Keywords
Oxidative stress (MDA), Antioxidants (SOD, CAT, GPx), Plant hormones (auxins, abscisic acids, brassinosteriods), maize (Zea mays L), Heavy metals, Kilembe mines
This article is included in the Agriculture, Food and Nutrition gateway.
This article is included in the Public Health and Environmental Health collection.
Heavy metal contamination of agricultural soils, particularly from mining activities, poses serious risks to food safety and public health. At the Kilembe mining site in Uganda, elevated levels of heavy metals have been reported in soils where maize (Zea mays L.) is widely cultivated. This study investigated the biochemical stress response and culturable rhizobacteria in maize grown in heavy metal-contaminated soils to understand their mechanisms of adaptation.
Maize seeds were planted on farms obtained at varying distances from the Kilembe mining site (0, 1, 3, and 5 km) and a control site in Bushenyi (95 km), serving as groups 1-5, respectively. Oxidative stress and hormone profiles of germinated leaves and rhizospheric bacterial composition were determined using standard analytical and microbiological methods.
Maize plants near the site had higher malondialdehyde levels and lower antioxidant enzyme activities (superoxide dismutase, catalase, and glutathione peroxidase). Brassinosteroid levels increased at 0 km, whereas auxin and abscisic acid levels decreased significantly across all distances compared to the control. Bacillus sp. was dominant at 39.71%, and Acinetobacter sp. was the least abundant (1.47%). The 3 km site had the highest bacterial load, while the 0 km site had high counts at lower dilutions. Plant growth-promoting rhizobacteria (Bacillus sp., Staphylococcus sp., and Klebsiella sp.) were highly distributed across sites.
The study showed that the presence of plant growth-promoting rhizobacteria, such as Bacillus spp., which were dominant, provided insight into the role of these rhizospheric organisms in conferring tolerance and is a probable adaptive mechanism that ensures the survival of maize plants grown around mining-associated farmlands, as seen in Kilembe, Uganda.
Oxidative stress (MDA), Antioxidants (SOD, CAT, GPx), Plant hormones (auxins, abscisic acids, brassinosteriods), maize (Zea mays L), Heavy metals, Kilembe mines
Food crops may serve as bio-monitors to evaluate the possible risks to public health resulting from polluted agricultural soils and food safety (Gizaw, 2019). Rapid global population expansion has led to an increase in food consumption, which in turn has caused an increase in the output of agricultural products (Majeed, 2018). Heavy metal contamination greatly impacts plant productivity and food safety due to the toxicities associated with mining operations or their activities (Alengebawy et al., 2021). One of the areas in Uganda that contributes significantly to heavy metal pollution is the Kilembe mining company, which is located in Kasese in the western region (Mwesigye et al., 2016). According to Mwesigye et al. (2019), soils from farmland in or around this mining area are heavily laden with heavy metals such as cobalt (Co), lead (Pb), cadmium (Cd), copper (Cu), and nickel (Ni) according to Mwesigye et al. (2019). Elevated levels of these heavy metals in the soil can be transported to food crops when planted in contaminated soils, especially maize fruits, which are among the predominant food crops consumed in the area (Mwesigye et al., 2019).
Plant metabolic processes are affected by the presence of heavy metals in soils owing to the oxidative damage they cause in plants. Heavy metals have the ability to overproduce reactive oxygen species when antioxidant defense is weakened or suppressed aasociated with increased levels of malondialdehyde (MDA) (Asiminicesei et al., 2024). However, plants have several strategies to combat the deleterious effects of heavy metal contamination induced by free radical production, and plants do so by either absorbing heavy metals or by preventing their uptake, which ensures plant survival and growth (Ifie et al., 2020; Nguyen et al., 2020). The production of excess ROS can induce an antioxidative cascade as a protective response to the harmful effects of ROS (Nguyen et al., 2020). Superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) are crucial plant antioxidant enzymes that protect plants against the detrimental effects of heavy metal (HM) stress (Briffa et al., 2020; Qamer et al., 2021). Rizvi and Khan (2019) showed that chromium, nickel, and cadmium affect biological traits, seed quality, antioxidant enzymes, and metal spread in maize, and malondialdehyde levels increased by 72%.
Plant hormones are required for the regulation of plant development and are crucial for plants to respond to metal toxicity by complementing the activities of antioxidant capacity, which in turn interferes with phytohormone biosynthesis and the mechanism of action to induce toxicity (Bücker-Neto et al., 2017; Lymperopoulos et al., 2018). The primary phytohormones involved in this process include abscisic acid (ABA), auxins (IAA), and brassinosteroids (BRs). Their suppression affects plant root growth, stomatal operation, and energy distribution during stress (Shaffique et al., 2023a). A previous study (Nomura et al., 2015) on Scopelophila cataractae in a Cu-rich medium showed that the plant was tolerant of copper by increasing the accumulation of auxin, which activates genes required for its growth and survival. Elevated concentrations of abscisic acid (ABA) have been observed in the roots of Phragmites australis, Typha latifolia, Oryza sativa, and potato tubers following exposure to cadmium (Fediuc et al., 2005; Hsu and Kao, 2005; Stroinski et al., 2010).
The involvement of abscisic acid (ABA), auxins (IAA), and brassinosteroids (BR) in the fight against heavy metal toxicity is influenced by the presence of rhizospheric microorganisms (Eichmann et al., 2021). Plant growth-promoting rhizobacteria (PGPRs) are unique rhizobacteria that have the ability to positively influence growth, even in the presence of heavy metal-induced stress, most notably when the levels of these heavy metals are high and have become toxic to plants (Sun et al., 2024a). Species of Azotobacter, Bacillus subtilis, B. pumilis, Clostridium, Pseudomonas aeruginosa, and Klebsiella are acknowledged examples of (PGPRs) (Chandran et al., 2021). Hence, investigating the diversity and functional traits of culturable rhizospheric bacteria in heavy metal-contaminated soils will provide a useful link between microbial ecology and plant physiological responses. Because there are limited data in this regard, the present study probes the biochemical stress responses of maize grown in heavy metal-contaminated soils by quantifying oxidative stress levels and analyzing antioxidant enzyme activities, addressing this to the disruption of phytohormonal regulation under heavy metal stress and further characterizing rhizospheric bacteria associated with maize grown around the Kilembe mining site with a keen focus on functional traits involved in alleviating metal-induced stress. The integration of this knowledge aims to provide solutions for crop management, plant-microbial interactions, and remediation strategies for sustainable agriculture in the mining-affected zones of Uganda and can be extrapolated globally.
The study area used in this research was the Kilembe Mining Site and its surroundings, Kasese District, and Bushenyi District in Western Uganda.
This study was an experimental laboratory-based investigation involving the collection of samples (soil and maize). Maize farm sites within the Kilembe mining site served as the study area, and maize farm sites in the Bushenyi district served as controls ( Figure 1). Group 1 was situated within the Kilembe mining site (0 km), Group 2 at 1 km, Group 3 at 3 km, and Group 4 at 5 km from the mining site. Group 5 (94 km), located in Ishaka, Bushenyi District, served as an uncontaminated control site.
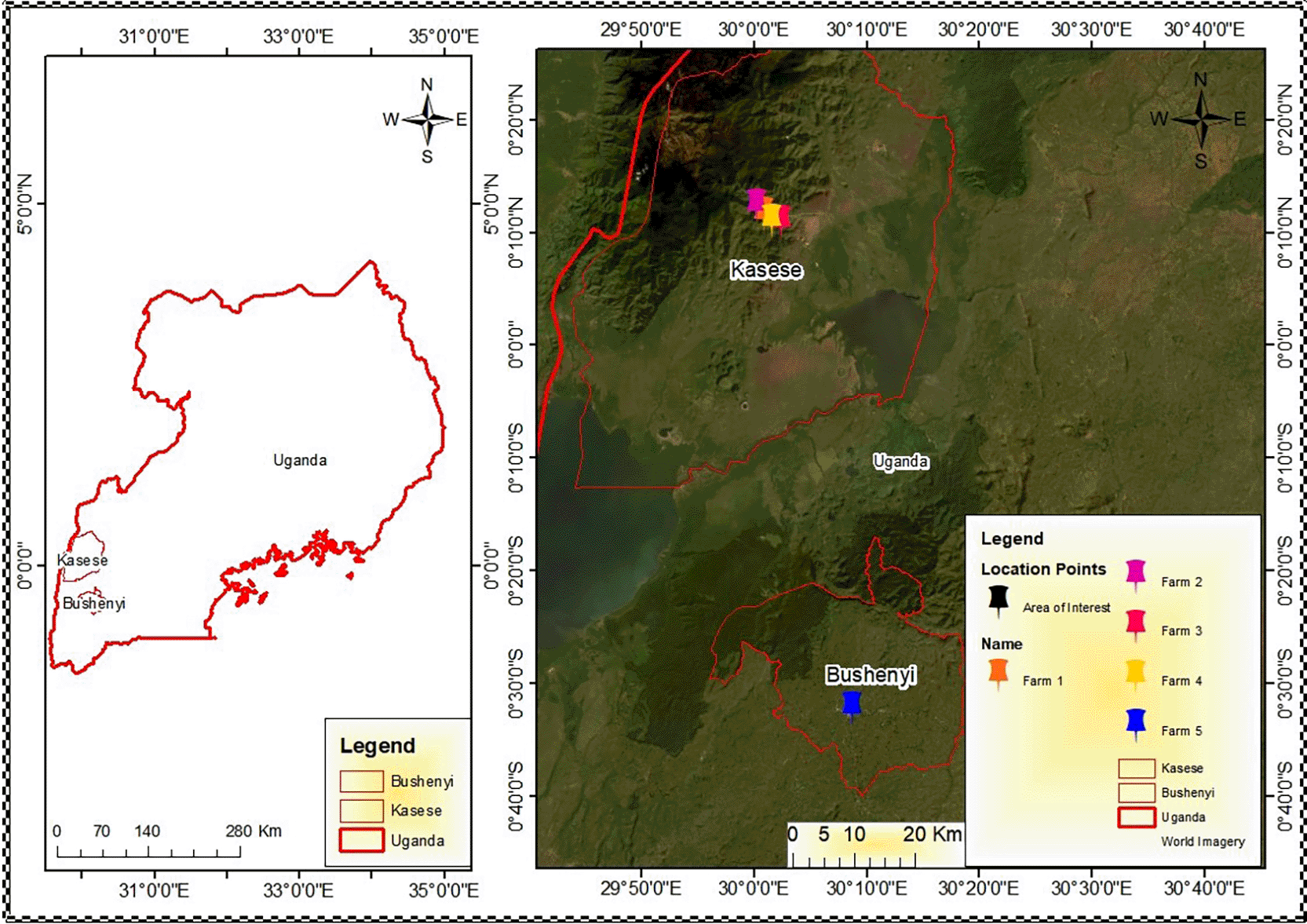
Group 1 (Farm 1) is situated within the Kilembe mining site (0 km). Group 2 (Farms 2) at 1km, Group 3 (Farms 3) at 3 km, and Group 4 (Farm 4) at 5 km from the mining site. Group 5 (Farm 5), located in Ishaka, Bushenyi District, serves as the uncontaminated control site.
Seeds of Zea mays (L) were obtained from an agricultural store where local farmers (living in or Kilembe district) obtain their seeds. The seeds were taken to a taxonomist at the Department of Botany, Mbarara University of Science and Technology, Uganda for identification and authentication (identification number: SOE001). Before planting, the farm sites were cleared and tilled, and the stones were removed. About 10-20 maize seeds were planted at a depth of 4 – 7 cm at the different experimental sites in March, the first normal planting season for maize in Uganda.
Determination of oxidative stress markers and the enzymatic antioxidant capacity in the leaves of the maize plant
Malondialdehyde (MDA) levels were assessed for oxidative stress, and the activities of superoxide dismutase, catalase, and glutathione peroxidase were assessed for enzymatic antioxidants. Maize leaves were harvested and homogenized according to the method described by Haida and Hakiman (2019). A clean knife was used to cut the plant leaves into smaller pieces, which were then transferred to a mortar and pestle on ice. 0.1 M Potassium phosphate buffer (pH 7.4) was then added for extraction and the leaves were ground thoroughly. The homogenate obtained from grinding was processed to determine oxidative stress and antioxidant enzyme levels.
Determination of the oxidative stress marker
Malondialdehyde was determined using the thiobarbituric acid reactive substances (TBARS) biomarker, as described by Prabhakar et al. (2012). The supernatant was obtained by centrifugation at 15,000 × g for 15 min. To 4.0 ml of 0.5% TBA in 20% TCA, 1 ml of supernatant was added, mixed, and heated in an electric oven at 95°C for 30 min. The solution was allowed to cool on ice and centrifuged for 10 min at 10,000 × g. The absorbance of the supernatant was measured at 532 nm and was used to determine the concentration of TBARS at an extinction value of 155 mM−1 cm−1.
Determination of the enzymatic antioxidants
The leaf homogenate was centrifuged at 3000 rpm for 15 min at 4°C to obtain the supernatant which containing the enzymes and separated into different tubes for the estimation of SOD and catalase. The supernatant (2 mL) and 2.5 mL of 0.05 M carbonate buffer (pH 10.2) were mixed and used to calibrate the spectrophotometer. Before then, 0.3 ml of freshly prepared 0.3 mM adrenaline was used. 2.5 ml of 0.05 M carbonate buffer (pH 10.2), 0.3 ml of adrenaline, and 0.2 ml of water were pipetted into a standard tube, and the change in absorbance (Abs) was read at 480 nm resulting from adrenochrome (Adreno) production at 30 and 150 s. SOD activity was assessed using the methodology described by Misra and Fridovich (1972). For catalase, the supernatant (0.5 ml of supernatant was transferred into 15 ml ice-cold tubes and 5 ml of 30 mM hydrogen peroxide) was added for initiation. Subsequently, 1 ml of 6M H2SO4 was added to terminate the reaction after 3 min. After the addition of 7 ml of 0.01 M potassium permanganate (KMnO4), the absorbance was read at 545 nm using spectrophotometer 30 to 60 seconds and the catalase activity was determined (Rasheed et al., 2021).
Glutathione peroxidase (GSH-Px) activity was quantified using a sandwich ELISA protocol, according to the manufacturer’s guidelines. Standards were serially diluted and distributed across ten wells (two per dilution), while one well was reserved as a blank control. The sample wells were loaded with 40 μL of sample dilution buffer and 10 μL of tissue homogenate, yielding a 1:5 dilution. Care was taken to dispense the samples at the bottom of each well without contacting the wall. plates were gently mixed and incubated at 37°C for 30 min under a closed membrane. The concentrated wash buffer was diluted at a ratio of 30:1 with distilled water. After incubation, the membrane was removed and the wells were aspirated. The wells were washed five times using diluted buffer, with a 30 s soak per cycle. Subsequently, 50 μL of horseradish Peroxidase -conjugate reagent was added to each well, except the control, followed by a second 30 mins incubation at 37°C. The closure membrane was then removed, the wells were washed again to prepare for substrate addition, and the final absorbance was measured at 450 nm using spectrophotometry.
Determination of the hormonal profile of the maize plants
Abscisic acid, auxins, and brassinosteroids were extracted and quantified from the leaves of germinated maize plants during field studies using a spectrophotometric assay.
The concentration of auxins was determined using the modified protocol described by Barkawi et al. (2010) in which 5 mL of methanol was used to homogenize 1 g of powdered leaf tissue. The homogenate was centrifuged at 10,000 rpm for 10 min and the extraction process was repeated. Both supernatants were subjected to sequential purification by the addition of 1mL each of acetic acid, 1M NaOH, and dichloromethane, and centrifuged at 10,000 rpm for 10 min after each addition. 1mL of anhydrous Na2SO4 was added to the supernatant, which was then centrifuged at 10,000 rpm for 10 min. The absorbance of the supernatant was measured against known standards at 280 nm.
The extraction and quantification of abscisic acid (ABA) from leaf tissues were performed following a modified protocol described by Huang et al. (2014). First, 1 g of finely powdered plant tissue was homogenized in 5 mL of chilled methanol to facilitate ABA solubilization. The homogenate was centrifuged at 10,000 rpm for 10 min, and the supernatant was retained. This extraction step was repeated to maximize the yield. The combined extracts were purified sequentially by adding 1 mL of acetic acid, 1M sodium hydroxide, and dichloromethane, followed by centrifugation following each addition (10,000 rpm for 10 min). The final organic phase was treated with 1 mL of anhydrous sodium sulfate and centrifuged to remove residual moisture. 1 mL of ABA solution (1 mg/mL) was added to the sample and mixed thoroughly for derivatization. ABA was heated to 60°C for 30 minutes to convert it into its methyl ester and the ABA methyl esters were quantified using a spectrophotometer calibrated against known ABA standards at 254 nm.
The extraction and quantification of brassinosteroids from leaf tissue followed the modified vanillin-sulfuric acid colorimetric protocol described by Bajguz and Tretyn (2003). Briefly, 1 g of fresh plant leaves was homogenized in methanol and centrifuged to obtain the crude extract. The supernatant was purified with ethyl acetate to remove interfering pigments and non-steroidal compounds. The purified extract was sequentially treated with sodium hydroxide (NaOH), dichloromethane, anhydrous sodium sulfate (Na2SO3), sulfuric acid (H2SO3), and vanillin. After completion of the vanillin-BR reaction, the absorbance was measured at 520 nm against BR standards to determine BR concentrations.
Determination of the composition of culturable rhizospheric soil bacteria
Microbial load was determined using the spread plate technique, as described by Waturu et al. (2017), with slight modifications. Briefly, 1 g of rhizospheric soil was suspended in 9 mL of buffered peptone water and serially diluted through seven ten-fold steps. Each dilution was achieved by transferring 1 mL of the mixture into 9 mL of fresh buffered peptone water with intermittent pipette tip changes and vortexing for 10 s. A parallel dilution series was prepared by using Sabouraud Dextrose broth for fungal enumeration. Aliquots (0.1 mL) from the appropriate dilutions were plated in triplicate onto pre-prepared agar media using the spread plate method (Wamyil et al., 2023). The media included Violet Red Bile Agar (VRBA) for coliforms, Mannitol Salt Agar (MSA) for Staphylococcal and Bacillus species, and Plate Count Agar (PCA) for total aerobic counts. plates were incubated aerobically at 37°C for 24–48 h. Colonies were enumerated and expressed as colony-forming units per gram (CFU/g) of soil. Only plates with 30–300 CFU/g were considered statistically significant (Pepper, 2024).
The distinct colonies were purified via streak plate technique on VRBA, MSA, and Nutrient Agar. The purified isolates were incubated aerobically at 35°C for 24 h (Wamyil et al., 2023; Pepper, 2024). The bacterial isolates were characterized based on colony morphology, Gram staining, and biochemical assays, following the protocols of Robert et al. (1957) and Ameh and Kawo (2018). Biochemical profiling included catalase, indole, citrate utilization, oxidase, triple sugar iron, and hydrogen sulfide (H2S) tests to aid species-level identification. For catalase test, the test expressed the occurrence of catalase; an enzyme that catalyses the liberation of oxygen from hydrogen peroxide. The smear of the bacteria isolate was made on a clean grease-free slide and a drop of 3% hydrogen peroxide was then added to the smear. A catalase positive was observed by effervescence while no effervescence indicates a negative reaction. For the Indole test which is used in the identification of members of the enterobacteriaceae. Most strains break down the amino acid tryptophan with the liberation of indole. 0.5 mL of kovac’s reagent was then added to nutrient broth culture, was shaken properly to homogenize, and incubated for 24 – 48 h. This was examined every 60 seconds. The absence of red colour indicates the absence of Indole (Indole negative) and its presence is confirmed by the red colour formation (Indole formation). The citrate utilization test is used to differentiate among the enterobacteriaceae family based on citrate utilization as a sole source of carbon. 5 mL of Kose’s citrate medium was dispensed into McCartney bottles which were sterilized and allowed to cool down. After which, they are inoculated with an isolate from the suspension of bacterial isolate using a sterile wire loop. The inoculated bottles were incubated at 37°C for 24 – 48 h and the observed position reaction is seen by the increase in turbidity while a negative result is seen of growth in the medium. The oxidase test is mainly used for differentiating between pseudomonas and other gram negative bacteria. Dip a strip of filter paper into the oxidase reagent and then touch the surface of the suspected colony. A purple coloration produced within 5 – 10 s indicates a positive reaction.
Data processing and analysis
GraphPad Prism (Version 8) was used to conduct one-way analysis of variance on the data gathered for this study. The subsequent analysis used Dunnett’s post hoc multiple comparison test for mean comparisons. The data are presented as mean and mean ± standard deviation, where applicable, with all statistical analyses conducted at a 95% confidence level (p < 0.05).
The results of the oxidative stress marker; MDA ( Figure 2) showed significantly elevated levels in samples from groups 1, 2, and 3, which were 0–3 km from the mining site. However, the mean MDA value of Group 4 (5 km from the mining site) was insignificant when compared to the control. Interestingly, the enzymatic antioxidant enzyme (SOD, Cat, and GPx) activities in maize leaves showed a significant (p < 0.05) reduction in the leaves of maize plants grown 0, 1, 3, and 5 km from the mining site ( Figure 2).

(a): Malondialdehyde (MDA) levels, (b): Superoxide dismutase (SOD) levels, (c): Catalase (Cat) levels, and (d): Glutathione peroxidase (GPx). The mean values (n = 3) with asterisk ****p < 0.00005, ***p < 0.0005, **p < 0.005, *p < 0.05 are statistically significant when Groups’ mean values were compared to the control site (no mining activity). DFFCMS (Distance from Farm Central Mining Site).
Hormonal levels in the leaves determined during the vegetative stage of the maize plant are presented in Figure 3. Auxin levels were significantly reduced in groups 1 – 4 (0 – 5 km from the mining site) when compared to the control. Abscisic acid and brassinosteroid levels were significantly reduced in groups 2, 3, and 4 (1 – 5 km from the mining site) when compared to the control. Interestingly, group 1 (0 km within the mining site) was insignificantly higher for abscisic acids, while the mean value for brassinosteroids was elevated (p < 0.05) compared to the control.
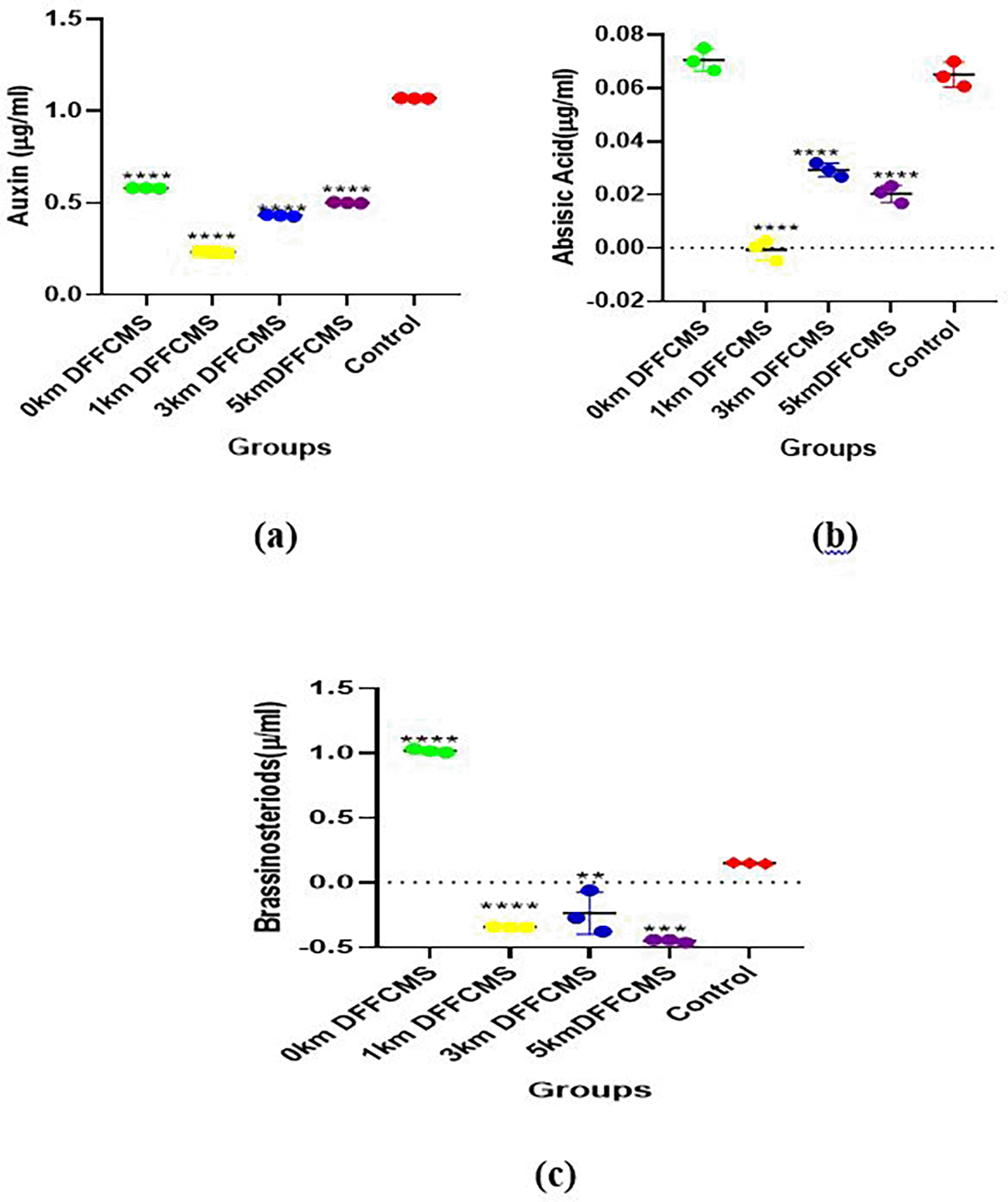
(a): Auxins levels, (b): Abscisic acids levels, (c): Brassinosteriods levels. The mean values (n = 3) with asterisk ****p < 0.00005, ***p < 0.0005, **p < 0.005, are statistically significant when Groups’ mean values were compared to the control site (no mining activity). DFFCMS (Distance from Farm Central Mining Site).
The morphological and biochemical characteristics used for the identification of bacterial isolates from the different sample sites are presented in Table 1. Gram staining was negative for colonies 1, 5, and 8 but positive for the others. Rod cell type was observed on colonies 1 and 5, Rods; colonies 3, 6, and 7, whereas colonies 2, 4, 8, and 9 showed filamentous rods, club rods, and cocci in tetrads and cocci, respectively. The identified bacteria were Acinetobacter sp., Actinomyces sp., Bacillus sp., Corynebacterium sp., Enterobacter sp., Klebsiella sp., Lactobacillus sp., Lactobacillus sp., and Staphylococcus sp. The percentage distribution of bacteria in the soil samples was Bacillus sp. The highest percentage (39.71%) was observed for Acinetobacter sp. (1.47%) ( Table 2). The bacterial loads across the different sample sites at dilution factors of 0.001 and 0.0001 are presented in Figure 4. At a dilution of 0.001, the soil samples collected at a distance of 3 km from the mining site showed the highest value (≥100 × 104 CFU/g). At an average CFU/g × 104 (colony-forming units) for group 1 (0 km within the mining site), the concentration was too high, leading to overcrowded plates where colonies grew so densely that they merged together. As a result, it becomes impossible to accurately distinguish and count individual colonies, as observed in samples and duplicates. However, at a dilution factor of 0.0001, the sample soils at a distance of 0 km from the mining site exhibited the highest value (>35 × 106 CFU/g). Similarly, at average CFU/g ×106 for control and group 3 (3 km from mining site), the microbial load was reduced enough to result in separate, well-isolated colonies, making it possible to perform an accurate colony count as observed in sample and duplicates. The distribution of the bacterial isolates from the different sample sites, as presented in Table 3, showed the presence of Bacillus sp. at both the control and sample sites. Whereas Staphylococcus spp. were found at 1 km, 3 km, and 5 km from the mining site, Klebsiella spp was only found in sample soils at 1 km and 3 km from the mining site.
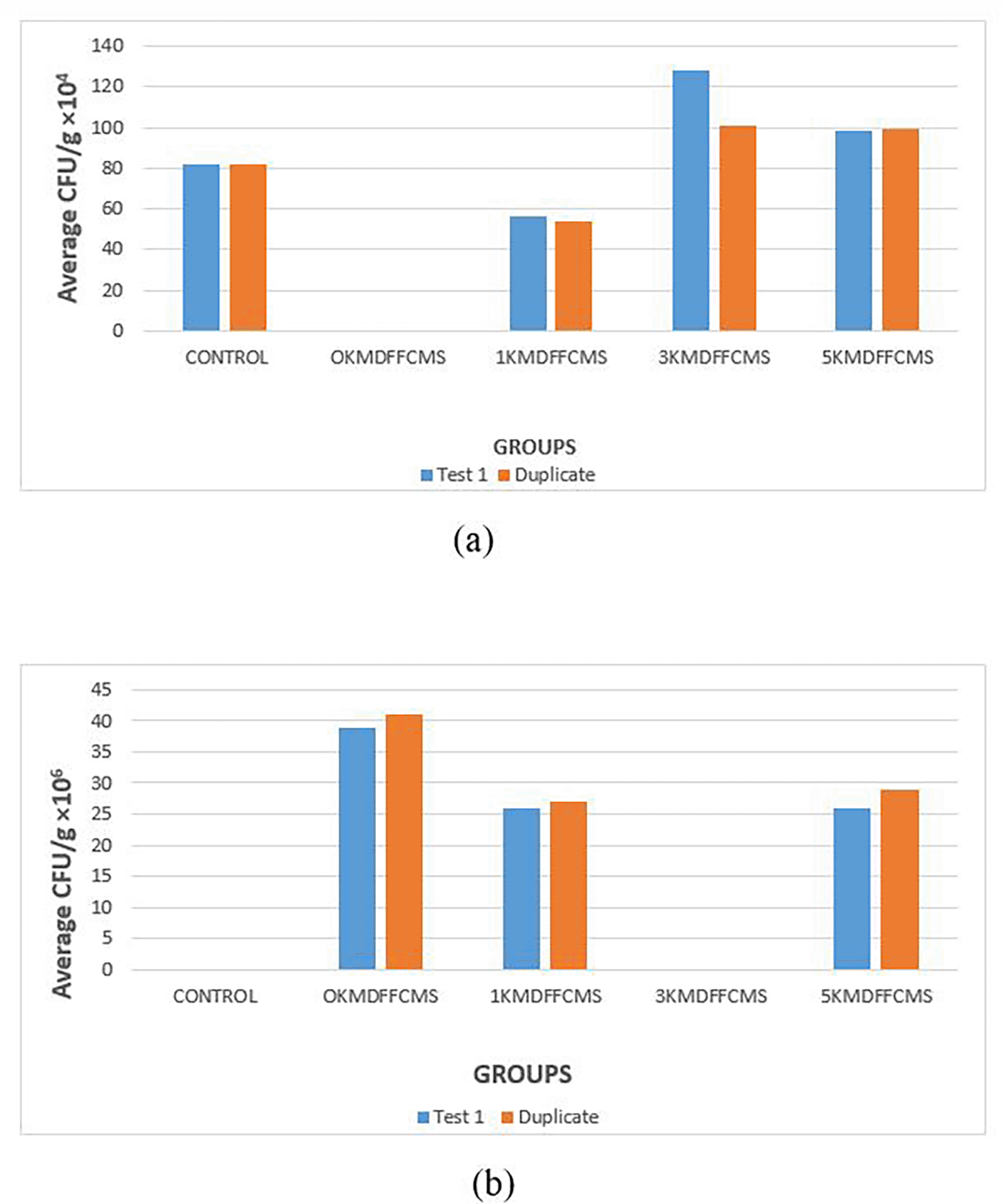
(a): 0.001 dilution factor, and (b): 0.0001 dilution factor. DFFCMS; Distance from Farm Central Mining Site.
The ability of heavy metals to bio-accumulate or bio-magnify in the environment is a serious safety concern for all organisms, especially plants, as toxic levels of these heavy metals can interfere with essential biomolecules of plants, such as DNA and nucleoproteins, which can induce the production of reactive oxygen species (Shaffique et al., 2023b). The interactions between the generated free radicals and essential biomolecules, such as unsaturated lipids located on the surfaces of the cell membrane, can result in lipid peroxidation, as well as protein oxidation and degradation (Ayala et al., 2014; Chaudhary et al., 2023).
Lipid peroxidation can be assessed by detecting MDA levels in the plant. Elevated MDA levels are anticipated in plants under stress, particularly because of heavy metal toxicity (de Oliveira et al., 2013; Onyango et al., 2020). The findings of this investigation indicated a substantial increase in MDA levels in the leaves of maize plants cultivated at distances of 0, 1, and 3 km from the mining site; however, the 5 km distance revealed no difference compared to the control site. The results indicate that the plants cultivated at 0 km, 1 km, and 3 km experienced oxidative stress, perhaps linked to the elevated levels of heavy metals identified in this study. The oxidative stress observed, marked by the increased MDA levels, may be a result of the presence of the heavy metals that induced lipid peroxidation, whereby the polyunsaturated fatty acids (PUFAs) were targeted. Free radicals in the form of hydroxyl radicals (•OH) or superoxide (O2-) can react with PUFAs to form lipid radicals, which react with molecular oxygen (O2) to produce lipid peroxyl radicals (LOO•) to initiate a chain reaction (Onyango et al., 2020). The decomposition of lipid hydroperoxides (LOOH) results in the production of malondialdehyde (MDA) as a by-product (Endale et al., 2023).
Heavy metals such as Ni, Fe, Mn, Co, Cd, Cu, Zn, Hg, and arsenic can accumulate in soils, and when they are absorbed, they affect plant metabolism and induce stress in plants, with evidence of increased MDA levels (Ghori et al., 2019). The selected heavy metals, Pb, Cu, and Cd, showed consistent increases in MDA levels, signaling the presence of stress when Dunaliella salina was exposed to these heavy metals (Gao et al., 2024). Increased MDA levels, a marker of oxidative stress, were corroborated by a study in which Ocimum basilicum L. was subjected to elevated levels of Ni, Zn, and Cu (Georgiadou et al., 2018). The MDA results from this study correspond with the results of Georgiadou et al. (2018), Ghori et al. (2019), Rizvi and Khan (2019), and Gao et al. (2024).
Antioxidative enzymes constitute the enzymatic antioxidant system that regulates minimum levels of certain radicals, notably superoxide dismutase, Catalase, and Glutathione peroxidase (Mansoor et al., 2023). In response to heavy metal stress, the expression of enzymes significantly increases to neutralize reactive oxygen species; therefore, plants that demonstrate resistance to the harmful effects of heavy metal-induced free radicals are expected to have increased levels of these enzymes (Chaudhary et al., 2023). The activities of the antioxidant enzymes SOD, CAT, and GPx were reduced significantly at the sample sites when compared to the control.
In the mitigation of toxicity by free radicals from heavy metal contamination, the first enzyme mobilized is superoxide dismutase, which acts as the first line of defense against oxidation, in which superoxides are converted into oxygen and hydrogen peroxide (Wang et al., 2018; Zheng et al., 2023). The produced hydrogen peroxide must also be metabolized to eliminate its toxic effects (Jomova et al., 2024). This detoxification activates supplementary enzymes from the antioxidant pathway, including CAT and GPx (Mansoor et al., 2023). Although these enzymes are organelle-specific, with catalase (CAT) demonstrating peak activity in peroxisomes or glyoxysomes and glutathione peroxidase (GPx) linked to chloroplasts and mitochondria, their primary role is to convert hydrogen peroxide into harmless byproducts: water and oxygen (Sandalio et al., 2021; Ugalde et al., 2021). Malar et al. (2016) identified decreased SOD activity while investigating growth and antioxidant enzyme levels in water hyacinths (Eichhornia crassipes) exposed to lead (Pb) toxicity. An in-depth analysis of several cowpea cultivars of TVu showed increased SOD activity in tolerant cultivars and reduced activities in stressed cultivars when exposed to different concentrations of NaCl (Praxedes et al., 2014). Significant increases in the relative gene expression and activities of enzymes such as CAT, SOD, GPX, and APX were observed in the leaves and shoots of wheat, Triticum aestivum L., when the effectiveness of antioxidative defense against lead toxicity was studied for tolerance (Navabpour et al., 2020).
Iftikhar et al. (2025) showed that the activity of catalase was significantly reduced when the P3939 cultivar of maize was exposed to varying concentrations of chromium. Activities of enzymatic antioxidants such as SOD. CAT, APx, and GPx levels were reduced in the leaves and shoots of Phaseolus vulgaris L. when exposed to increasing concentrations of cadmium (Gutiérrez-Martínez et al., 2020). Plants grown in soils contaminated with heavy metals, displaying lowered antioxidant enzyme levels, may utilize alternative tolerance mechanisms beyond the antioxidant cascade, as diminished antioxidant capacity signifies plant stress, whereas increased capacity indicates tolerance (Laxa et al., 2019; Angulo-Bejarano et al., 2021). The findings of this study imply that maize plants experience stress, indicating that their survival may not be associated with the antioxidant pathway, which is consistent with several studies demonstrating reduced antioxidant enzyme activity in stressed plants.
Phytohormones can act as chemical messengers that induce signaling pathways involved in the induction of certain tolerance genes, thus providing a basis for the management of heavy metal-induced stress (Bücker-Neto et al., 2017). Among the notable plant hormones which function to prevent the occurrence of the toxic effect of heavy metals in plants when exposed to mostly abiotic stress are the bscisic acid, auxin, and brassinosteroids (Waadt et al., 2022). Auxins are essential plant hormones that facilitate the management of heavy metal (HM) stress, and their production is triggered by the absorption of HMs (Zhang et al., 2022). Auxins are produced in the shoot region of plants and are necessary for various metabolic processes such as cell division, elongation, and differentiation (Kean-Galeno et al., 2024). Auxins affect how plants respond to external stimuli such as light and gravity and are involved in root and fruit development (Mazzoni-Putman et al., 2021). The results of this study demonstrated that auxin concentrations in germinated maize plants from the sampled soils were significantly lower (p < 0.05) than those in the control soil. Auxin concentrations in the sampled soils were highest at the mining site (0 km), whereas the concentrations at 1 km from the site were the lowest.
Exposure of wild-type (WT) Arabidopsis seedlings to Cd stress resulted in a significant decline in growth (Hu et al., 2013). In their study, Cd treatment led to a significant reduction in auxin levels with a corresponding increase in indole acetic acid (IAA) oxidase and alterations in the expression of many potential auxin biosynthesis and catabolic genes. In a study to determine the ability of Acutodesmus obliquus to tolerate the presence of lead through the external application of different auxins (IAA, IBA, PAA) (Piotrowska-Niczyporuk et al., 2020), the findings showed that lead treatment led to a decrease in intracellular concentrations of IAA (by 33%) and PAA (by 45%) in A. obliquus cells compared to the control group. Heavy metal toxicity may reduce auxin levels in plants by interfering with auxin synthesis, transport, and signaling (Moeen-Ud-Din et al., 2023). Auxin oxidase is activated by oxidative stress induced by heavy metals, and the increased activity of auxin leads to increased degradation of auxin. This degradation affects root development, shoot elongation, and plant growth (Moeen-Ud-Din et al., 2023). In addition, the presence of heavy metals influences auxin transportation by changing the functions of PIN proteins associated with auxin transport and distribution (Cha et al., 2022). In this study, the auxin levels determined showed decreased values and may be interpreted to mean that the maize plant experienced stress, and the tolerance mechanism may not include the increased synthesis of auxin. This observation is consistent with several studies showing that tolerance involves increased auxin biosynthesis, and stressed plants have reduced auxin concentrations in response to heavy metal presence (Hu et al., 2013; Piotrowska-Niczyporuk et al., 2020; Cha et al., 2022; Moeen-Ud-Din et al., 2023).
Abscisic acid is a complex plant hormone that is essential for the life cycle of plants with respect to seed development and dormancy is abscisic acid (Bücker-Neto et al., 2017). Abscisic acids, in association with other hormones, such as ethylene and gibberellin, are known to be important regulators of plant responses to abiotic stress; abscisic acids are the most essential principal regulatory and signaling hormones (Sah et al., 2016; Bücker-Neto et al., 2017; Shu et al., 2018; Pan et al., 2020). The results of this study showed reduced levels of ABA in the 1-5KM km mining sites compared to the control. However, the levels of ABA at 0 km within the mining site increased. Heavy metals, such as lead, mercury, cadmium, and arsenic, significantly affect ABA signaling pathways, which influence plant metabolism (Hu et al., 2020). These heavy metals induce oxidative stress by amplifying stress signals, thus triggering ABA biosynthesis as a mitigating strategy for tolerance (Rai et al., 2024). Increased biosynthesis is associated with the induction of genes and the activation of enzymes linked to ABA synthesis and their increased levels (Zhao et al., 2023). The activity of ABA catabolic enzymes, such as 8’-hydroxylase, is inhibited by heavy metals at minimal concentrations and therefore reduces the degradation of ABA, leading to increased levels and induction of tolerance (Mo et al., 2024).
While an increase in the endogenous levels of ABA is associated with the mitigation strategy of plants in the presence of heavy metals, decreased levels of ABA are observed during plants’ susceptibility to heavy metal-induced oxidative stress, especially with prolonged exposure, as observed in this study, in which the levels of ABA were reduced at 1 – 5 km. This may stem from the disruption of ABA biosynthetic pathways or the inactivation of ABA-associated enzymes (Chen et al., 2021). Heavy metals like cadmium (Cd) and lead (Pb) may impede the expression or functionality of essential enzymes in the ABA biosynthesis pathway, notably NCED (9-cis-epoxycarotenoid dioxygenase), a rate-limiting enzyme in ABA biosynthesis (Zhang et al., 2022). This enzymatic inhibition may result from heavy metal-induced damage to chloroplasts, where ABA biosynthesis commences (via carotenoid cleavage), the endoplasmic reticulum, and peroxisomes, where ABA processing transpires, and downregulation of gene expression through epigenetic modifications or transcriptional repression (Zdunek-Zastocka et al., 2024). Heavy metals may augment the activity of ABA catabolic enzymes, such as ABA 8’-hydroxylase, encoded by CYP707A genes, resulting in the accelerated breakdown of ABA into phaseic acid and other inactive metabolites. Thus, ABA levels are reduced in plants (Li et al., 2024).
ABA concentrations significantly increase during drought conditions, as well as in reactions to salt and low temperatures (Kim et al., 2013). Heavy metals affect plant metabolism by interacting with guard cells, which regulate stomatal opening and closing, whereas drought is known to induce stomatal closure (Bücker-Neto et al., 2017). It is well known that heavy metals behave like drought, another abiotic stressor, as seen in the exposure of cowpeas to iron toxicity (Ifie et al., 2020). Endogenous ABA concentrations were significantly increased in Panicum virgatum L. as a strategy for drought tolerance (Aimar et al., 2014). Triticum aestivum L. seedlings showed tolerance to drought stress through the exogenous application of ABA (Wei et al., 2015) whereas increased concentrations of ABA were observed in the roots of Typha latifolia and Phragmites australis, Oryza sativa, and potato tubers after exposure to Cd, indicating tolerance (Fediuc et al., 2005; Hsu and Kao, 2005; Stroinski et al., 2010). Mercury, copper, and cadmium produce varying levels of ABA in wheat seeds exposed to these heavy metals (Munzuroğlu et al., 2008). ABA levels were significantly reduced when Boehmeria nivea L. was exposed to Cd toxicity, and this decrease was associated with stress and/or susceptibility according to Chen et al. (2021). However, a significant increase in endogenous ABA levels was observed in African (Oryza glaberrima Steud) seedlings when they were exposed to acute iron toxicity, which accounts for tolerance (Majerus et al., 2009). The results of the present study correspond with those of previous studies, indicating that increased ABA levels are associated with plant tolerance to heavy metal toxicity, whereas decreased levels suggest susceptibility to oxidative stress. These findings suggested that maize plant tolerance does not involve endogenous ABA biosynthesis.
Plant growth, light-induced development, seed germination, stomatal function, and senescence in plants are affected by brassinosteroid levels of brassinosteroids (Bücker-Neto et al., 2017). Antioxidant activities are modulated by brassinosteroids to confer tolerance on plants to heavy metal-induced oxidative stress and are achieved by the induction of the expression of antioxidant genes (Hayat et al., 2007; Hafeez et al., 2021). Abiotic stressors, such as salt and low temperature, induced increased endogenous brassinosteroid levels in microalgal species, which postulates the significance of the involvement of brassinosteroids in plant defense against heavy metal-induced stress and tolerance (Stirk et al., 2018). The results obtained in this study showed that the levels of brassinosteroids at the 1 – 5 km distance of the mining site were significantly reduced, whereas they were increased at 0 km when compared to the control. This reduction may be an indication of the effect of distance and location on the heavy metal concentrations within the mining site inducing stress, but the higher value observed for the 0 km treatment may be due to tolerance or initial increase triggered by heavy metals.
Heavy metals contribute to the downregulation of critical brassinosteroid biosynthetic genes, including DWF4 (DWARF4) and CPD (Constitutive Photomorphogenesis and Dwarfism), which encode cytochrome P450 enzymes vital for brassinosteroid synthesis, as well as BR6ox (Brassinosteroid-6-Oxidase), which facilitates the final step in brassinosteroid biosynthesis (Chung and Choe, 2013; Zhou et al., 2024). Oxidative stress induced by heavy metals compromises cell membranes and proteins, disrupts normal metabolic functions, particularly hormone biosynthesis, and impedes the absorption of essential nutrients such as Mg, Fe, and Zn, which are vital cofactors for BR biosynthetic enzymes (Rajewska et al., 2016; Chang et al., 2024). In certain instances, particularly at reduced heavy metal concentrations or during the initial stress phases, plants may transiently elevate brassinosteroid production to initiate defense mechanisms by augmenting antioxidant enzyme activity, regulating ion homeostasis and detoxification pathways, and facilitating growth recovery and damage repair (Li et al., 2022; Song et al., 2025).
Overexpression of the brassinosteroid biosynthetic gene DWF4 simultaneously increased seed yield and stress tolerance in Brassica napus (Sahni et al. 2016). A study that involved the exposure of Oryza sativa to Cd stress showed that BR could reduce the amount of Cd that accumulated in rice shoots by reducing the amount of Cd that was translocated from roots to shoots; thus, the rice became tolerant to Cd toxicity (Sun et al., 2024b). The exogenous application of brassinosteroids increases the tolerance to seed germination and seedling growth of Brassica juncea L. upon exposure to lead (Soares et al., 2020). The results of this study, therefore, are consistent with various studies that imply stress in plants with decreased brassinosteroids and tolerance.
Plant roots produce a wide variety of compounds that serve as chemical attractants for different metabolically distinct populations of soil microorganisms, which influences the composition of exudates, plant species, and physiological conditions (Upadhyay et al., 2022). A special class of microorganisms that is capable of colonizing the roots of plants as well as enhancing plant growth activity via different mechanisms is plant-growth-promoting rhizobacteria (Sun et al., 2024a). These rhizobacteria are special organisms that are found around or on the root surfaces and are involved in the metabolic processes of plant growth, either directly or indirectly, by regulating plant hormonal levels and the uptake of minerals (Bhat et al., 2023).
The morphological and biochemical characteristics for the identification of the bacterial isolates from the different sample sites for this study showed that Gram staining was negative for colonies 1, 5, and 8, while the others were positive. Rod cell type was observed on colonies 1 and 5, Rods; colonies 3, 6, and 7, whereas colonies 2, 4, 8, and 9 showed filamentous rods, club rods, and cocci in tetrads and cocci, respectively. The identified bacteria were Acinetobacter sp., Actinomyces sp., Bacillus sp., Corynebacterium sp., Enterobacter sp., Klebsiella sp., Lactobacillus sp., Lactobacillus sp., and Staphylococcus sp. The percentage distribution of bacteria in the soil samples was Bacillus sp. The highest percentage (39.71%) was observed for Acinetobacter sp. (1.47%). Bacterial load across different sample sites at a dilution factor of 0.001. The soil samples collected at a distance of 3 km from the mining site showed the highest value (≥100 × 104 CFU/g). However, at a dilution factor of 0.0001, the sample soils at a distance of 0 km from the mining site exhibited the highest value (>35 × 106 CFU/g). The distribution of the bacterial isolates from the different sample sites showed the presence of Bacillus sp. at both the control and sample sites. Whereas Staphylococcus spp. were found at 1 km, 3 km, and 5 km from the mining site, Klebsiella spp was only found in sample soils at 1 km and 3 km from the mining site.
The presence of both Gram-negative and Gram-positive bacteria highlights the variability in microbial populations, which may have been influenced by the presence of heavy metals. The microbial load was significantly higher at 0 km from the mining site at a dilution factor of 0.0001 (>35 × 10 CFU/g), indicating possible enrichment due to the presence of heavy metals or organic residues favoring microbial survival. At a distance of 3 km, the bacterial density remained high (≥100 × 104 CFU/g at 0.001 dilution), suggesting that contamination or favorable growth conditions extend beyond immediate proximity to the mining activity. The presence of Bacillus sp. found in both control and sample sites suggests that it maintains a stable presence regardless of environmental stress (heavy metal), reinforcing its usefulness and important roles as a PGPR (Tsotetsi et al., 2022). Bacillus sp. dominance (39.71%) is notable given its role in plant growth promotion through mechanisms such as nitrogen fixation, phosphate solubilization, and stress (Tsotetsi et al., 2022).
Staphylococcus spp. (1 km, 3 km, and 5 km) and Klebsiella spp. (1 km, 3 km) indicated site-dependent variability, possibly linked to soil composition, pH, or contamination levels, as well as heavy metal-induced chelation and possible growth of maize plants. Klebsiella spp. and Enterobacter spp., which are known for their nitrogen-fixing abilities, may offer insights into soil fertility restoration in contaminated areas (Ji et al., 2020; Youseif et al., 2025). Nitrogen fixation, which is the conversion of atmospheric nitrogen into forms usable by plants, is crucial for healthy plant growth and development. It provides plants with the nitrogen necessary to build proteins, DNA, and other essential compounds (Mahmud et al., 2020). Heavy metals significantly reduce nitrogen fixation in plants by inhibiting the activity of nitrogen-fixing bacteria and the enzymes involved in nitrogen assimilation. This can lead to decreased nitrogen availability in the soil, negatively affecting plant growth and the overall nitrogen cycle (Zayed et al., 2023). Heavy metals, particularly lead and cadmium, can negatively impact the activity of nitrogen-fixing bacteria, such as rhizobia in legume nodules and other soil microorganisms involved in nitrogen transformation (Jach et al., 2022). Thus, the identification of Acinetobacter sp., Actinomyces sp., Bacillus sp., Corynebacterium sp., Enterobacter sp., Klebsiella sp., Lactobacillus sp., and Staphylococcus sp. suggests a mix of both beneficial and potentially opportunistic bacteria that induce tolerance in maize plants for their growth, despite the presence of heavy metals.
The findings of this study clearly show that pollution of farmlands as a result of heavy metal mining has a significant effect on the antioxidant capacity of maize plants, as evidenced by increased malondialdehyde, decreased antioxidant enzymes, superoxide dismutase, catalase, and glutathione peroxidase, and varied hormonal responses. The presence of plant growth-promoting rhizobacteria, such as Bacillus spp., which were dominant, provided insight into the role of these rhizopheric organisms in conferring tolerance and ensuring the survival of maize plants in the presence of heavy metals. The study also highlighted the need for the application of external plant hormones and plant growth-promoting rhizobacteria as mitigation strategies in soils contaminated with heavy metals, as is the case with farmlands around the Kilembe mining site, Kasese, and Uganda, and the results can be extrapolated globally.
However much this study did not involve human nor animal studies, this research study received ethical approval from the Kampala International University Western Campus Research Ethics Committee (approval number: KIU-2024-397) and was registered with the Uganda National Council for Science and Technology (registration number: NS809ES).
Due to ethical considerations and local data governance policies as it concerns the Kilembe Mining Company around where the study was conducted, raw data are not publicly archived. However, the data collected, generated, and analyzed during this study which includes the levels of MDA, SOD, CAT, GPx, and microbial loads will be made available by the corresponding author (Dr. Ifie Eseoghene Josiah) through the email; josiahifie@gmail.com upon reasonable request.
Our appreciation goes to the head and members of the Institute of Biomedical Research (IBR, Biochemistry and Microbiology and Immunology laboratories, Kampala International University, Western Campus, Uganda where the SOD, Catalase and microbial analysis were performed. Our gratitude also goes to Mr. Rubeihayo Randloph and other members of the Analytical Biosciences Laboratory, Makerere University, Kampala, Uganda where the analysis for the glutathione peroxidase was performed. Mr. Jackim Nabona, Mr. Danladi Makeri and Mr. Pius Theophilus are appreciated for their inputs in the microbial analysis.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Plant sciences, Ecotoxicology, Environmentaal Biolgy
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Relevant to this article
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Remediation of heavy metals contaminated soil
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 05 Sep 25 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)