Keywords
Malaria, economic evaluation, perennial malaria chemoprevention, Benin, Cameroon, Cote d'Ivoire
This article is included in the Pathogens gateway.
This article is included in the Global Public Health gateway.
Malaria continues to impose a significant mortality and morbidity burden, particularly among young African children. Children under two years of age account for one-third of the estimated 597,000 annual global malaria deaths. Perennial malaria chemoprevention (PMC) is a strategy recommended by the World Health Organization to reduce malaria in this high-risk age group. However, uptake remains low in many endemic countries. Given the limited resources available for health and the stagnation of malaria budgets over the last decade, it is important for policymakers to understand the costs and cost-effectiveness of PMC to support future decision making.
We describe the design of an economic evaluation of PMC in Benin, Cameroon, and Côte d’Ivoire, where it is being implemented at scale by the ministries of health through the Essential Programme of Immunization (EPI) with support from Population Services International. We will take a disaggregated societal perspective, collecting data on resources provided for PMC by 45 health facilities across 12 districts in these three countries, higher levels of the health services, donor-supported activities, and households. This study will assess the financial and economic costs per dose of PMC and calculate the incremental cost per malaria case averted and per disability-adjusted life-year averted by PMC compared to the usual standard of care. We will model the costs and effects of scaling up PMC to other regions in the three countries where PMC is deemed suitable.
This economic evaluation aims to provide robust evidence from three countries, each implementing different PMC delivery models, regarding whether, where, and under what circumstances investing in PMC may represent an efficient and equitable use of scarce resources. We expect these findings to be valuable for the governments of Benin, Cameroon, and Côte d’Ivoire and other countries and international donors in designing the optimal mix of malaria control interventions.
Malaria, economic evaluation, perennial malaria chemoprevention, Benin, Cameroon, Cote d'Ivoire
In 2023, 263 million malaria cases and 597,000 deaths occurred globally, nearly all (94%) in the World Health Organization (WHO) African Region.1 Children under two years of age account for one-third (36%) of global malaria deaths.2 Since 2010, the WHO has recommended intermittent preventive treatment of malaria in infants (IPTi) with three doses of sulfadoxine-pyrimethamine (SP) in areas of sub-Saharan Africa with moderate to high malaria transmission, where SP is still effective.3,4 However, only Sierra Leone implemented IPTi on a programmatic scale.5 To address the stagnating progress in malaria control in recent years, the WHO and national governments have pursued new tools, including vaccines, monoclonal antibodies, and improved insecticide-based vector control interventions, and have sought to maximize the potential of existing tools, notably chemoprevention.6 In June 2022, the WHO recommended perennial malaria chemoprevention (PMC), which expanded and added flexibility to IPTi guidance in four main ways: first, extending the target age range to include children older than 1 year of age; second, allowing delivery of PMC doses alongside any suitable delivery strategy; third, removing the restriction on the target number of PMC doses; and fourth, allowing flexibility in the choice of antimalarial drug combination.7 Given the stagnation of malaria budgets over the last decade, policymakers require evidence on whether and under what circumstances PMC may represent an efficient and equitable use of scarce resources to support decision making on how to prioritize PMC relative to other malaria and wider health interventions.
Cost-effectiveness analyses conducted alongside randomized controlled trials (RCTs) found a three-dose schedule of PMC to be cost-effective in areas of moderate to high malaria transmission intensity when delivered alongside the Essential Programme on Immunization (EPI).8,9 The low cost of SP and integration with the existing EPI delivery system were key determinants of the low intervention costs observed; effects were greater in areas of higher transmission, but low coverage, and the growing problem of SP resistance reduced the effectiveness and, therefore, cost-effectiveness of the intervention.10 All existing economic evaluation evidence has assessed the implementation of three-dose schedules via the EPI platform within trials. In recommending PMC, the WHO Guidelines Development Group identified several evidence gaps, including “updating estimates of the costs and cost-effectiveness of PMC delivered through the EPI, including in settings with low coverage of routine childhood immunization, and by evaluating the costs of alternative approaches to delivering PMC”.7 Additional investments will be required to increase the coverage of existing EPI delivery channels and to expand to community-based delivery, as well as to provide more doses of SP to a wider age range of children. However, by averting more malaria cases, PMC may also avert substantial malaria treatment costs, reduce the disease burden, and potentially generate long-term economic benefits as part of overall efforts to reduce the malaria burden.11 To understand whether investing in PMC represents a cost-effective and equitable use of scarce resources, rigorous economic evaluation evidence is needed.
We outline a protocol for a multi-country economic evaluation of PMC conducted in three West/Central African countries (Benin, Cameroon, and Côte d’Ivoire) as part of the Unitaid-funded Plus Project; a linked economic evaluation in Mozambique is described separately.12 The overall project aimed to support governments in co-designing and implementing tailored PMC delivery models in these four countries, which were chosen based on their interest in implementing PMC, high malaria transmission, SP resistance profiles, and good EPI coverage, and to generate evidence to inform the scale-up of PMC in these and other countries. The specific objectives of the economic evaluation in Benin, Cameroon, and Côte d’Ivoire are as follows:
1. Estimate the costs associated with implementing PMC through alternative delivery models at scale.
2. Analyze the cost-effectiveness of adopting alternative PMC models in addition to existing malaria control practices, relative to current practice, and compare with alternative interventions targeting children under 2 years of age.
3. Assess the expected budget impact of scaling up PMC from the provider’s perspective.
4. Analyse the equity of adopting PMC in terms of costs and health outcomes.
Stakeholders from the National Malaria Control Programme (NCMP), EPI, and other government bodies, non-governmental organizations (NGO), donors, and community organizations collaboratively co-designed PMC delivery models in each country through workshops in October-December 2021 and dialogue before and after. The funder was not present in the co-design but required a target population of children under the age of 2 years, use of SP, and ≤8 doses. Benin and Cameroon opted for an 8-dose schedule and Côte d’Ivoire a 5-dose schedule, co-delivered within the existing EPI delivery schedule (vaccines, Vitamin A supplementation, or growth checks) ( Table 1); the latter decision by Côte d’Ivoire was based on the assessment that coverage at other contact points was insufficient for effective PMC co-delivery.
Country stakeholders also selected the PMC pilot implementation districts ( Table 2). Areas receiving seasonal malaria chemoprevention (SMC) were excluded. The remaining districts were scored using the following criteria: duration of malaria transmission >6 months, high malaria prevalence, high vaccination coverage, and acceptable SP resistance (<50% prevalence of 540E). In Benin and Côte d’Ivoire, stakeholders selected one health district in each of the three regions (north, center, and south) to account for variation in epidemiological profiles. In Cameroon, stakeholders selected the Centre Region because it has the country’s highest malaria burden and chose six health districts from the 32 within the region based on high malaria incidence and the majority of rural populations ( Figure 1). Across the three countries, the ownership mix of facilities delivering PMC varied ( Table 2). Cameroon’s national policy supports a 5-dose PMC schedule in areas with perennial transmission, while Benin and Côte d’Ivoire do not currently implement PMC beyond the Plus Project-supported districts.
| Country | Region | District | Population <2 years (2023 data) | Malaria prevalence (6-59 months)13,14,15 | Administrative coverage of DTP3 (2022)* | Total Health Facilities | Total health facilities providing PMC | Start of PMC implementation | Total health facilities sampled for economic evaluation data collection |
|---|---|---|---|---|---|---|---|---|---|
| Benin | Zou | Zogbodomey-Bohicon-Zakpota (ZoBoZa) | 37,448 | 37% | 137% | 86 (32 public, 54 private) | 32 (32 public, 0 private) | 30-Nov-22 | 6 (6 public, 0 private) |
| Borgou | Bembèrèkè-Sinendé (BS) | 21,000 | 45% | 131% | 48 (35 public, 13 private) | 35 (35 public, 0 private) | 31-Mar-23 | 6 (6 public, 0 private) | |
| Couffo | Klouékanmè-Toviklin-Lalo (KTL) | 31,758 | 51% | 103% | 36 (32 public, 4 private) | 32 (32 public, 0 private) | 31-Mar-23 | 6 (6 public, 0 private) | |
| Cameroon | Centre | Bafia | 11,546 | 49% | 68% | 74 (37 public, 37 private) | 45 (31 public, 14 private) | 01-Dec-22 | 0 |
| Nkolbisson | 9,634 | 49% | 75% | 77 (4 public, 73 private) | 47 (4 public, 43 private) | 01-Dec-22 | 5 (1 public, 4 private) | ||
| Soa | 3,203 | 49% | 129% | 38 (11 public, 27 private) | 21 (10 public 11, private) | 01-Dec-22 | 5 (2 public, 3 private) | ||
| Ngoumou | 3,092 | 49% | 62% | 34 (29 public, 5 private) | 33 (28 public, 5 private) | 01-Dec-22 | 0 | ||
| Obala | 9,574 | 49% | 79% | 32 (17 public, 15 private) | 32 (17 public, 15 private) | 01-Sep-23 | 0 | ||
| Ntui | 9,176 | 49% | 69% | 46 (25 public, 21 private) | 46 (25 public, 21 private) | 01-Aug-23 | 5 (3 public, 2 private) | ||
| Côte d’Ivoire | Indenie-Djuablin | Abengourou | 28,174 | 28% | 87% | 40 (36 public, 4 private) | 40 (36 public, 4 private) | 23-Nov-22 | 4 (3 public, 1 private) |
| Marahoue | Bouaflé | 39,025 | 48% | 88% | 37 (37 public, 0 private) | 37 (37 public, 0 private) | 19-Apr-23 | 4 (4 public, 0 private) | |
| Worodougou | Seguela | 22,873 | 53% | 94% | 39 (39 public, 0 private) | 39 (39 public, 0 private) | 25-Apr-23 | 4 (4 public, 0 private) |
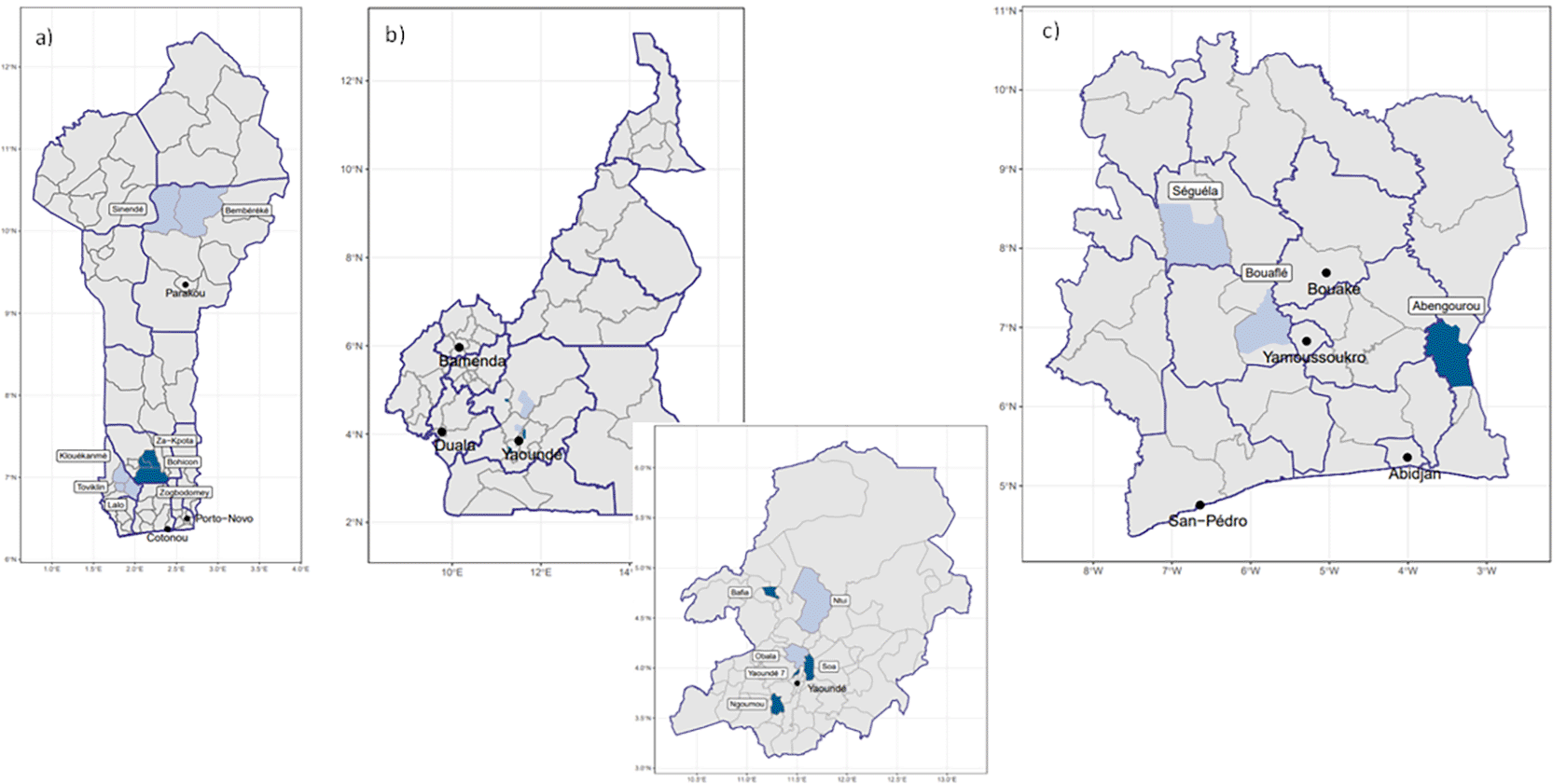
Dark blue indicates phase 1 districts; light blue indicates phase 2 districts.
The Ministry of Health (MoH) is delivering PMC with support from Population Services International (PSI), an international NGO, in the creation of training and routine monitoring materials, procurement of SP, and co-delivery of supportive supervision. In all three countries, health workers deliver PMC through health facility-based EPI clinics and outreach clinics, and community health workers (CHWs) support community mobilization activities. In Cameroon, CHWs have also been trained to administer PMC to children aged >6 months who received their first dose at a health facility, with priority given to areas with gaps in outreach coverage.
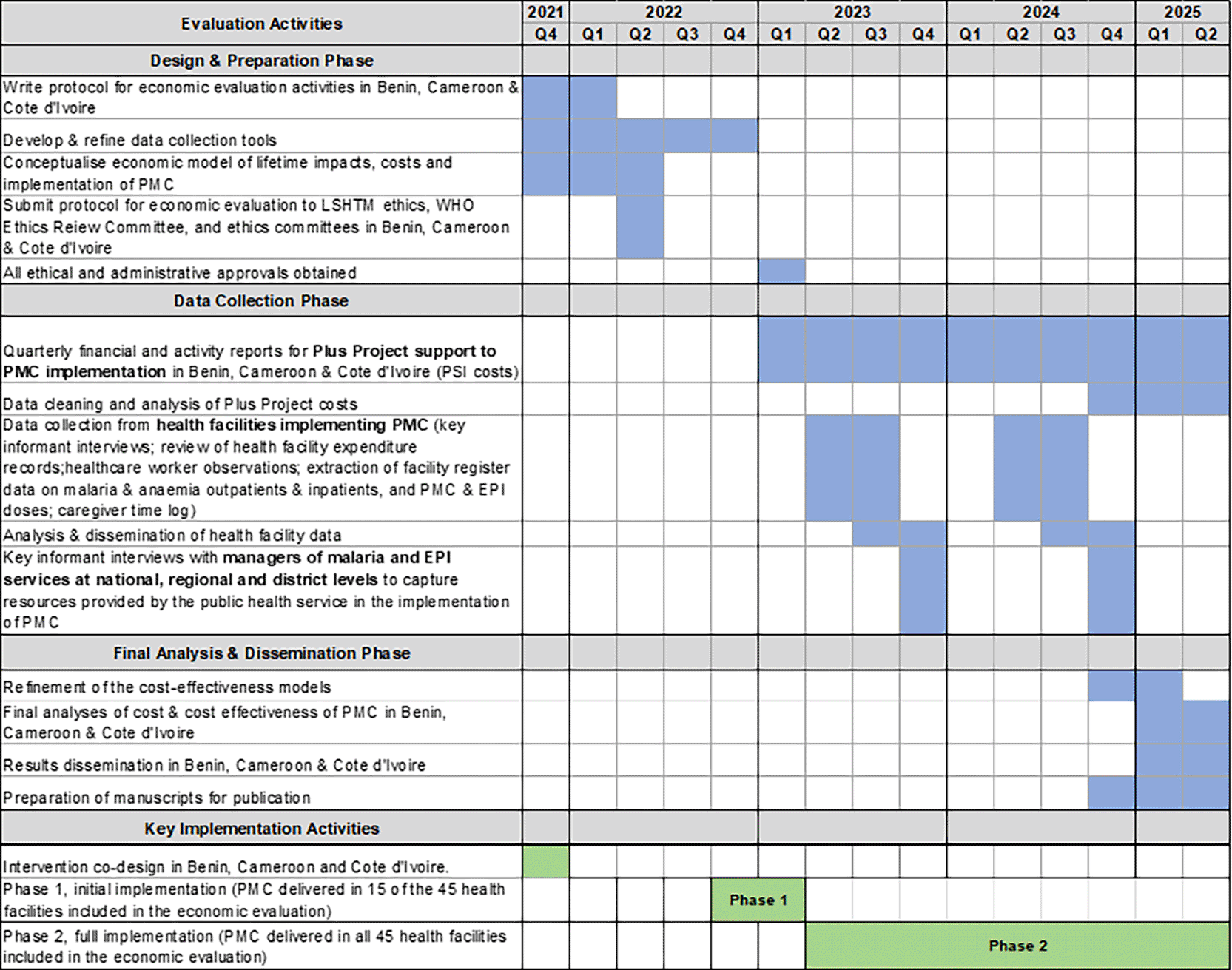
PMC implementation was planned in two phases: Phase 1 districts began delivering PMC in November-December 2022, with Phase 2 districts starting in March-June 2023 ( Table 2). Plus Project support for implementation in phase 1 and 2 districts will continue until March 2025 in Benin and Côte d’Ivoire, and until June 2025 in Cameroon.
The 12 selected implementation districts across the three countries have a combined target population of 226,000 children under 2 years. The Plus Project is supporting PMC delivery at 435 health facilities. Malaria transmission is perennial and intense, with malaria prevalence among children aged 6-59 months of around 50% (range across districts: 34-51%) ( Table 2). The administrative coverage of DTP1, a marker of EPI coverage, was ≥68% in all districts (range across districts: 69-156%) in 2023.
In Benin, the project will support PMC delivery in all 99 public health facilities (and their outreach services) currently providing EPI in the three selected health districts, whose combined target population is approximately 90,000 children under two years; preventive services (EPI and PMC) are not routinely provided in private health facilities in Benin (although they represent an important source of care for curative services, including malaria treatment13) ( Table 2). In Cameroon, the project will support PMC delivery in 224 health facilities (115 public and 109 private), approximately two-thirds of the total facilities providing EPI in the six selected districts. Around half of the facilities are public and half are private, including both faith-based not-for-profit and for-profit facilities, reflecting the distribution of healthcare providers in the intervention area. The total target population is approximately 46,000 children under two years. In Côte d’Ivoire, the project will support PMC delivery in all 112 public health facilities and in the four faith-based not-for-profit health facilities currently providing EPI in the three selected districts. The total target population is approximately 90,000 children under two years.
The study period for the economic evaluation was from January 2022 to July 2025 ( Figure 2). The decision problem motivating the economic evaluation is whether decision-makers in Benin, Cameroon, and Côte d’Ivoire should add PMC to their current package of malaria interventions, and if so, where and using which delivery model(s), reflecting the opportunity costs of this investment. Key decision-makers include managers within the NMCP and EPI of the Ministry of Health, as well as international donors supporting malaria control. We will take a disaggregated societal perspective, meaning that we will consider the costs to the provider (including the donor and national health service) and the household, both separately and together. We assess incremental financial costs, which reflect additional expenditure, and incremental economic costs, which reflect the opportunity cost of all resources used. The latter includes, for example, the time of health service staff, whose contributions may not result in direct financial expenditure but still represent an important cost to the health service. We will include the cost savings from reductions in malaria and anemia cases. Impact evaluations were used to assess the effectiveness of PMC in Cameroon and Côte d’Ivoire in reducing malaria and anemia incidence.16 In Benin, effectiveness will be modelled using efficacy estimates from genotype-specific modelling of the duration of protection of SP17 (further detail provided in the “Identification, measurement, and valuation of effects” section below).
Cost-effectiveness analyses will compare the incremental costs and incremental effects in the intervention areas of the co-designed PMC delivery models relative to a hypothetical counterfactual comprising the absence of PMC or, in Cameroon, the national 5-dose delivery model if they had been delivered in the same area. We present the incremental cost per malaria case averted and disability-adjusted life-year (DALY) averted. DALYs combine length and quality of life into a single measure of effect, and are a standard metric used in economic evaluation to allow comparison of results with other studies. We will model the costs and effects of scaling up PMC to all regions of each focus country where PMC may be suitable using information on malaria epidemiology and health system structure.
As detailed below, we will collect primary data, combine it with secondary data, and use various forms of modelling to meet our objectives.
Resource use data will be collected from multiple sources using various methods ( Table 3). We prospectively collected detailed financial data from PSI on project expenditures by activity. The economic costs of MoH personnel time involved in co-design, training, etc. will be captured through detailed record keeping of workshops and meetings by PSI. Key informant interviews (KIIs) will be conducted with managers of malaria and EPI services at national, regional and district levels to capture resources provided by the public health service in the implementation of PMC. Drug procurement costs are obtained from national and international price lists.
To capture resources required for PMC delivery and malaria and anaemia treatment at health facilities, we had a target sample size of 12-18 facilities per country; this allows for a purposive sample that is sufficiently large to include health facilities with varying characteristics (ownership, size, and rurality), while remaining logistically feasible. The final number selected in each country differed owing to the geographic spread and the available budget. We purposively sampled 45 health facilities (18 in Benin, 15 in Cameroon, and 12 in Côte d’Ivoire) from the 435 designated to provide PMC to obtain the widest possible variation in PMC costs, thereby allowing us to understand the key drivers of the variation in costs. Therefore, we sampled health facilities from three districts in each country with varying ownership, size, and rurality. This type of purposive (rather than random) sampling is standard in economic evaluation and allows maximum possible insights to be gleaned from a relatively small number of health facilities. Therefore, we did not perform power-based sample size calculations. Two data collection rounds (February-June 2023 and March-July 2024) will allow comparison of Phase 1, when only approximately one-third of the facilities had initiated early stage implementation, and Phase 2, when PMC implementation was established. The same facilities were visited in both the rounds. Small field teams of data collectors and their supervisors spent approximately 10 days in each health facility carrying out the following activities:
• Key-informant interviews (KIIs) with the facility in-charge, EPI focal point, and 1-3 health workers directly involved in delivering EPI and PMC to understand workload organization and resources required to provide PMC alongside usual EPI services.
• Review of health facility expenditure records for the calendar year prior to data collection (round one: 2022; round two: 2023), guided by the KIIs, will identify the health facility resources necessary for implementing PMC, as well as treating malaria and anemia. Recurrent cost data will be collected and categorized into the following cost categories: personnel, nonmedical supplies, utilities, communication, maintenance, insurance, training, meetings, and transport. Capital costs will be estimated during the analysis as a percentage of the total recurrent costs.
• Healthcare worker observations during facility-based clinics and outreach clinics, where feasible. Structured data collection forms captured the time spent on different activities, including setting up and preparation for the day, health education messaging, preparation and delivery of vaccine doses, PMC and other EPI services (including Vitamin A), clearing up, and administrative tasks, as well as the amount of non-productive time. The total number of children attending the clinic and the total number of PMC doses, EPI vaccines, and Vitamin A supplements administered on the day of observation provide the denominator for the average health worker time per EPI client and average health worker time per dose of PMC.
• Extraction of monthly totals from facility registers of inpatient and outpatient malaria and anemia cases and monthly totals of childhood vaccines and PMC doses for the calendar year prior to data collection for three age categories: 0-11 months, 12-23 months and 24-35 months.
• Extraction of detailed information on diagnostic tests and treatment from facility registers for a systematic sample of up to 50 malaria and 30 anemia outpatients aged <3 years per facility, and up to 50 malaria and 30 anemia inpatients <3 years per facility (for those facilities providing inpatient services) across the calendar year prior to data collection. These numbers were chosen to obtain a sufficiently large sample of records to capture the variation in treatment costs, balanced with the length of time available to complete the task.
• Caregiver time logs at entry and exit to measure the time children and their caregivers spend at the facility or outreach clinic when attending for their vaccinations and PMC. This was on the same day as the health workers’ observations with up to 50 caregivers included per clinic observed. Upon exit, the caregivers were asked about the age of their child (ren) and whether they received any vaccination, dose of Vitamin A dose of PMC, or any other services while at the facility/clinic.
Other costs of accessing PMC (and vaccinations and Vitamin A) and costs of malaria treatment to households will be collected through household surveys within the process evaluation activities described elsewhere.
To calculate the number of DALYs averted by PMC, we will estimate the total number of malaria-related DALYs in children <2.5 years in a standardized population of 10,000 children <2.5 years (i) for each comparator and (ii) for the PMC intervention model(s) implemented. Although the intervention targets children aged <2 years, we will include children <2.5 years because some doses scheduled for children aged ~24 months may be delivered slightly later and may confer benefits at the start of the third year of life.
For each PMC model explored in each country, the number of incident malaria cases per 10,000 children <2.5 years will be estimated by multiplying estimates of malaria case incidence in the comparator scenario by the rate ratio (RR) of malaria cases with implementation of PMC. Effectiveness will be estimated in two ways: 1) directly, based on impact evaluations in focus countries where these are conducted (Cameroon and Côte d’Ivoire), and 2) indirectly, by using modelling to combine estimates of coverage and efficacy of SP doses.
Efficacy estimates will be obtained from genotype-specific modelling of the duration of protection of SP,17 using data from parasite clearance and prevention of infection (PCPI) studies being conducted in Cameroon and Zambia,18 and parasite resistance genotype mapping activities. Detailed estimates and an understanding of coverage will be obtained from the Plus Project process evaluations. The direct estimates from the impact evaluations will be used to validate and calibrate the approach to indirectly modelling effectiveness. The (indirect) modelling will then be used to estimate effectiveness in all areas of the focus countries where PMC is considered potentially suitable based on SP efficacy, the absence of SMC, and other factors. The incidence of mild, moderate, and severe anemia with PMC will be estimated in similar ways.
For each health facility, we entered expenditure records directly into an Excel-based spreadsheet developed for the project. At least two supervisors reviewed the data for quality control purposes. We will use Visual Basic for Applications (VBA) macros to extract data from these individual health facility spreadsheets into a single Excel workbook in a long format for analysis, alongside data from Mozambique. We entered register data directly into ODK forms on encrypted tablets and use paper forms for healthcare worker observations and caregiver time logs before entry into ODK forms. ODK forms had built-in data validation controls and checks with additional regular reviews by the data manager. Further checks were conducted by the data manager.
We recorded KIIs using the same encrypted tablets and transcribed them in French, ensuring the use of unique identification codes without names to preserve the anonymity of the participants. The transcripts were checked against the original audio for quality control.
All electronic data were captured using encrypted devices and stored in password-protected devices and database systems. After verification, and as soon as the Internet was available, data was be uploaded to a central secure server. Data – whether on central computers and servers, remote computers, or handheld devices – was backed up daily to a central secure data server. We will delete the original audio recordings from all devices once the transcription is complete. A data manager in each country has full access to data through the server.
Detailed financial data from PSI will be provided regularly by the PSI program management team as an Excel-based transaction list from their financial reporting system. Transactions will be coded against activities and expenditure categories using standard fields and reporting codes within the reports and verified through discussion with the PSI team.
Costs of implementing PMC (objective 1)
We will code and analyze KIIs thematically in French using NVivo 1419; we will seek to understand how EPI and PMC services are organized and identify information on resource use.20 We will analyze quantitative data captured via ODK in Stata 1821 using descriptive statistics to explore variations within and between health facilities and over time with respect to health worker and caregiver time on EPI clinic days, which will then be incorporated into cost analyses in Excel.
We will analyze resource use and cost data collected from health facility expenditure records and PSI records in Excel. We estimate the provider costs incurred within health facilities of delivering PMC using a combination of step-down costing (to estimate the cost per EPI visit) and micro-costing (to estimate the cost of SP and associated supplies).8,22 Financial data from PSI will be analyzed to estimate the fixed and variable provider costs of supporting PMC delivery, including SP procurement.
We will estimate the cost per PMC dose delivered and the total costs for PMC implementation across the eligible areas of each country. We will disaggregate costs in various ways, including by cost center (personnel, materials, SP drugs and storage, meetings and training, equipment, transport, and monitoring), perspective (i.e., donor, national health service, EPI, NMCP, community level, household, etc.), financial and economic costs, and start-up and recurrent costs.
Cost-effectiveness of PMC (objective 2)
Qualitative analysis will be used to refine our understanding of decision makers and decision-making processes around PMC, which will inform modelling choices. Our main outcome metric is the incremental cost-effectiveness ratio (ICER).
The costs of the intervention and comparator strategies reflect the combined costs of implementing PMC and treating malaria and anemia cases:
In this way, the ICER will reflect the additional costs of implementing PMC in the intervention strategy, minus the cost savings from having fewer malaria and anemia cases to treat than in the comparator strategy.
The cost to the public health provider per malaria case and per anemia case diagnosed and treated will be estimated separately for outpatients and inpatients from our health facility data following a similar approach as for PMC costs. Step-down costing will be used to estimate the cost per outpatient consultation and per inpatient day for children <2.5 years at each health facility. For each case sampled in the detailed register review, we will generate a cost by applying these unit costs per outpatient or inpatient day and unit costs of diagnostics and medicines obtained from national and international sources to patient-specific resource use data. Analyses of these individual patient data reflect clustering at health facilities and purposive sampling.
A decision tree model will be used to combine primary data on costs and effects with additional primary and secondary data. We express all estimates of costs and effects for a standardized population of 10,000 children under the age of 2.5 years. The implementation costs and cost savings will be presented separately and in combination.
A base case analysis for each country will explore the cost-effectiveness of PMC using the most plausible set of assumptions. We will use deterministic analyses to explore heterogeneity; that is, how our results may vary across settings. We use deterministic and probabilistic analyses to explore the sensitivity of our findings to uncertainty in methodological choices, including input parameters.
The costs of implementing PMC will be estimated over a period of five to ten years to be decided in consultation with relevant stakeholders. The costs and effects of incident malaria cases and anemia cases occurring during this implementation period will be modelled over a lifetime. A 3% annual discount rate will be used for both costs and effects in the base case.23 DALYs will be modelled without age weighting and using standard DALY weights24 and life tables25 as well as other secondary data (e.g., duration of an episode of malaria or anemia).
To understand whether PMC is likely to represent an efficient use of scarce resources, we will compare the ICERs we estimate with appropriate cost-effectiveness thresholds, such as those recommended by Ochalek et al.26 Cost-effectiveness acceptability curves will be generated, and probabilities calculated that adopting PMC in a given context and for a given cost-effectiveness threshold will represent an efficient policy choice. ICERs for PMC will also be compared to ICERs from alternative interventions targeting children aged <2 years, based on a review of evidence at the time of analysis.
Budget impact (objective 3)
A qualitative analysis of interviews with national- and regional-level stakeholders will inform our understanding of budgets and budgeting processes. A budget impact analysis will be performed to explore whether introducing PMC may be affordable within the relevant provider budgets in each country. We will compare the net incremental financial costs of PMC (including both implementation costs and cost savings from reduced malaria incidence) to specific budgets where possible. The time horizon will be limited to a period relevant to the budget holder, usually 5-10 years.
Equity (objective 4)
We will assess the equity of implementing PMC by exploring the variation in costs and the effects of PMC models across sub-populations. The main equity analyses will use wealth quintiles as a measure of socioeconomic status. Wealth quintiles will be created using principal component analysis27 based on standard asset ownership questions included in the impact evaluation cohort census survey and process evaluation household surveys.
Ethical review committees approved this study in Benin (Comité national d’éthique pour la recherche en santé) (103/MS/DC/SGM/CNERS/SA), Cameroon (Comité national d’éthique de la recherche pour la santé humaine) (2023/01/1512/CE/CNERSH/SP), Côte d’Ivoire (Comité national d’éthique des sciences de la vie et de la santé) (001-23/MSHPCMU/CNESVS-km), WHO (ERC.0003756), and LSHTM (27531). Following sensitization to the study and presentation of ethics approvals, administrative authorization was obtained from the relevant national, regional, and/or district authorities.
Before commencing data collection from health facilities, written informed consent was obtained from the head of the health facility. We also obtained written informed consent from all participants in the primary data collection (i.e., key informant interviews, health worker observations, and caregiver time capture). For participants who were unable to read the information sheet, a witness verified that the information had been explained correctly. We used assent forms for participants younger than the age of the majority (<21 years in Cameroon; <18 years elsewhere).
We ensured that the locations of the interviews provided privacy. Respondents may participate but choose not to quote, even anonymously; specific consent was sought for the use of anonymous quotes. We will present the results in ways that prevent the identification of individuals or health facilities.
Research team members attend quarterly chemoprevention Technical Working Groups (TWGs) in each country to present updates on evaluation activities and are in regular communication with PSI country teams for two-way communication on implementation and evaluation updates. After each round of activities, we will disseminate preliminary results through the national as well as international chemoprevention TWGs attended by a range of relevant stakeholders, including the MoH, particularly the NMCP and EPI, and their partner agencies (NGOs, technical agencies and implementing partners, donor organizations, WHO, UNICEF). The results will also be widely disseminated in each country through a series of meetings that will include regional and district health authorities, as well as the national level, and the health facilities and communities where we are working. The results will be shared more widely through scientific publications in peer-reviewed journals, at national and international conferences, and other fora, as appropriate.
Integrating PMC into existing EPI contacts is expected to be a low-cost approach.9,10,28 However, expanding from no PMC to a new PMC strategy (or, in the case of Cameroon, from a 5-dose to 8-dose PMC schedule) will require additional investments of both financial and non-financial resources, including healthcare worker time in facilities and time of MoH managers and others at all levels. Further investments will be required if coverage of existing EPI delivery channels (such as outreach clinics) needs to be increased and/or expanded to community-based delivery to provide more doses of SP to a wider age range of children. By averting more malaria cases, PMC may reduce the burden of malaria, including malaria deaths, avert substantial malaria treatment costs, and potentially contribute to long-term economic benefits associated with malaria burden reduction.11
The strengths of our study design include the scale of data collection, which included 45 health facilities across the three countries. Along with the purposive sampling approach, this provides the opportunity to explore variations in the costs of PMC implementation and malaria diagnosis and treatment, which will be used to estimate the costs of scale up more accurately. We will collect data at two time points: before implementation/very early implementation of PMC and after approximately 12 months of implementation, when we expect PMC to be established. This provides an opportunity to look for changes and directly measure the incremental costs of introducing PMC. Furthermore, using a standardized approach and tools (with contextual adaptations) across the three West African countries included in this protocol and Mozambique (reported elsewhere12) allows us to compare and contrast economic evaluation outputs across different settings. Our use of qualitative data alongside more traditional quantitative economic data is another strength that will inform the collection of relevant resource-use data and support our analysis, interpretation, and reporting of findings in a way that is understanding of the context and can support the transferability of findings.20
Limitations of our design reflect various challenges, including the inherent difficulties in measuring health workers’ time. For example, observing the time use of health workers is challenging. We can only observe one or two clinics per health facility in each round, which may not be “typical” clinics in terms of staff providing services and/or caregivers attending; similarly, coinciding the field team’s presence with planned outreach clinics may not always be possible given the sporadic nature of outreach clinics. Triangulating observation data with information collected through qualitative interviews and facility register review will strengthen our understanding of clinic organization and personnel time use. It is also important to acknowledge the difficulties in evaluating the costs (and effects) of an intervention being implemented at scale under routine conditions and a changing policy context; for example, malaria vaccines were introduced in some of the intervention areas during PMC implementation. However, these challenges are outweighed by the benefits of transferability owing to our ability to adapt the cost-effectiveness model design and answer the most relevant current questions for decision makers.29
In conclusion, this economic evaluation will provide rigorous evidence from three countries, each implementing different PMC delivery models, regarding whether, where, and under what circumstances investing in PMC may represent an efficient and equitable use of scarce resources. Updated WHO guidelines recommend PMC but indicate that more evidence is needed on cost and cost-effectiveness to support countries and donors in developing the most optimal mix of malaria prevention and control interventions30; our findings will respond to this call for evidence.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)