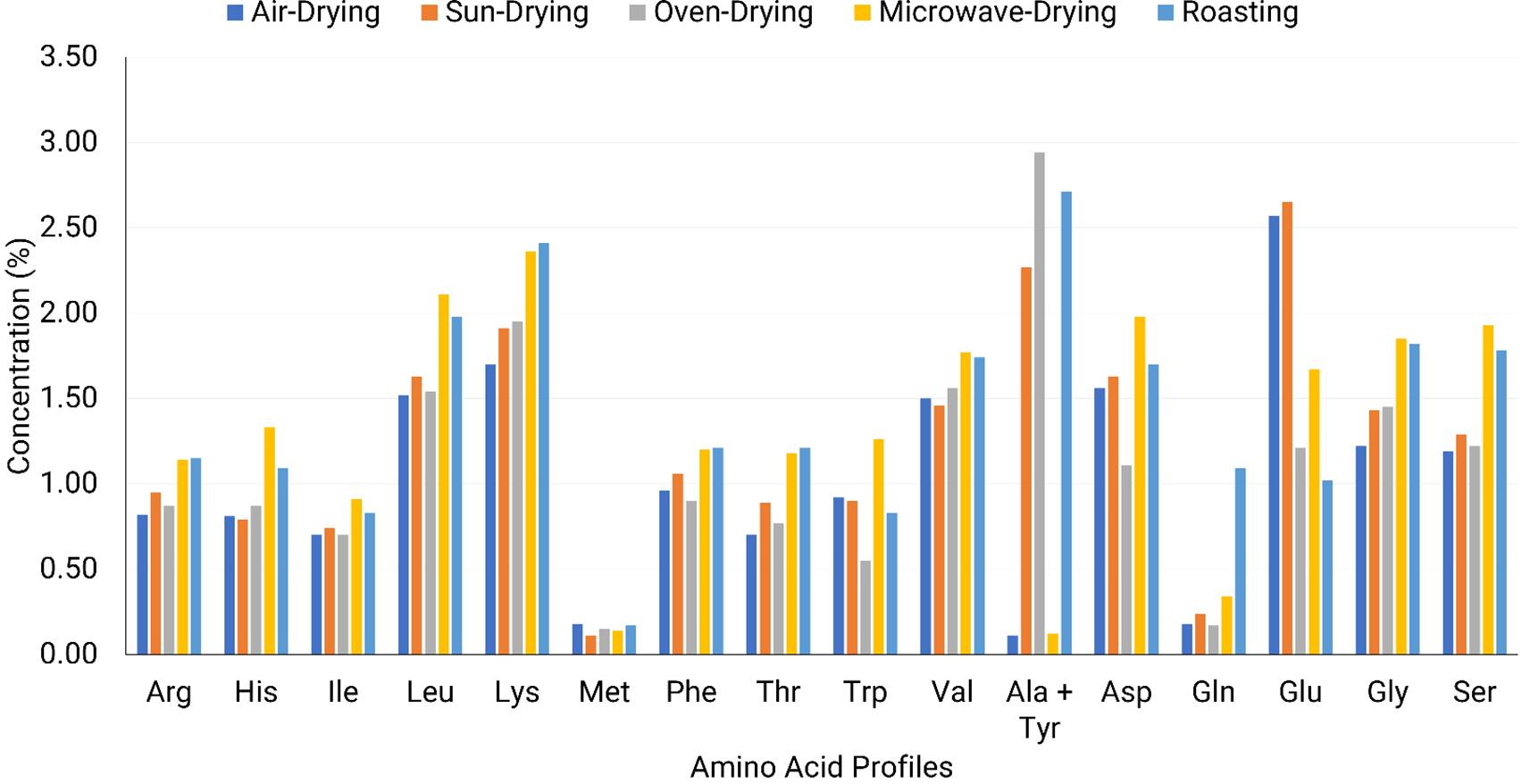
The results in Figure 1 indicate that BSF larvae meal contained substantial amounts of essential amino acids, particularly lysine (1.76–2.41%), leucine (1.46–2.11%), and valine (1.41–1.77%). Previously, it was also reported that BSF larvae meal contained substantial amount of lysine, leucine, and valine (Spranghers et al., 2017; Huang et al., 2019; Fuso et al., 2021; Zulkifli et al., 2022; Miron et al., 2023). Lysine, leucine, and valine are crucial for protein synthesis, muscle development, and metabolic functions in animal nutrition (McDonald et al., 2011). These amino acids were retained across all drying methods, highlighting the resilience of BSF larvae meal as a protein-rich feed ingredient.
Arg: Arginine, His: Histidine, Ile: Isoleucine, Leu: Leucine, Lys: Lysine, Met: Methionine, Phe: Phenylalanine, Thr: Threonine, Trp: Tryptophane, Val: Valine, Ala + Tyr: Alanine + Tyrosine, Asp: Aspartic acid, Gln: Glutamine, Glu: Glutamic acid, Gly: Glycine, Ser: Serine.
Among the processing methods, microwave drying resulted in the highest retention of lysine (2.36%), leucine (2.11%), and valine (1.77%), indicating that rapid heating effectively minimized nutrient degradation. The preservation of lysine, an essential amino acid that is often heat-sensitive, suggests that microwave drying may be the most efficient method for maintaining protein quality in BSF larvae meal. The higher heat sensitive amino acids could be attributed to shorter times during microwave drying, which limit thermal degradation and oxidative damage to amino acids (Lier et al., 2024).
Roasting also demonstrated high retention of lysine (2.41%), leucine (1.98%), and valine (1.74%), suggesting that short, high-temperature exposure might have concentrated amino acids due to moisture reduction (Muthee et al., 2024). Oven drying, conducted at 60°C for 36 hours, retained substantial amounts of lysine (1.95%), leucine (1.54%), and valine (1.56%), indicating that moderate heat application was effective in preserving these essential amino acids (Fombong et al., 2017).
Sun drying and air drying also preserved lysine (1.91% and 1.76%, respectively), leucine (1.63% and 1.46%), and valine (1.46% and 1.41%), suggesting that low-temperature drying methods were effective in minimizing amino acid loss. However, the longer drying duration may increase the risk of oxidation and microbial contamination, potentially affecting protein digestibility and feed safety (Mutungi et al., 2019).
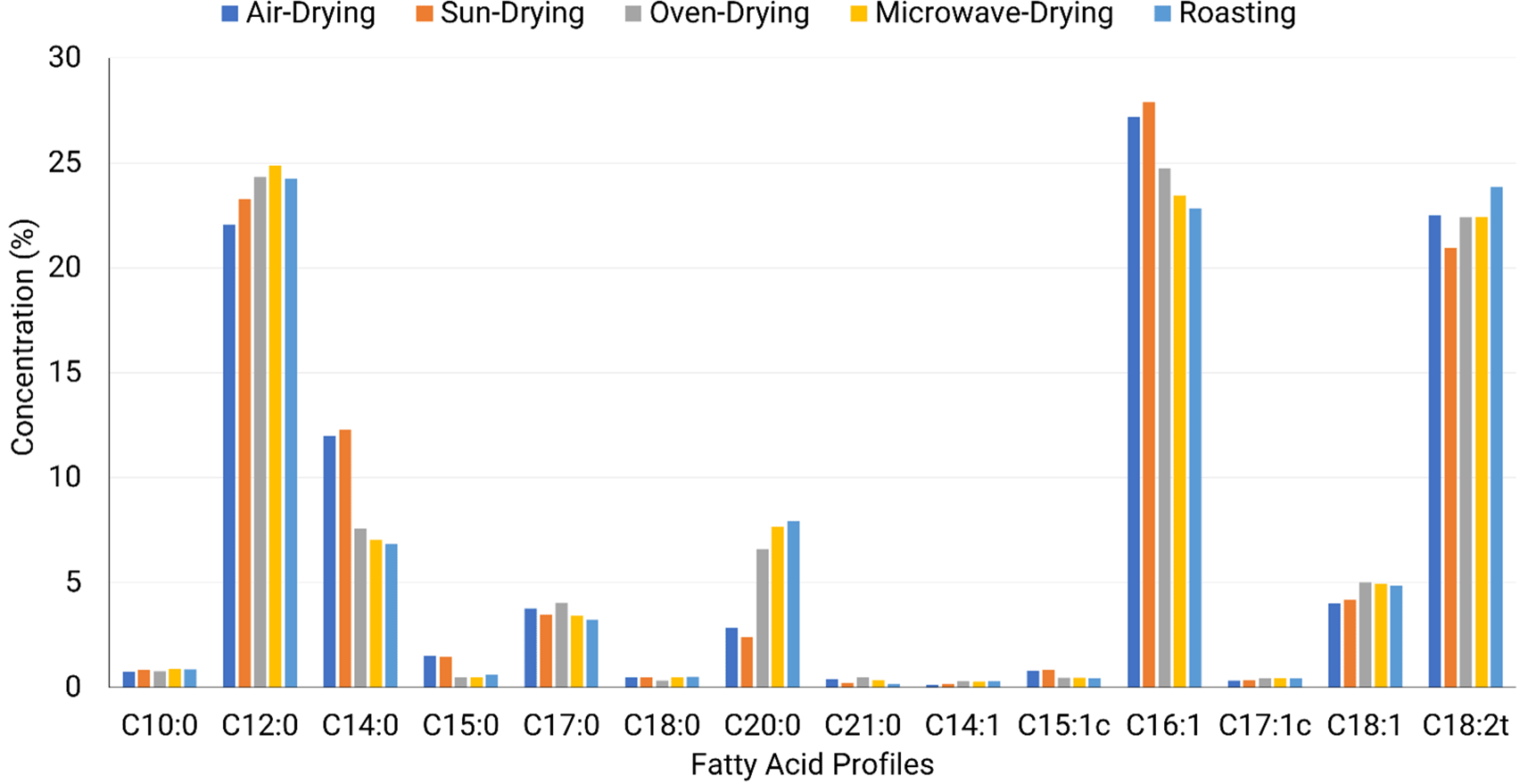
According to Figure 2, the fatty acid profiles of BSF larvae meal were predominantly composed of lauric acid (22.07–24.88%), palmitoleic acid (22.84–27.89%), and linolelaidic acid (20.95–23.86%), all of which consistently exceeded 20% across different processing methods. Previous studies have also reported that BSF larvae meal contains a substantial amount of lauric acid (Danieli et al., 2019; Ewald et al., 2020; El-Dakar et al., 2021; Rodrigues et al., 2022). The composition of fatty acids in BSF larvae meal is largely influenced by the growing media (Li et al., 2022; Siddiqui et al., 2022; Cattaneo et al., 2023). Lauric acid is classified as a saturated fatty acid, while palmitoleic acid is a monounsaturated fatty acid, and linolelaidic acid is a polyunsaturated fatty acid. These fatty acids are essential for the nutritional value of BSF larvae meal, playing a key role in energy metabolism and improving digestibility in animal feed applications (McDonald et al., 2011).
C10:0: Decanoic acid, C12:0: Lauric acid, C14:0: Myristic acid, C15:0: Pentadecanoic acid, C17:0: Heptadecanoic acid, C18:0: Stearic acid, C20:0: Arachidic acid, C21:0: Heneicosanoic acid, C14:1: Myristoleic acid, C15:1c: cis-10-Pentadecenoic acid, C16:1: Palmitoleic acid, C17:1c: cis-10-Heptadecenoic acid, C18:1: Oleic acid, C18:2t: Linolelaidic acid.
Among the drying methods, microwave drying effectively retained lauric acid (24.88%), confirming that rapid and uniform heating minimized oxidation and thermal degradation. Similarly, linolelaidic acid (22.41%) and palmitoleic acid (23.45%) remained stable under this method, demonstrating that controlled heat exposure helped preserve key fatty acids in BSF larvae meal. The effective preservation of these fatty acids highlights the advantage of microwave drying in maintaining their stability and composition (Lenaerts et al., 2018).
Oven drying resulted in comparable retention of lauric acid (24.32%) and linolelaidic acid (22.4%), showing that moderate heat exposure was sufficient to maintain these fatty acids. Palmitoleic acid (24.73%) was well preserved, suggesting prolonged drying at 60°C for 36 hours did not cause significant fatty acid degradation. The stable presence of these fatty acids across oven drying highlights its effectiveness in maintaining the fatty acids profile (Fombong et al., 2017).
Roasting demonstrated strong retention of linolelaidic acid (23.86%), indicating that short, high-heat exposure effectively preserved this fatty acid. Lauric acid (24.24%) and palmitoleic acid (22.84%) were also well retained, suggesting that roasting can be a suitable drying method when properly controlled. Due to its effectiveness in preserving key fatty acids, roasting presents a practical and efficient drying approach (Kinyuru, 2021).
Sun drying resulted in the highest retention of palmitoleic acid (27.89%), confirming that low-temperature drying effectively preserved monounsaturated fatty acids. Lauric acid (23.28%) and linolelaidic acid (20.95%) remained stable, indicating that sun drying was viable for maintaining fatty acid integrity. The effectiveness of sun drying suggests that prolonged exposure to moderate environmental conditions did not significantly alter the fatty acid profiles (Kinyuru, 2021).
Air drying showed similar results to sun drying, with palmitoleic acid (27.25%), lauric acid (22.07%), and linolelaidic acid (22.56%) all well maintained. These results suggest that air drying effectively retained the key fatty acids despite the extended drying period. While air drying requires a longer duration, its ability to preserve fatty acid composition makes it a practical method for processing BSF larvae meal in resource-limited settings (Hernández-Álvarez et al., 2021).
Selecting a suitable drying method for BSF larvae meal depends on nutrient retention and practical feasibility. Microwave drying emerged as the most effective method for preserving both amino acids and fatty acids, making it ideal for operations prioritizing nutritional integrity and processing speed. However, its high energy consumption may limit its scalability (Melgar-Lalanne et al., 2019). Oven drying provides a balanced retention of essential nutrients, making it a viable alternative for controlled processing, especially in facilities with access to moderate heat drying equipment. Roasting effectively maintained key fatty acids and certain amino acids, suggesting that short, high-heat exposure may be beneficial. However, its impact on heat-sensitive amino acids should be considered (Liang et al., 2024). Sun drying and air drying demonstrated good preservation of monounsaturated fatty acids and key amino acids, making them cost-effective and accessible options, especially in regions with abundant sunlight and limited drying infrastructure. However, the extended drying duration may pose risks of oxidation and microbial contamination, which could affect the overall feed quality (Parniakov et al., 2022). Given these findings, the choice of processing method should be guided by resource availability, drying efficiency, and specific nutritional goals.
Black Soldier Fly larvae meal is a nutritionally rich feed ingredient, containing substantial amounts of lysine, leucine, and valine, along with lauric acid, palmitoleic acid, and linolelaidic acid, making it a valuable amino acids and fatty acids source for animal feed. The results confirm that BSF larvae meal retains essential nutrients across various drying methods, reinforcing its potential as a sustainable alternative protein source. However, microwave drying proved to be the most effective method, preserving both amino acids and fatty acids while minimizing degradation. These findings highlight the importance of selecting an optimal drying method based on processing efficiency and resource availability to maximize nutritional retention. Future research should focus on developing hybrid drying techniques, optimizing processing conditions, and evaluating the long-term stability of BSF larvae meal to further enhance its viability as a high-quality feed ingredient.
This study was approved by the Institutional Ethical Clearance Committee of Universitas Brawijaya under registration number 247/EC/KEPK/08/2023.
Figshare: ARRIVE checklist for “Amino Acid and Fatty Acid Profiles of Black Soldier Fly Larvae Meal Reared on Rice Bran, Milk Waste, and Slaughterhouse Waste Combined Substrate Under Different Processing Methods”. https://doi.org/10.6084/m9.figshare.28724408.v1 (Isnaini and Andri, 2025b)
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Figshare: Amino Acid and Fatty Acid Profiles of Black Soldier Fly Larvae Meal Reared on Rice Bran, Milk Waste, and Slaughterhouse Waste Combined Substrate Under Different Processing Methods. https://doi.org/10.6084/m9.figshare.28723397.v2 (Isnaini and Andri, 2025a)
The project contains the following underlying data:
− Raw data of amino acid profiles of black soldier fly larvae meal under different processing methods.
− Raw data of fatty acid profiles of black soldier fly larvae meal under different processing methods.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Livestock production system
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 08 Jul 25 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)