Keywords
carotenoids, fermented milk, bioavailability, human
In humans, the bioavailability of β-carotene from vegetables is approximately 5˗10%. To maximize the health benefits of dietary carotenoids it is important to enhance their bioavailability. We reported previously that co-ingestion of fermented milk (100 g) plus vegetables enhanced the bioavailability of carotenoids.
The objective of this study was to compare the differences in plasma levels of various carotenoids after co-ingesting them with fermented milk.
We conducted a randomized crossover study of 10 healthy males in two groups across two periods. A beverage containing carotenoids with and without fermented milk (50 g) was consumed. Blood samples were collected before ingestion and 2, 4, 6, and 8 h after ingestion and plasma β-carotene, lycopene, lutein, astaxanthin in the triacylglycerol-rich lipoprotein (TRL) fraction and crocetin in all fractions were measured.
The incremental area under the curve of the TRL fraction of plasma β-carotene, lycopene, lutein, astaxanthin, and all fractions of plasma crocetin were significantly higher after the ingestion of carotenoids with fermented milk versus carotenoids alone.
These results suggest that the absorption of various carotenoids may be improved when consumed with fermented milk. Furthermore, it was also found that carotenoid absorption was enhanced at lower doses than previously reported for fermented milk.
Clinical trial registry number and website: UMIN clinical registry system (trial ID: UMIN000052407), https://center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000059825
carotenoids, fermented milk, bioavailability, human
Carotenoids are a class of yellow to red natural pigments commonly found in fruits and vegetables. More than 750 types of carotenoids have been identified from sources such as microorganisms, animals, and plants. Carotenoids exhibit a host of physiological functions (Bendich and Olson 1989). For instance, β-carotene, found in carrots, is known to help maintain night vision and the health of skin and mucus membranes (Eggersdorfer and Wyss 2018). β-carotene is also known as a precursor of vitamin A, and vitamin A deficiency is common in developing countries, especially in regions with limited access to fruits, vegetables, and animal products and can lead to night blindness, weakened immune systems, and increased risk of infections (World Health Organization 2009). Lutein, present in spinach, can alleviate eye fatigue (Ma and Lin 2010). Lycopene, found in tomatoes, has been reported to increase high-density lipoprotein (HDL) cholesterol in the blood and reduce blood pressure (Ried and Fakler 2011). In humans, the bioavailability of β-carotene from raw carrot or spinach is approximately 5 to 10% (van Lieshout, West, and van Breemen 2003). To maximize the health benefits of carotenoids, it is important to enhance their bioavailability in the diet.
Carotenoids are lipid-soluble substances; thus, the concurrent consumption of vegetable fats such as canola oil or soybean oil and vegetables will result in a higher blood concentration of carotenoids (Brown et al. 2004; Goltz et al. 2012). Cooking vegetables with oil or consuming them together with an oil-based dressing is a suitable method by which to augment dietary carotenoid absorption. We have reported previously that when fermented milk, produced by fermentation of lactic acid bacteria, and β-carotene were administered concurrently to rats, the serum β-carotene concentration was higher than when β-carotene alone was administered; the active components involved in this enhanced effect were found to be milk proteins and lactic acid bacterial metabolites (Morifuji et al. 2020). Furthermore, we have reported that co-ingestion of 100 g of fermented milk and vegetables (carrots, tomatoes, spinach) resulted in an increased concentration of vegetable-derived carotenoids in plasma compared with when vegetables alone were ingested (Morifuji et al. 2020). However, the minimum amount of fermented milk required to be consumed to elicit this effect remains unclear. Furthermore, increasing attention is being paid to the health benefits of carotenoids found in non-vegetable sources, such as crocetin in saffron and in astaxanthin, which is abundant in seafood such as salmon.
Therefore, the purpose of this study was to verify the effect of fermented milk on the absorption of various carotenoids in healthy participants.
This trial was approved by the Chiyoda Paramedical Care Clinic Ethics Review Board (September 15, 2023, approval number: 23091502) which was composed of third parties not involved in the trial. Our research was conducted in accordance with the ethical principles stipulated by the Helsinki Declaration, for medical research involving human subjects (as announced by the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare) and was registered in the UMIN clinical registry system (trial ID: UMIN000052407, registration date: October 4, 2023). This trial was performed at the Chiyoda Paramedical Care Clinic (Tokyo, Japan). A screening test was conducted on October 12, 2023, and the intake tests were done on November 9 and 23 of 2023. Our clinical trial ended on March 31, 2024.
The investigating clinicians thoroughly explained details of the study to the prospective participants based on the informed consent document. After confirming that the participants fully understood the information, the clinicians obtained written consent. Consenting participants subsequently underwent a screening test. This included a physical examination, physical assessment (height, weight, body mass index [BMI], blood pressure, and pulse rate), blood analyses (hematological, biochemical, and infectious disease tests), and measurement of plasma carotenoid concentrations. The participants were enrolled in the study if they met the study selection criteria (described below) and did not have any characteristics that would disqualify them according to the exclusion criteria.
The number of research participants was calculated from a preliminary trial (UMIN trial ID: UMIN000030890) which used the same fermented milk planned for consumption in this study. Using the difference in mean values and standard deviation, the effect size (Cohen’s d) for this study was estimated to be 0.91. In an experiment with 10 participants, the possibility of detecting a difference with an effect size of 0.91, at a significance level of 5%, gave us an 80% chance of detecting an effect when one existed. We determined the number of cases required to evaluate the difference in blood carotenoid concentrations for this study.
The selection criteria for study participants included those who had received a thorough explanation about the purpose and content of the research and were able to understand and voluntarily provide their consent in writing by exercising their free will. Participants were to be male subjects aged between 20 and 35 years at the time consent was provided, and individuals with a body mass index of 18.5 to <25.0 kg/m2 at the time of screening.
The exclusion criteria disqualified from study participation those who [1] had consumed medicines, non-medicinal products, supplements, functional foods, or carotenoid-enhanced products at least twice per week in the month prior to the screening test, and those planning to continue consuming these products during the test period, [2] had severe or progressive illness or symptoms, [3] were diagnosed by a trial supervising physician, or trial sub-physician, as having abnormalities in lipid metabolism, glucose metabolism, liver function, or renal function, [4] had food allergies, [5] were intolerant to lactose, [6] had previously undergone gastrointestinal surgery, [7] had smoked within the month prior to the screening test, [8] had donated 200 mL of blood within the month prior to the initial screening or who, within three months prior, had donated 400 mL of blood, [9] participated in another clinical trial or a monitoring study within the month before the initial screening, or who were planning to participate in another clinical trial during the study period, or [10] were physicians or co-investigators responsible for the trial.
The study food allocation manager, who belongs to an organization independent of other study personnel, randomly assigned the 10 selected study participants into two groups of 5 people using plasma β-carotene concentration as an allocation factor. Group A received carotenoids alone and group B received carotenoids + fermented milk.
The test drink product comprised 50 g of a carotenoid-containing drink and 50 g of fermented milk. The carotenoid drink was prepared by adding a carotenoid preparation to water. The carotenoid preparation included 5.0 mg of β-carotene, 4.0 mg of lycopene, 2.3 mg of lutein, 6.2 mg of astaxanthin and, 5.6 mg of crocetin (per 50 g serving).
The fermented milk was prepared by adding to a milk base product (concentrated skim milk), fermented milk starters (Lactobacillus delbrueckii subsp. bulgaricus OLL1251 and Streptcoccus thermophilus OLS3290). Fermentation continued until the count of each bacterium reached 1×107/g. The nutritional composition of the fermented milk was as follows, per 100 g serving: energy, 35 kcal; carbohydrates, 4.9 g; proteins, 3.6 g; fat, 0.1 g; ash, 0.8 g; water content, 90.6 g; β-carotene, 0.01 mg; and exopolysaccharide, 8.0 mg.
The study was conducted as a two-group, two-period randomized crossover trial ( Figure 1). A 13-day washout period was required between each test day. The study subjects consumed prescribed meals (breakfast, lunch, and dinner) the day before the test. The nutritional components of the meal were 2280 kcal of energy, 332 g of carbohydrates, 57 g of proteins, and 67 g of fats. Only water was permissible as a drink. Participants were instructed to finish their meal before 21:00 h on the day before a test.
On the day of the test, the participants attended the clinic without eating or drinking and were prohibited from consuming anything other than the designated food and drink items until after completion of all tests. Upon arrival at the facility, prior to consuming the test drink a physical examination, a compliance check, and a physical examination (including weight, BMI, blood pressure, and pulse rate) were performed. Administration of the test drink (50 g carotenoids + 50 g water, or 50 g carotenoids + 50 g fermented milk) was initiated between 9:00 and 10:00 h, followed by completion within 20 min of the start. Instructions were provided to ensure that ingestion times of the test drink did not differ significantly across the three periods. Blood was collected in ethylenediaminetetraacetic acid (EDTA) tubes and inverted gently several times before the test and, 2, 4, 6, and 8 h after ingestion of the test drink. Each collected blood sample was separated into plasma (1730 × g, for 10 min at 4°C) and was kept at ˗80°C until measurement. To avoid photodecomposition of carotenoids in the blood, the collection tubes were maintained in darkness until analyzed.
To ensure the safety of the research participants, water was provided from the time participants consumed the test drink through until just before the last 8-h blood collection. After the 4-h blood collection participants could consume an offered snack (300 g of rice with salt, 880 kcal, carbohydrates 203 g, and protein 13 g).
During the study period, the participants were asked to adhere to the following restrictions and prohibitions related to food, beverages, and other indulgences, such as: [1] refrain from making significant changes to one’s usual diet (in terms of meal quantity, meal content, snacking habits, etc.); [2] while the intake of beverages such as green tea and coffee remained unrestricted in terms of quantity and frequency, there could be no change in the volume or type of these items consumed; [3] no extreme dieting or overeating of any food ingredients, dishes, or drinks that were rich in carotenoids (i.e., green and yellow vegetables, seaweed, etc.); [4] alcohol consumption was generally permissible provided it did not exceed the participant’s usual amount or frequency, although, it was prohibited from the day prior to the start of the test day until the testing ended; and [5] smoking during the study period was prohibited.
Medicines, health foods—including functional foods, special use/indicated foods, and carotenoid fortified foods—quasi-drugs, and supplements were prohibited because of the potential risk of affecting the test results. Participants were also asked to refrain as much as possible from making significant lifestyle changes.
We measured plasma concentrations of the carotenoids (i.e., β-carotene, α-carotene, lycopene, lutein, zeaxanthin, β-cryptoxanthin, astaxanthin, and total carotenoids) in the triacylglycerol-rich lipoprotein (TRL) fraction, and crocetin in all fractions. Total carotenoids were calculated by adding together β-carotene, α-carotene, lycopene, lutein, zeaxanthin, β-cryptoxanthin, and astaxanthin. We evaluated the change from pre-ingestion value to zero (delta value) and the incremental area under the curve (iAUC) as indicators of bioavailability.
The TRL fraction is a known indicator of postprandial carotenoid contents in plasma (van Vliet, Schreurs, and van den Berg 1995). Because of the high concentration of endogenous carotenoids present in blood, and thus the data from the TRL fraction was chosen for analysis. Crocetin was the carotenoid of choice for the kinetics analysis since it could be assessed in all plasma fractions.
Symptoms, severity, outcome, and frequency of adverse events and side effects that occurred from the start to the end of the study period by all study subjects who had consumed the study substance at least once were recorded, and the association with the study substance was evaluated for all events.
To measure plasma carotenoids, 500 μL of plasma layered with 500 μL of saline solution with a density of 1.006 g/mL was ultracentrifuged at 15,500 × g, for 30 min, at 20°C to obtain a TRL fraction. The blood concentration of carotenoids in the total fraction and TRL fraction were analyzed by extracting carotenoids in accordance with previous reports, and using high-performance liquid chromatography (HPLC) (Morifuji et al. 2020).
The measurement of crocetin was conducted using the following methodology (Umigai et al. 2011): 50 μL of plasma was mixed with 200 μL of chilled methanol. After centrifugation at 12,000 rpm for 10 min, at 4°C, the supernatant was collected. The pellet was washed with a volume of 80% methanol, and the supernatant was collected following centrifugation. The collected supernatant was dried using a desiccator, then re-dissolved in 85% methanol to prepare the sample. Crocetin was quantified using HPLC (LC-20AT, SPD-M20A). All the analyses were performed on a 4.6-mm I.D. x 150-mm column (COSMOSIL Packed Column 5C18-MS-II). The mobile phase A consisted of 0.5% acetic acid and the mobile phase B methanol. The total running time was 5 min. The eluent flow was 1.0 mL/min, and the column temperature was set at 40°C. The mobile phase A was mixed with mobile phase B in a ratio of 15% to 85%, respectively, and eluted isocratically. The absorption wavelength was set to 425 nm.
Nutrients and carotenoid measurements were performed by Japan Food Research Laboratories, Tokyo. The moisture content was measured using the vacuum drying method, protein content was determined using a combustion procedure, fats were extracted for measurement using the method of Röse-Gottlieb, ash content was evaluated using a direct ashing method, and carotenoids were measured using High performance liquid chromatography (HPLC).
The per-protocol set (PPS) analysis was used for participants who fulfilled the compliance requirements. The changes in plasma carotenoid concentrations over time were analyzed using a two-factor repeated-measures ANOVA post-hoc paired Student’s t test. The iAUC over 0–8 h was calculated using the trapezoidal rule. Postprandial carotenoid concentrations were baseline-corrected using fasting values. The iAUC data for plasma carotenoid concentrations were analyzed using the paired Student’s t-test. Differences between groups were significant at P < 0.05.
The flow chart related to enrolment, allocation, tracking, and data analysis of the study is shown in Figure 1. A screening test was conducted on 21 participants who consented to participate in the study to determine their eligibility. Four participants met the exclusion criteria. A further 3 participants withdrew from the study for personal reasons. Because an analysis of changes in the blood concentration of β-carotene could potentially produce errors when the intrinsic plasma carotenoid concentration is high, it was decided to select 10 subjects for the study, based on their lowest whole blood plasma β-carotene concentrations during the screening test, and allocated them to one of two groups (n = 5).
One individual discontinued study participation due to poor health condition during the experiment. The remaining nine selected study participants completed the entire research schedule. No participants had a compliance violation during the research study. The safety analysis set (SAS) and the full analysis Set (FAS) comprised all ten participants. The number of participants in the per protocol set (PPS) was nine.
Table 1 presents the characteristics of the study participants measured at baseline (PPS).
Figure 2 shows the changes for the TRL fractions of β-carotene, lycopene, lutein, astaxanthin, total carotenoids, and all fractions of crocetin. The plasma β-carotene concentration in the TRL fraction 4, 6, 8 h post-consumption significantly increased with the co-ingestion of carotenoids and fermented milk compared with the ingestion of carotenoids alone.
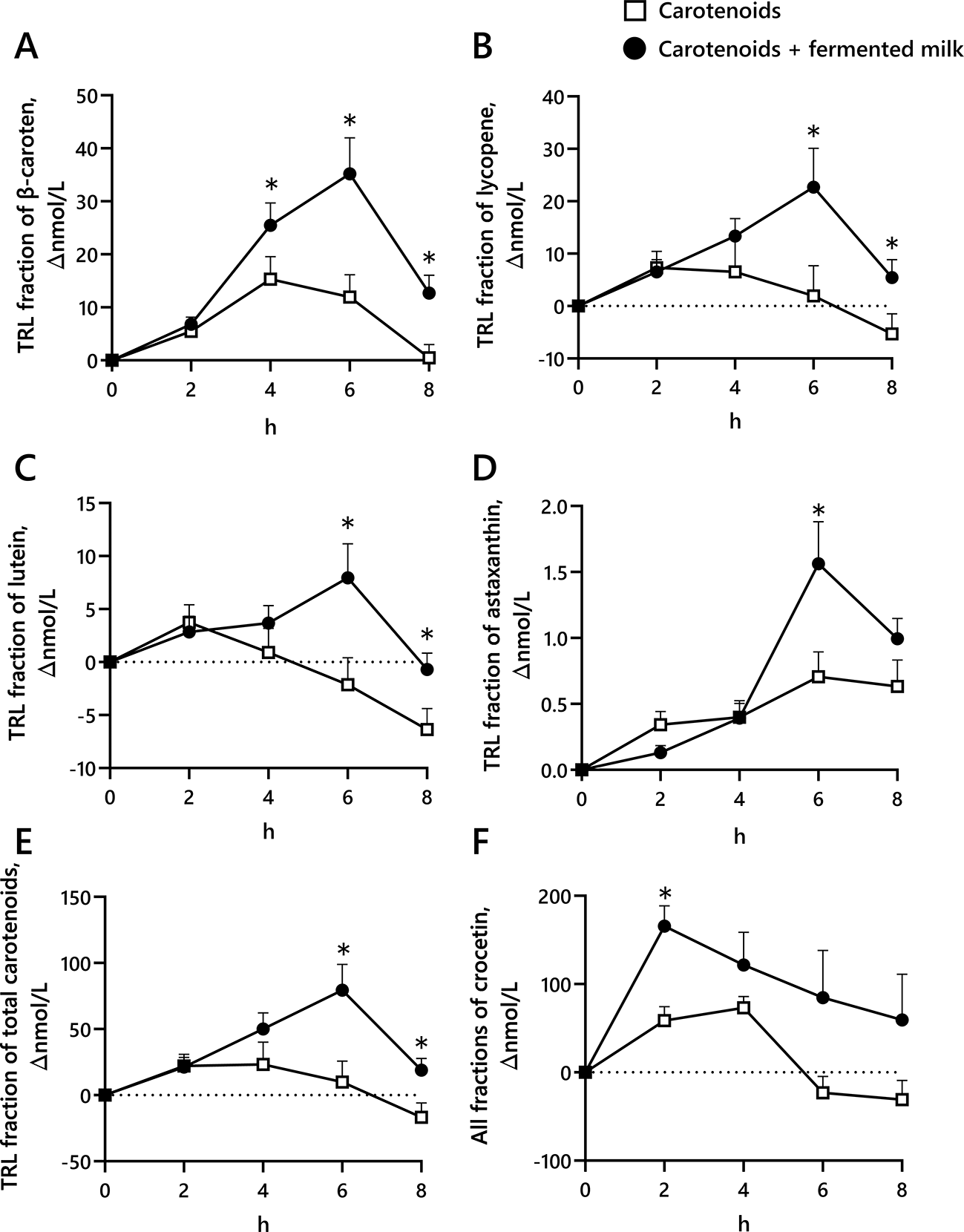
Blood was collected before ingestion and 2, 4, 6, and 8 h after ingestion of the test drinks (containing carotenoids or carotenoids + fermented milk).
Total carotenoids were calculated by adding together β-carotene, α-carotene, lycopene, lutein, zeaxanthin, β-cryptoxanthin, and astaxanthin.
Mean ± standard error (N = 9: per protocol set analysis)
* P < 0.05 versus a carotenoid mixture at a given time (using a repeated-measures two-factor ANOVA post-hoc paired Student’s t test for analysis).
In the TRL fraction—when comparing the concurrent intake of carotenoids and fermented milk with the intake of carotenoids alone—the plasma lycopene concentration became significantly higher 6 and 8 h after consumption of the test drink; the plasma lutein concentration became significantly higher after 6 and 8 h, the plasma astaxanthin concentration significantly increased 6 h post-consumption, and the total carotenoids concentration significantly increased 6 and 8 h after intake. The crocetin concentration in the all fraction of plasma was significantly higher 2 h after the carotenoids and fermented milk compared with the ingestion of carotenoids alone.
Moreover, the iAUC for the TRL fraction of the plasma β-carotene, lycopene, lutein, astaxanthin, total carotenoids and all fraction of plasma crocetin concentrations significantly increased with the co-ingestion of carotenoids and fermented milk compared with consumption of carotenoids alone ( Figure 3).
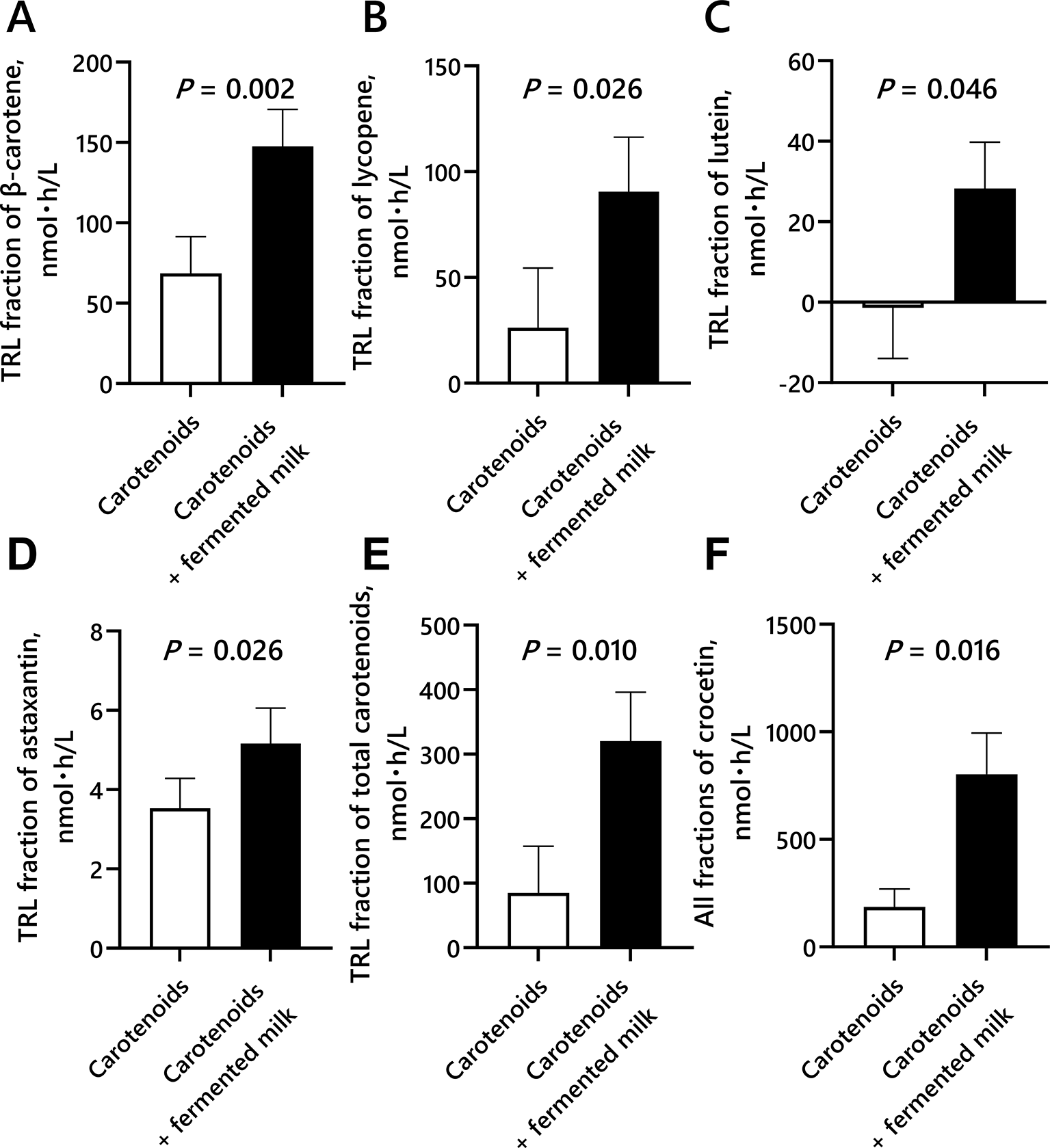
Blood samples were collected before ingestion and 2, 4, 6, 8 h after ingestion of the test drinks (containing carotenoids or carotenoids + fermented milk).
The iAUC data for plasma carotenoid concentrations were analyzed using the paired Student’s t test. Differences between groups were considered significant at P < 0.05.
Mean ± standard error (N = 9, per protocol analysis set).
Total carotenoids were calculated by adding together β-carotene, α-carotene, lycopene, lutein, zeaxanthin, β-cryptoxanthin, and astaxanthin.
The co-ingestion of carotenoids and 50 g of fermented milk was shown to increase the plasma concentrations of β-carotene, lycopene, lutein, astaxanthin, and crocetin compared with the ingestion of carotenoids alone, suggesting possible enhancement of the absorption of various carotenoids when their intake is accompanied by fermented milk.
We have reported that when vegetables and fermented milk are consumed together, the absorption of vegetable-derived carotenoids, specifically α-carotene, β-carotene, lycopene, and lutein, is improved. In addition to these carotenoids, for the first time we report that the absorption of carotenoids with different structures, including astaxanthin and crocetin, was also improved. These results suggest that fermented milk likely facilitates the absorption of various carotenoids that have a similar absorption mechanism. Astaxanthin, newly confirmed to have absorption-enhancement effects, reportedly functions to preserve skin moisture, reduce eye fatigue, and reduce oxidation of lipids in the blood (Davinelli, Nielsen, and Scapagnini 2018). Crocetin has been reported to improve sleep quality and mitigate eye strain (Guo et al. 2022). The co-ingestion of carotenoids with fermented milk is expected to increase the efficacy of these carotenoids by increasing their concentration in the blood.
Lactic acid bacteria and milk proteins may contribute as components of this fermented milk product in promoting the absorption of carotenoids derived from vegetables. The cell surface of lactobacilli can play a role in emulsification. The proteins and lipids on the cell membrane can interact with both water and oil phases, contributing to emulsion stability (Pimentel-González et al. 2009). Further, lactobacilli can also produce exopolysacccharide, which are complex sugars that can stabilize emulsions by increasing viscosity and preventing the oil droplets from coalescing (Brüls et al. 2024). It has been shown that in rats, the more exopolysaccharides in the fermented milk, the higher the area under the curve (AUC) of β-carotene in the blood increases (Morifuji et al. 2020). Furthermore, casein and whey protein, a major component of milk proteins, has unique properties such as high amphipathicity and promotion of micelle formation, and has been shown to be implicated in drug delivery research. In rats, the blood concentration of β-carotene is higher with a combination of β-carotene and skim milk powder than with β-carotene alone, and the enhancement of β-carotene absorption as a result of the addition of skim milk powder is not observed when skim milk powder is hydrolyzed by protease (Morifuji et al. 2020). This suggests the possibility that lactic acid bacteria and milk protein in the fermented milk drink contribute additively to the absorption of carotenoids. To what extent each ingredient contributes to the absorption of carotenoids and the dose-dependence of this fermented milk for promoting absorption remains an unknown and will require further investigation.
In previous experiments, it was reported that the absorption of carotenoids derived from vegetables increases when 100 g of vegetables added 100 g fermented milk are consumed together. However, it is unclear by how much the amount of fermented milk can be reduced while maintaining this effect. Therefore, we evaluated the effectiveness of fermented milk when the amount was halved from 100 g to 50 g. As a result, similar to previous experiments with 100 g of fermented milk, we reported an increase in the blood concentration of carotenoids, such as β-carotene, lycopene, and lutein. This study did not evaluate doses less than 50 g. If the required amount of fermented milk to increase the absorption of carotenoids decreases, it may be less challenging to add an effective amount of fermented milk to a variety of meals containing carotenoids.
An identified limitation of the study is its design; only males were included as participants. There are reports suggesting that the concentration of carotenoids in the blood tends to be higher in females (Allore et al. 2019). Conversely, there is no reported difference in the absorption of carotenoids between sexes (O’Neill and Thurnham 1998). Therefore, we speculate a similar effect in females is highly likely.
Co-ingestion of carotenoids and fermented milk increases the plasma concentrations of carotenoids such as β-carotene, lycopene, lutein, astaxanthin, and crocetin, more so than when only carotenoids are consumed. These results suggest that fermented milk promotes absorption of various carotenoids. Minimizing the dosage of fermented milk to maximize the bioavailability of carotenoids could play a crucial role in unleashing its health benefits. According to a report by the World Health Organization, a deficiency in Vitamin A, which is produced from β-carotene, is a serious health concern in most countries around the world (World Health Organization 2009). The new concept of improving nutritional efficiency through combinations of foods may potentially contribute to the resolution of various global issues, such as health problems caused by malnutrition from dietary imbalances and food shortages.
This trial was approved by the Chiyoda Paramedical Care Clinic Ethics Review Board (September 15, 2023, approval number: 23091502). Our research was conducted in accordance with the ethical principles stipulated by the Helsinki Declaration, for medical research involving human subjects (as announced by the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare) and was registered in the UMIN clinical registry system (trial ID: UMIN000052407, registration date: October 4, 2023).
The investigating clinicians thoroughly explained details of the study to the prospective participants based on the informed consent document. After confirming that the participants fully understood the information, the clinicians obtained written consent.
The data that support the findings of this study are openly available.
The name of the repository: Zenodo
Checklist title: CONSORT-2010-Checklist.doc
Data title: Data.xlsx
Working DOI: 10.5281/zenodo.16631274
License: Creative Commons Zero v1.0 Universal
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)