Keywords
Hyaline membrane disease, Newborns, Secondary research, low income country.
Many primary articles in Ethiopia approach the prevalence and factors associated with respiratory distress syndrome, but discrepancies were seen among those articles. This study aimed to assess the pooled prevalence and factors associated with neonatal respiratory distress syndrome in Ethiopia.
Primary studies were searched using different data sources. Thirteen primary studies were included. The data were extracted using a Microsoft Excel spreadsheet and exported to Stata version 14 for analysis. Publication bias was confirmed using Egger’s regression test.
This study included thirteen primary articles that met the inclusion criteria. The pooled prevalence of respiratory distress syndrome in Ethiopia was 30.28%. Neonates with birth weights less than 1500gm and preterm neonates had a significant factor for respiratory distress syndrome.
We found a high pooled prevalence of respiratory distress syndrome. Thus, to reduce this problem, the relevant body should put special emphasis on the variables mentioned above.
Hyaline membrane disease, Newborns, Secondary research, low income country.
Respiratory distress syndrome (RDS) is a surfactant-deficient (dysfunctional) lung disease caused mostly by lung immaturity and characterized by cyanosis, grunting, apnea, nasal flaring, poor feeding, tachypnea, and retractions in the intercostal, subcostal, or suprasternal areas. These manifestations develop either at or shortly after birth.1–4
RDS is one of the most common health problems encountered in preterm neonates and the most frequent cause of neonatal respiratory failure and death.5,6 It accounts for death among neonates 12.8% in Poland, 46.9% in Nigeria, and 45% in Ethiopia.7–9 It is the leading cause of morbidity and mortality among premature neonates in Ethiopia.10
RDS is a known risk factor for intracerebral/intraventricular hemorrhage, which increases the likelihood of cerebral palsy and late epilepsy. It also often leads to morbidities such as bronchopulmonary dysplasia and requires prolonged hospitalization resulting in high costs that lead to economic burdens on the family, society, and health care systems.11–14
According to studies conducted in Iraq, India, Nigeria, and Egypt, the prevalence of RDS was 34.7%, 57%, 26.2%, and 48.5%, respectively.7,15–17 Many studies have suggested that prematurity, low birth weight, sepsis, perinatal asphyxia, gestational diabetes mellitus, and placental insufficiency are factors significantly associated with RDS.1,18–22 If RDS is not identified early and treated effectively, it may cause fatal consequences or respiratory failure may result in death.6,10 Although several primary studies on the prevalence of RDS were conducted in Ethiopia, the reports from this research revealed disparate and inconsistent findings. Furthermore, there have been no prior systematic reviews or meta-analyses of the pooled prevalence and risk factors for RDS in Ethiopian newborns. Therefore, determining the pooled prevalence and its associated factors of RDS can be used as baseline evidence for the concerned stakeholders to address the problem and the potential factors for neonatal RDS.
The protocol for this study is registered with the International Prospective Register of Systematic Reviews (PROSPERO) under ID CRD42023491295. Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, this study was conducted in Ethiopia to determine the combined prevalence of neonatal respiratory distress syndrome and its contributing factors.23
Using published studies up to December 28/2023, this systematic review and meta-analysis were carried out. Several electronic search engines, including PubMed/Medline, Google, Google Scholar, Web of Science, CINAHL, and EMBASE, were searched for the published articles. The keywords and search strategies used for the PubMed database were (“Prevalence” OR “Magnitude” OR “Epidemiology” OR “Proportion” OR “Pattern of admission” OR “Burden” OR “Incident”) AND (“associated factors” OR “risk factors” OR “determinants” OR “predictors”) AND (“respiratory distress syndrome” OR “hyaline membrane disease”) AND (“neonatal intensive care unit”) AND (“neonates” OR “newborns”) AND “Ethiopia”.
All primary articles that reported the prevalence and associated factors of respiratory distress syndrome among neonates in various regions of Ethiopia, published between 2016 and 2023, were included. Only full-text articles in English, based on cross-sectional and retrospective follow-up study designs, were considered, without restrictions on the study area. However, this study excluded case reports, case studies, journals lacking full text, articles in other languages, and qualitative findings.
After a thorough examination of the titles, abstracts, and full texts of the included studies, two writers (GD and GT) independently extracted all necessary data from the primary articles using a standardized data extraction format in Microsoft Excel. This format included the first author’s name, publication year, study design, region of study, sample size, and prevalence of respiratory distress syndrome. For the second objective, the writers also extracted risk factors associated with respiratory distress syndrome, along with the 95% confidence interval and odds ratio. To any disagreements that arose during the data extraction process, the two writers discussed the issues with the third author (SF), and any conflicting data were re-extracted.
There are two key results from this systematic review and meta-analysis. The primary outcome variable of the study was respiratory distress syndrome, while the secondary outcomes examined the risk factors associated with neonatal respiratory distress syndrome in Ethiopia. The prevalence reported in each primary study was used to calculate the pooled prevalence of respiratory distress syndrome, and the findings on the correlation between each risk factor and the disorder were employed to determine the pooled odds ratio (AOR).
The quality of the primary studies included in this systematic review and meta-analysis was assessed using a standard critical appraisal tool. Specifically, the Joanna Briggs Institute Quality Appraisal Checklist was employed to evaluate the methodological quality of these studies24 (S1 File). This checklist addresses several key aspects of study methodology, including sample representativeness, appropriate participant recruitment, comprehensive participant description, use of reliable standard measurements, adequate statistical analysis, and the identification of subgroups or confounding variables.
Studies were classified based on their appraisal scores: high quality for scores of 8 or above, moderate quality for scores between 5 and 7, and low quality for scores between 0 and 4. Only articles with quality scores of 5 or higher were included in this systematic review and meta-analysis. Any disagreements among authors regarding the quality evaluation of the included articles were resolved by the third author (TM) to reach a consensus.
The data synthesis was completed by tabulation, producing a clear and thorough descriptive summary of the included studies. The results of the current study were outlined and presented using texts, forest plots, and tables. Microsoft Excel spreadsheet was used to extract the numerical data and STATA 14.0 was used for analysis. The standard I2 was used to measure heterogeneity.25 Because of the significant heterogeneity, a random-effect meta-analysis model was utilized to determine the pooled effect size of all the included studies at a 95% confidence interval. Additionally, subgroup analysis was done using publication year, regional state and study design to identify the possible sources of heterogeneity. Sensitivity analysis was conducted to check the effects of a single study on the pooled prevalence. By visually examining a funnel plot for symmetrical distribution, publication bias was assessed.26 To further evaluate publication bias, Egger weighted regression tests were used and it was considered when the value of p was less than 0.05.27
Patient and public involvement: The public and/or patients were not involved in the design, execution, reporting, or dissemination plans of this meta analysis.
After a search on the above-listed databases and other search engines, 319 studies were found. Of those, 124 studies were excluded because of duplication. The remaining articles were further reviewed by topic and abstract, leading to exclude 171 articles conducted outside Ethiopia. Additionally, 24 studies were checked through a review of the full-text articles for eligibility using the inclusion criteria and 11 studies were excluded. Lastly, 13 articles were included in the current systematic review and meta-analysis ( Figure 1).
In the current study, nine primary studies that were published between 2016 and 2023 were included. Out of the included studies, seven studies in Amhara, two in SNNPs, one in Addis Ababa, another one in (Addis Ababa, Gondar and Jimma) and the remaining two study in Oromia were conducted with a total population of 10,015. Regarding the study designs, nine were cross-sectional designs and four were retrospective cohort study designs. From the included studies, the minimum sample size was 291 and the maximum sample size was 4919. The prevalence of respiratory distress syndrome (RDS) in the study area ranged from 8.3% to 49.83% ( Table 1). According to the Joanna Briggs Institute (JBI), 69% of the studies demonstrated high quality, while the remaining 31% were rated as medium quality.
| Authors | Year of publication | Study design | Study area | Sample size | Prevalence of RDS (%) |
|---|---|---|---|---|---|
| Ebissa et al.44 | 2019 | Cross-sectional | Oromia | 341 | 11.9 |
| Bogale et al.45 | 2023 | Cross-sectional | SNNPs | 395 | 45.1 |
| Yared et al.46 | 2020 | Retrospective follow-up study | Addis Ababa | 571 | 43.4 |
| Shambel et al.47 | 2023 | Cross-sectional | Amhara | 422 | 26.9 |
| Abayneh et al.48 | 2017 | Cross-sectional | Amhara | 769 | 10.9 |
| Wubet et al.49 | 2023 | Retrospective follow-up study | Amhara | 423 | 47.16 |
| Yalemtsehay Dagnaw et al.50 | 2022 | Retrospective follow-up study | Amhara | 291 | 49.83 |
| Binyam et al.51 | 2021 | Retrospective follow-up study | Amhara | 535 | 40 |
| Belete et al.52 | 2020 | Cross-sectional | SNNPs | 412 | 34 |
| Mehretie Kokeb & Teshome Desta53 | 2016 | Cross-sectional | Amhara | 335 | 19.7 |
| Birtukan Assefa et al.54 | 2021 | Cross-sectional | Amhara | 228 | 11.8 |
| Zemene Tigabu et al.9 | 2020 | Cross-sectional | Ethiopia | 4919 | 45.3 |
| Jorge Seboka et al.55 | 2018 | Cross-sectional | Oromia | 384 | 8.3 |
In this systematic review and meta-analysis, thirteen studies were included to determine the overall prevalence of respiratory distress syndrome among neonates in Ethiopia. The pooled prevalence of RDS in Ethiopia was found to be 30.28% (95% CI: 20.54%, 40.02%) and the weighted prevalence of RDS was also determined ( Figure 2).
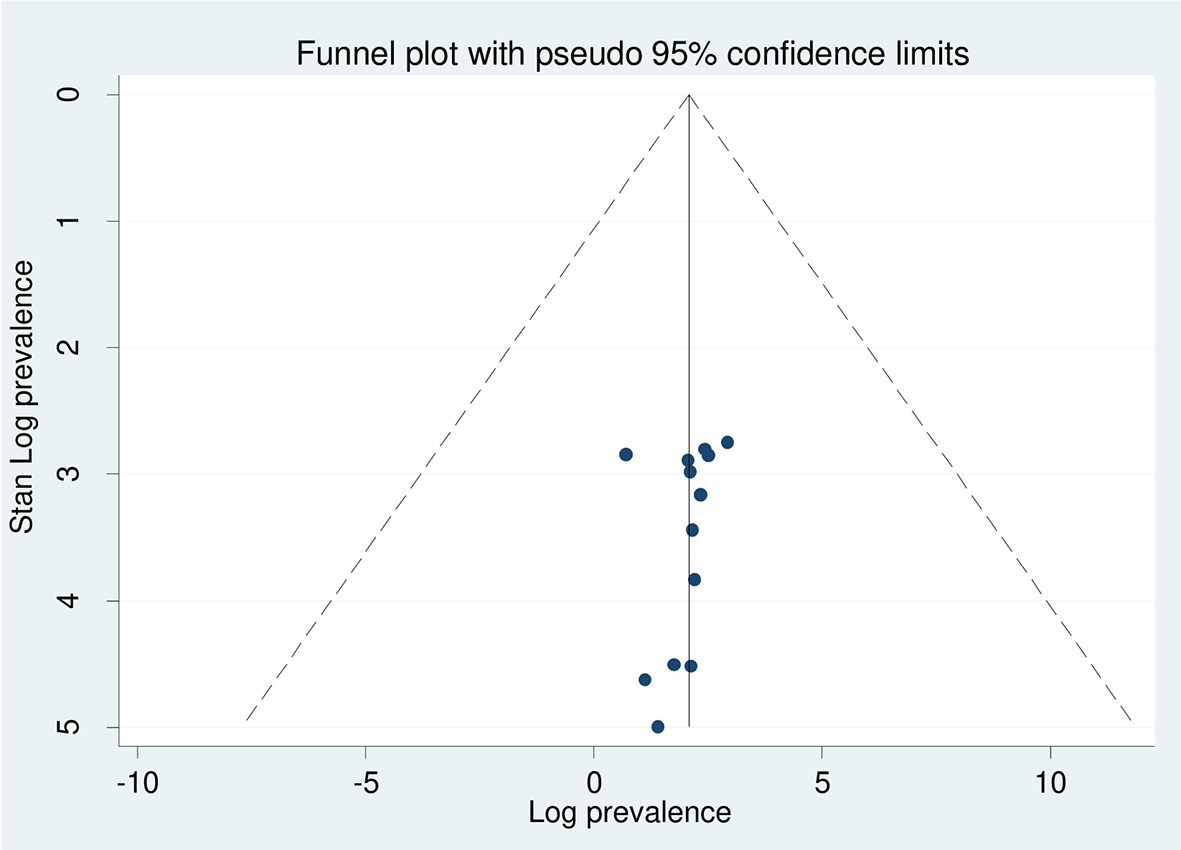
The test statistic showed that there was significant heterogeneity between the included articles (I2 = 99.1%, p = 0.000) ( Figure 2). To check the publication bias, two techniques were used such as funnel plot ( Figure 3) and Egger’s tests showed that there was no statistically significant evidence for the presence of publication bias (p = 0.553) ( Table 2).
Because heterogeneity affected the pooled prevalence of respiratory distress syndrome, we conducted a sub-group analysis based on the year of publication, the regional state and study design. As subgroup analysis showed, the pooled prevalence of respiratory distress syndrome among neonates was found to be 39.51% (95% CI: 28.64, 50.34) in the SNNPs region, 9.95% (95% CI: 6.43%, 13.46%) in Oromia region, and 29.38% (95% CI: 17.33%, 41.44%) in Amhara region. Furthermore, the subgroup analysis indicated that the pooled prevalence of respiratory distress syndrome was greater in the studies conducted after 2021 with a prevalence of 36.74% (95% CI: 24.74%, 48.74%) than in the studies carried out before 2021 with the prevalence of 24.78% (95% CI: 10.24%, 39.31%). Based on the study design, the pooled prevalence of respiratory distress syndrome among neonates that was conducted with cross sectional study design was lower when compared to retrospective follow-up studies: 25.65% (95% CI: 13.31%, 37.98%) and 41.95% (95% CI: 38.02%, 45.89%), respectively ( Table 3).
The sensitivity analysis was accomplished to determine the heterogeneity of the included studies systematically by keeping out one study to determine the impact of each study’s findings on the pooled prevalence of respiratory distress syndrome. As the analysis shows, all of the values were within the expected 95% CI, supplying that the exclusion of a single study did not significantly change the prevalence of the current study ( Table 4).
This systematic review and meta-analysis demonstrated that two risk factors were significantly associated with respiratory distress syndrome among neonates in Ethiopia. A neonate with a birth weight of less than 1500 gm was significantly associated with respiratory distress syndrome in two studies and being preterm neonate was significantly associated with respiratory distress syndrome in three studies that were included in this systematic review and meta-analysis.
Based on the findings of this systematic review and meta-analysis, the likelihood of respiratory distress syndrome occurrence was 2.92 times higher among neonates with a birth weight of less than 1500 gm than neonates with a birth weight of 1500 gm and above (AOR: 2.92; 95% CI: 1.72, 4.94). Neonate being born preterm was 3.8 times more likely to develop respiratory distress syndrome as compared to term (AOR = 3.82; 95% CI: 2.80, 5.23) ( Figure 4).
To mitigate neonatal respiratory distress syndrome (RDS), a comprehensive approach that emphasizes both prevention and treatment is essential. Key strategies include antenatal corticosteroid therapy, which accelerates fetal lung maturation and reduces the incidence of RDS in preterm neonates, and surfactant replacement therapy, which enhances lung function in affected infants. Non-invasive respiratory support, such as continuous positive airway pressure (CPAP), helps minimize ventilator-induced lung injury. Preventing preterm birth through proper antenatal care, tocolytics, and maternal nutrition also decreases the overall risk of RDS. Additionally, early neonatal monitoring, stringent infection control measures, and improved regionalized neonatal care and referral systems contribute to better survival outcomes. Strengthening healthcare provider training, expanding access to life-saving interventions, and enhancing neonatal transport networks are crucial for reducing RDS-related neonatal mortality, particularly in resource-limited settings such as Ethiopia.
Addressing neonatal respiratory distress syndrome (RDS) in Ethiopia necessitates improved preventive measures, standardized research designs, and additional studies. Strengthening antenatal care through the timely administration of corticosteroids, infection prevention, and enhanced maternal nutrition can mitigate critical risk factors. Utilizing standardized research methods will enhance data accuracy, while further studies should investigate region-specific determinants and refine interventions. Policymakers must implement evidence-based neonatal care guidelines to ensure equitable access to surfactant therapy, continuous positive airway pressure (CPAP), and specialized care, ultimately reducing neonatal mortality.
The current study was carried out to assess the pooled prevalence and risk factors associated with neonatal respiratory distress syndrome in Ethiopia. This is the first systematic review and meta-analysis in the study setting and it can assist program planners and policymakers in determining the best course of action to address issues affecting this vulnerable population.
In this systematic review and meta-analysis, the pooled prevalence of respiratory distress syndrome among neonates in Ethiopia was found to be 32.76% (95% CI: 22.24%, 43.27%). This finding is consistent with the primary studies conducted in Nigeria (26.2%),7 Iraq (34.7%),16 Nepal (34%),28 and India (33.4%).29 However, the pooled prevalence of respiratory distress syndrome in this study is lower than studies conducted in Egypt (45.8%),15 Saudi Arabia (54.7%),5 India (57%),17 Poland (54.29%),30 and Cameroon (47.5%).21 On the other hand, the pooled prevalence of respiratory distress syndrome is higher than studies conducted in Pakistan (4.6%),31 Portugal (8.83%),32 Nepal (17.8%),33 and Bulgaria (15%).34 The possible reason for this disparity may be due to the differences in study design, sample size, study population, the population’s awareness of health services, health care quality and coverage. The other reason for this variation might be due to the fact that the other studies conducted in different countries had a single result while the current systematic review and meta-analysis pooled prevalence from different studies.
Meta-analysis showed that neonates with a birth weight of less than 1500 gm and preterm neonates were significantly associated with respiratory distress syndrome. The current study identified that the odds of having respiratory distress syndrome among neonates with a birth weight of less than 1500 gm were 2.9 times higher compared with those neonates with a birth weight of 1500 gm and above. This finding was supported by the study conducted in Italy.35 This could be because low birth weight newborns are more likely to have immature physiological and anatomical structures, like fragile brain capillaries, a large surface area to body mass ratio, lack of subcutaneous tissue, and surfactant deficiencies end up with respiratory distress syndrome.36 Furthermore, respiratory distress syndrome in low birth weight neonates is primarily caused by the immaturity of their lungs, specifically the surfactant-producing cells (type II pneumocytes). Without sufficient amounts of surfactant, the alveoli become unstable and tend to collapse, resulting in impaired oxygen exchange and respiratory distress syndrome.37
This review also disclosed that preterm neonates were at higher risk of developing respiratory distress syndrome than term neonates. This is consistent with studies conducted in Poland,30 India,38 and China.1 This could be because, respiratory distress syndrome occurs primarily due to the immaturity of the lungs, and premature birth interferes with the normal development of the respiratory system. As gestational age increases (during the last trimester of pregnancy), the lungs undergo a crucial stage of development where the production of surfactant begins which reduces the surface tension in the alveoli, the tiny air sacs in the lungs and keeps the alveoli open and prevents their collapse during exhalation.39,40
Joanna Briggs Institute Quality Appraisal Checklist was used to evaluate the quality of eligible studies. Funnel plot and Egger weighted regression tests were used to assess the risk of bias of the included studies. Only quantitative observational studies were included in this systematic review and meta-analysis; case series, case reports, and qualitative data were not included. Furthermore, subgroup analysis was identified in different strata; heterogeneity was observed in specific stratified groupings. Lack of sufficient studies on prevalence and factors associated with neonatal respiratory distress syndrome in Ethiopia.
The result of systematic review and meta-analysis revealed that the pooled prevalence of respiratory distress syndrome among neonates in Ethiopia was found to be high. It also revealed that a neonate with a birth weight of less than 1500 gm and being preterm neonate were both significantly associated with respiratory distress syndrome. According to the findings of the subgroup analysis that was conducted using publication year, and regional state, there is a difference in the pooled prevalence of neonatal respiratory distress syndrome. As a result, to minimize the complication of respiratory distress syndrome in neonates, the concerned stakeholders should give special emphasis on neonates delivered with preterm and birth weight of less than 1500 gm. The findings of this study emphasize the urgent need for targeted interventions to reduce neonatal respiratory distress syndrome (RDS) in Ethiopia, with significant implications for both policy and clinical practice. Policymakers should prioritize integrating antenatal corticosteroid administration into routine maternal care to ensure that all eligible preterm pregnancies receive timely treatment. Additionally, the study highlights the necessity of expanding access to surfactant therapy and CPAP in neonatal intensive care units (NICUs), particularly in underserved regions. Strengthening regionalized neonatal care and referral systems can facilitate timely interventions, ultimately reducing neonatal mortality. By incorporating these evidence-based strategies into national neonatal care guidelines, Ethiopia can improve neonatal survival rates, lower healthcare costs associated with prolonged hospital stays, and enhance overall maternal and child health outcomes.
Asnake Tadesse and Gezahagn Demsu: Conceived and designed the studies, performed the studies, analyzed and interpreted the data, analysis tools or data, and wrote the report. Gebresilassie Tadesse and Setegn Fentahun: Conceived and designed the studies, performed the studies, and analyzed and interpreted the data. Tsehayu Melak: Contributed materials, analysis, tools, or data, and wrote the paper.
Figshare: RDS outcome data Excel.xlsx: https://doi.org/10.6084/m9.figshare.29476340.v141
Data are available under the terms of the: https://creativecommons.org/licenses/by/4.0/
Figshare: Online resource for PRISMA Checklist of items to be included in reports of observational studies in epidemiology Checklist for cohort, case-control, and cross-sectional studies (combined): Abate, Asnake (2025). PRISMA Checklist.docx. figshare. Journal contribution.
https://doi.org/10.6084/m9.figshare.29476388.v142
Data are available under the terms of the: https://creativecommons.org/publicdomain/zero/1.0/
Figshare: Supporting file: https://doi.org/10.6084/m9.figshare.29476475.v143
Supporting file 1: Quality assessment of the included studies.
Data are available under the terms of the: https://creativecommons.org/licenses/by/4.0/
Patient consent for publication: Not required.
We would like to express our gratitude to all authors of the articles included in this systematic review and meta-analysis.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)