Keywords
General Surgery, Valentino’s Syndrome, Acute appendicitis, Perforated gastric ulcer
This article is included in the Health Services gateway.
This article is included in the Rare diseases collection.
Valentino’s Syndrome is a rare affliction caused by the perforation of a peptic ulcer which simulates deviously a picture of acute appendicitis. The diagnosis of this condition is not simple (often intraoperative) and requires a high degree of suspicion.
A 90-year-old man arrived at the Emergency Room with abdominal pain mainly localized in the right iliac fossa (Blumberg +, McBurney +). The patient had a history of previous perforated peptic ulcer treated with surgery. A diagnosis of suspected perforated acute appendicitis was made based on clinical and radiological findings and the patient underwent urgent surgical intervention. Laparoscopic exploration revealed a picture of diffuse choleperitonitis due to a probable perforation of a duodenal ulcer. Considering the patient’s age and the severity of the clinical and surgical picture, characterized by the presence of indissociable intra-abdominal adhesions, it was decided to forgo further surgical procedures, except drainage of the abdominal cavity and palliative medical treatment, due to a poor prognosis.
This syndrome, although very rare, should always be suspected in cases of right iliac fossa pain in the presence of certain risk factors for perforated peptic ulcer.
Patient’s ethnicity: Italian.
General Surgery, Valentino’s Syndrome, Acute appendicitis, Perforated gastric ulcer
Valentino’s Syndrome is a very rare clinical condition caused by the perforation of a peptic ulcer which manifests itself with an atypical clinical presentation characterized by the presence of pain in the right iliac fossa, thus simulating the clinical picture of acute appendicitis,1 due to the leakage of gastric contents and their resulting accumulation in the right iliac fossa,2 which leads, sometimes, to a secondary chemical appendicitis caused by peritoneal irritation. This syndrome was first described in 1926 and it was named after the Italian-American actor Rodolfo Valentino, who, after collapsing in a New York hotel, underwent an emergency appendectomy with a diagnosis of acute appendicitis and afterwards died at just 31 years of age a few days after being discharged when he was hospitalized again for septic peritonitis with multiorgan failure secondary to an undiagnosed perforated peptic ulcer, discovered only after the autopsy.3 Perforated peptic ulcer disease (PUD) affects 4 million people annually all over the world, (prevalence: 5%-10%), 5% of whom will progress to perforation. Perforated PUD still has a high rate of morbidity (48.5%) and mortality (9.3%).4 The diagnosis is made through clinical examination, laboratory tests and instrumental investigations (x-ray, CT), which, unfortunately, are not always helpful in obtaining a conclusive diagnosis and, sometimes, can be misleading. Consequently, after having suspected the diagnosis of acute appendicitis, an appendectomy is opted for, until the exploration of the abdominal cavity reveals the presence of a perforated peptic ulcer, whereas the appendix is normal or simply slightly inflamed. Hence, Valentino’s Syndrome is surely a rare clinical entity, but, in spite of this, it should be kept in mind by the physician when assessing a patient with right lower quadrant abdominal pain,5 in order to decrease the mortality and treat this syndrome appropriately.
Herein we report a case of Valentino’s syndrome in an elderly man who underwent emergency exploratory laparoscopy at our institution, describing the clinical presentation and the diagnostic-therapeutic process. Lastly, we present and discuss a review of 34 cases found in literature published between 2004 and 2024.
A 90-year-old man was transported to the Emergency Room for intense abdominal pain localized in the right iliac fossa that lasted for 4 days, which radiated to the remaining lower quadrants and the renal lodges. The vital parameters upon admission to the E.R. were: BP 92/56 mmHg, HR 100 bpm, SpO2 95%, CT 97,88°F (36.6 °C). Abdominal pain was associated with other symptoms, such as diarrhea (constipation in the previous days), asthenia and an episode of reported “dark” vomiting a day earlier. Hyperchromic urine for 4 days was also reported. His medical history included arterial hypertension in antihypertensive therapy and a previous peptic ulcer for which he most likely underwent laparoscopic surgery in 2009. During physical examination we found a painful and tense abdomen that was diffusely tender and responsive to palpation, suggesting peritoneal irritation, with Blumberg + and McBurney +. On auscultation the peristalsis was poor. Visual inspection also revealed the presence of laparoscopic trocar scars.
Thus, in light of the objective findings, a clinical suspicion of acute appendicitis was raised, and the patient was investigated accordingly. Laboratory tests showed an increase of inflammation markers (WBC 24.900/mm^3, CRP 15.3 mg/dL), elevated serum amylase (1737 UI/L), and elevated serum creatinine (2.76 mg/dL). Lactate levels were in the range (1.2 mmol/L). An abdominal CT scan with contrast medium was performed, which showed the presence of abscess collections in the right pararenal space, in communication with perihepatic effusion and with some collections close to the appendiceal worm (no intra-abdominal free air), that is to say a radiological picture highly suspicious for acute perforated appendicitis with involvement of the retroperitoneum. On completion, a chest x-ray was also carried out, which showed a slight pleural effusion on the right side.
Since the clinical, laboratory and radiological findings were compatible with acute appendicitis, it was decided to have the patient undergo laparoscopic appendectomy: surgical exploration revealed severe choleperitonitis, with the presence of bile fluid both intra and retroperitoneally, in particular in the right renal lodge, right parietocolic region and in the supra and subhepatic area. Furthermore, a picture of widespread adhesions was found in which the hepato-biliary-pancreatic region was engulfed by inseparable adhesions which made exploration of the duodenal region difficult. The appendix was visualized and did not appear to be affected by acute inflammation. Hence, an intraoperative diagnosis of diffuse biliary peritonitis due to a probable perforation of a posterior duodenal ulcer was made ( Figure 1). Given the patient’s age and the severity of the clinical and surgical condition, characterized by the presence of inseparable adhesions (probably the result of previous surgery for peptic ulcer), it was decided to postpone further surgical procedures, except for drainage of the subhepatic lodge. At the end of the procedure, two abdominal drains are placed: one in the subhepatic compartment and the other in the hypogastrium. Finally, a methylene blue test was performed, which was present in both drains. A nasogastric tube and urinary catheter were left in place.
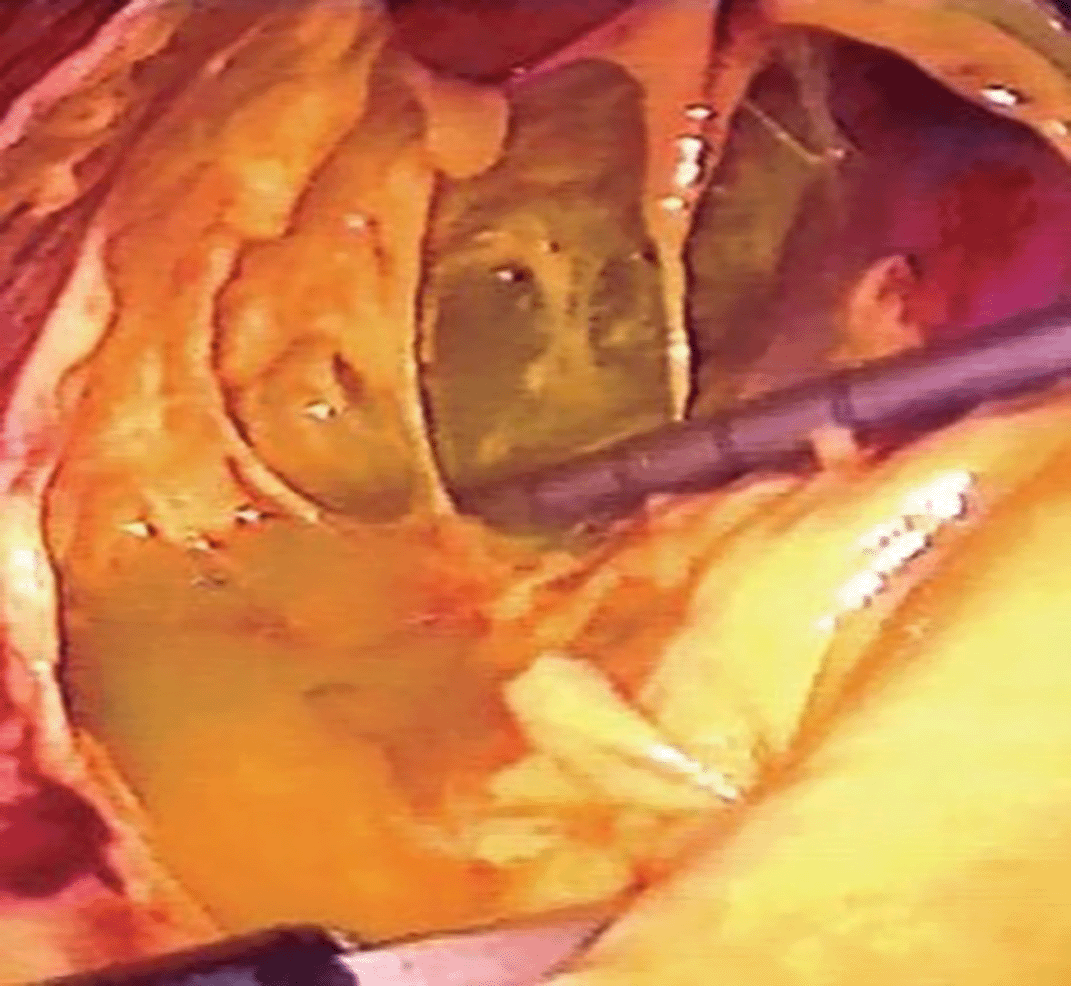
Given the patient’s critical condition and a picture of a perforated peptic ulcer not amenable to surgical resolution, after a joint discussion with the anesthesiologists and in agreement with the patient’s relatives, it was decided not to proceed with resuscitation. Finally, admission to the General Surgery Unit was agreed upon to ensure supportive palliative care. Appropriate postoperative pharmacological therapy was established (iv rehydration therapy, antibiotic therapy with piperacillin/tazobactam, TPN, analgesics, gastroprotectives and antithrombotic prophylaxis with LWMH. Although the patient arrived in the ward in a soporific state and was initially given an unfavorable prognosis, during his hospital stay he recovered consciousness and showed a modest improvement in his clinical picture, with good pain control through analgesic therapy. In particular, a very slow, but progressive reduction in inflammation markers (with a peak of CRP in the second POD that reached 36.4 mg/dL) was highlighted in blood tests, together with a decrease in serum amylase and a return of serum creatinine to the normal range. With regards to the two abdominal drains, there was persistent leakage of abundant quantities of corpuscular biliary-enteric material (up to 1000 mL/24 h), for which reason investigations were carried out aimed at searching for bile leakage from the duodenum or biliary tree (abdominal MR imaging with i.v. liver-specific contrast medium and abdominal CT scan with oral contrast medium), which however showed the absence of extra-luminal extravasation of contrast medium outside the biliary tree, the gallbladder and the duodenum. Also, the dosage of amylase and bilirubin from the drainage fluid was negative. The post-operative course was characterized by a progressive reduction of the gastro-biliary debt from the nasogastric tube (removed in the eighth POD) and of the bile fluid in the drains, gradual resumption of oral feeding and bowel movement. Oral methylene blue was administered, which was absent from the drains and was eliminated regularly in the urine. During the final days of hospitalization, it was proposed that the patient undergo further imaging studies with contrast to determine the exact location of the perforation, as well as endoscopic procedures (EGD) and interventional radiology (PTBD placement). However, given the patient’s age and clinical condition (sufficient, but not perfect), the decision was made not to perform these procedures, trusting in the spontaneous resolution of the lesion. Finally, after more than 2 weeks of hospitalization, the patient was discharged and transferred to a hospice in average clinical conditions (with inflammation markers still slightly above the limits and with a portion of retroperitoneal effusions in the right quadrants that remained unchanged at the last control CT scan), and with a urinary catheter and two abdominal drains in place (still containing a fair amount of corpuscular material).
Peptic Ulcer Disease (PUD) is a common illness that is widespread in all countries of the world, but with a different epidemiology and degree of diffusion depending on the risk factors present in a given population, which are mainly H. pylori, infections, consumption of NSAIDs, potus, smoking, obesity, coffee consumption and genetic factors.7 Perforated Peptic Ulcer (PPU) is a severe complication of PUD that affects approximately 4 million people annually all over the world,4 whose morbidity is about 50%, while short-term mortality can reach, in some cases, 30%.6 It is a rare occurrence (second to peptic ulcer bleeding in frequency6) that happens in 2% to 10% of patients with PUD.8 Previous history of PUD,6 but also NSAIDs abuse and smoking, have been recognized as predisposing factors to a greater risk of perforation.9 The typical clinical presentation consists of the onset of sudden and very intense epigastric pain which becomes generalized leading to frank peritonitis, although in certain patients (elderly, immunocompromised, obese) the symptoms might be aspecific, making it impossible to exclude other pathologies which must therefore be considered in the differential diagnosis, including acute appendicitis. Sometimes, unfortunately, radiological diagnostic investigations are not always diriment.
From a physiopathological point of view, VS arises when the biliary enteric fluid leaks from the perforated ulcer through the right parietocolic gutter, reaching the right iliac fossa, causing a localized chemical peritonitis with consequent positive McBurney.10 For this reason, in some cases, the pain can be of a migratory type from the periumbilical/epigastric region to the RLQ, sometimes with a sudden onset and worsening, otherwise with a slower progression and lasting a few days.10 Blood tests may show an increase in serum amylase and lipase.11 Abdominal CT scan can also allow visualization of small amounts of free intra-abdominal gas: in addition to peritoneal and retroperitoneal effusions, it is possible to detect pneumoperitoneum (gastric ulcer), pneumo-retroperitoneum (duodenal ulcer), particularly posterior to the right kidney (the “veiled right kidney sign”, Figure 2)12 and sometimes thickening of the gastric/duodenal wall. However, it is not always possible to visualize the exact location of the perforation. PPU can be treated “conservatively” (fasting, nasogastric tube, i.v. antibiotics and PPI) so as to promote spontaneous healing of the ulcer after a period of observation, which will be confirmed with water-soluble contrast imaging.6 Alternatively, it can be treated with surgery (laparoscopically or laparotomically), using various possible surgical techniques (depending on the characteristics of the ulcer and the patient), among which we mention: gastroraphy (with or without omentoplasty), Graham’s repair (free omental Graham’s patch) or Cellan-Jones repair (pedicled omentoplasty).13 In case of lesions >2 cm, nevertheless, gastric resection may be necessary.13
Valentino’s Syndrome is a rare clinical entity and very few cases are described in literature. It is a real diagnostic challenge for the physician, who should be able to recognize and manage it as quickly as possible, as it may become a serious threat to the patient’s life, in addition to the fact that this, from a medical-legal point of view, could be mistaken for medical malpractice, making the physician liable to possible legal action. Here is a report of 34 cases of patients affected by VS between 2004-2024 ( Tables 1, 2, 3). The search was made by entering the terms “Valentino’s Syndrome”, “Valentino’s Syndrome appendicitis” or “Valentino’s Syndrome perforated peptic ulcer” into the PubMed/Google Scholar search engine. In this group, 27 out of 34 are aged <60 years old (79,4%), of which 6 are under 20 years old (17.6%). The youngest of them, a 12-year-old male adolescent, did not even appear to have any risk factors for PUD.14 Blundell et al.15 described, however, a case of VS in a 14-year-old boy with strong family history of PPU. Furthermore, in this small case series, we can note that 76.4% (26 out of 34) are male, confirming the fact that PUD seems to be more widespread in male population,16 in which a higher prevalence of gastroduodenal perforations appears to be evident in cases affected by PUD.17 As regards the opposite gender, the differential diagnosis might be even more arduous, since it is necessary to take into account gynecological pathologies such as ruptured ectopic pregnancy, ovarian torsion, endometriosis, infarcted leiomyoma or pelvic inflammatory disease.2 Wijegoonewardene et al.18 even reported, as a noteworthy clinical eventuality, a rare case of a 30-year-old female with PPU mimicking appendicitis which manifested itself a few hours after the removal of an intrauterine contraceptive device, thus suggesting that also a possible complication of intrauterine contraceptive devices removal could be taken into consideration in the differential diagnosis in female subjects. Furthermore, as we can notice in this small 34-case series, a case of VS has also been described in the literature in a 23-year-old pregnant woman in the 20th week of gestation,19 in whom, however, a past medical history of untreated duodenal ulcer must be highlighted. In pregnant patients, indeed, acute abdominal pain could become a real diagnostic enigma, made even more complex by the impossibility of seeking help from conventional x-ray investigations, thus determining a dangerous diagnostic delay for both the mother and the fetus.19 This brief research, in addition to confirming well-known risk factors and predisposing conditions (PUD, chronic gastritis, chronic H. pylori infection, prolonged intake of NSAIDs),2,3,12,15,19–22 it highlights other emerging ones of no less importance including, in addition to chronic alcohol ingestion or smoking,10,11,21,23,24 also drug abuse10,21 or even irregular eating habits,25 although the literature on these last two topics is rather limited.26–28 While the sudden onset of abdominal pain in the epigastric/mesogastric region could immediately suggest the diagnosis of PPU with a rather narrow margin of doubt, it is precisely its subsequent migration or irradiation to the right iliac fossa region accompanied by a McBurney + that may be deceitful, leading us into error. This becomes even more so especially in cases where the abdominal pain is initially localized in the right lower quadrant, in the absence of previous pain in the upper quadrants,1,3,12,18,20,22,29–33 hence the importance of performing an anamnesis as accurate as possible on the characteristics of onset, irradiation and duration of the pain which, in addition to the identification of possible risk factors for PUD, could sometimes lead to having a light bulb moment. Not all cases, although the majority (55,8%), showed quite clear warning signals, such as alterations in vital parameters5,14,19,24,25 or peritonism,1,2,14,15,18,20,23–25,30,32–34,36–39 in some cases associated with fever.11,12,15,20,22,25,30,32,33,36 As for the laboratory tests, in addition to neutrophilic leukocytosis and elevated CRP, 4 cases presented with slightly elevated serum amylase or lipase,3,11,25,34 although these last two markers are rather non-specific in situations of acute abdominal pain, which, therefore, could sometimes be altered in case of appendicitis.35 Diagnostic imaging, in most cases (about 82.3%) highlighted non-specific radiological signs, such as the presence of intra-abdominal fluid collections, sometimes peri-appendiceal, even associated with thickening of the appendiceal wall as if from inflammation, which, only intraoperatively, will be recognized as “secondary appendicitis”. Generic signs of intestinal perforation (intra-abdominal free air pockets, subdiaphragmatic free air) have also been found among these cases. Only in 6 out of 34 cases (20.4%) there were more specific imaging findings: pyloric thickening,12,40 periduodenal fluid and air collections,1,3,22,29 including the rarer but more radiologically significant “veiled right kidney sign”, which is the visualization of retroperitoneal air surrounding the right kidney.12,22 Furthermore, although Karmo et al.1 described the case of a male whose CT scan revealed an uncertain focal discontinuity in the anterior pyloric wall, it is almost never possible to pinpoint the exact site of the perforation on CT scan. In 2 cases intraoperative EGDS was used to facilitate diagnosis.12,38 On the other hand, in the case described by Villamil-angulo et al.37 it was decided that there was no need to resort to instrumental diagnostic investigations due to the high suspicion of appendicitis simply on the basis of the “characteristic” symptoms (pain migrating towards the RLQ) associated with leukocytosis, assigning an Alvarado score of 8.
Turning to treatment, this report appears to be roughly split in two regarding the initial surgical approach, with just over half (52.9%, 18 out of 34) preferring laparotomy to laparoscopy, and one laparotomic conversion33 due to the lack of a cleavage plane between the stomach and gallbladder associated with the finding of intense diffuse peritoneal inflammation. There was no surgical intervention in the case described by Wang et al.,12 who reported the case of a 72-year-old man with Valentino’s Syndrome with pain in the right lower abdomen, but with radiological signs of retroperitoneal perforation (the “Veiled Right Kidney Sign” already mentioned), so that the patient was immediately subjected to endoscopy - which showed multiple gastroduodenal ulcers - and, finally, treated conservatively with i.v. antibiotics. Different surgical techniques have been used to repair the perforation (gastric raffia, Graham’s or Cellan-Jones patch), associated or not with appendectomy, either due to its secondary involvement or “prophylactic”, in the absence of clear signs of appendiceal inflammation. Two cases underwent re-operation following the development of post-operative complications21,29: Chavez et al.21 reported the case of a 76-year-old man, who, 5 days after being discharged from the hospital following laparoscopic appendectomy, developed severe abdominal pain associated with bilious vomiting and fever, and was subsequently readmitted and underwent laparotomic repair of the perforated ulcer. Rodrigo et al.29 described the case of a 31-year-old woman who underwent 2 relaparotomies after laparotomic appendectomy: the first (POD 5), a PPU repair with omental patch following bile-stained discharge from the surgical wound associated with abdominal pain and fever, and the second (POD 7), an emergency right hemicolectomy after sudden abdominal distension and feculent discharge from appendectomy wound and drain which even led to hospitalization in the ICU for severe dyspnea and desaturation with the need for tracheal intubation. In the remaining cases the postoperative course was substantially regular.
To conclude, in brief, some of the data illustrated in this paragraph are summarised here in the graphs of Figures 3 and 4.
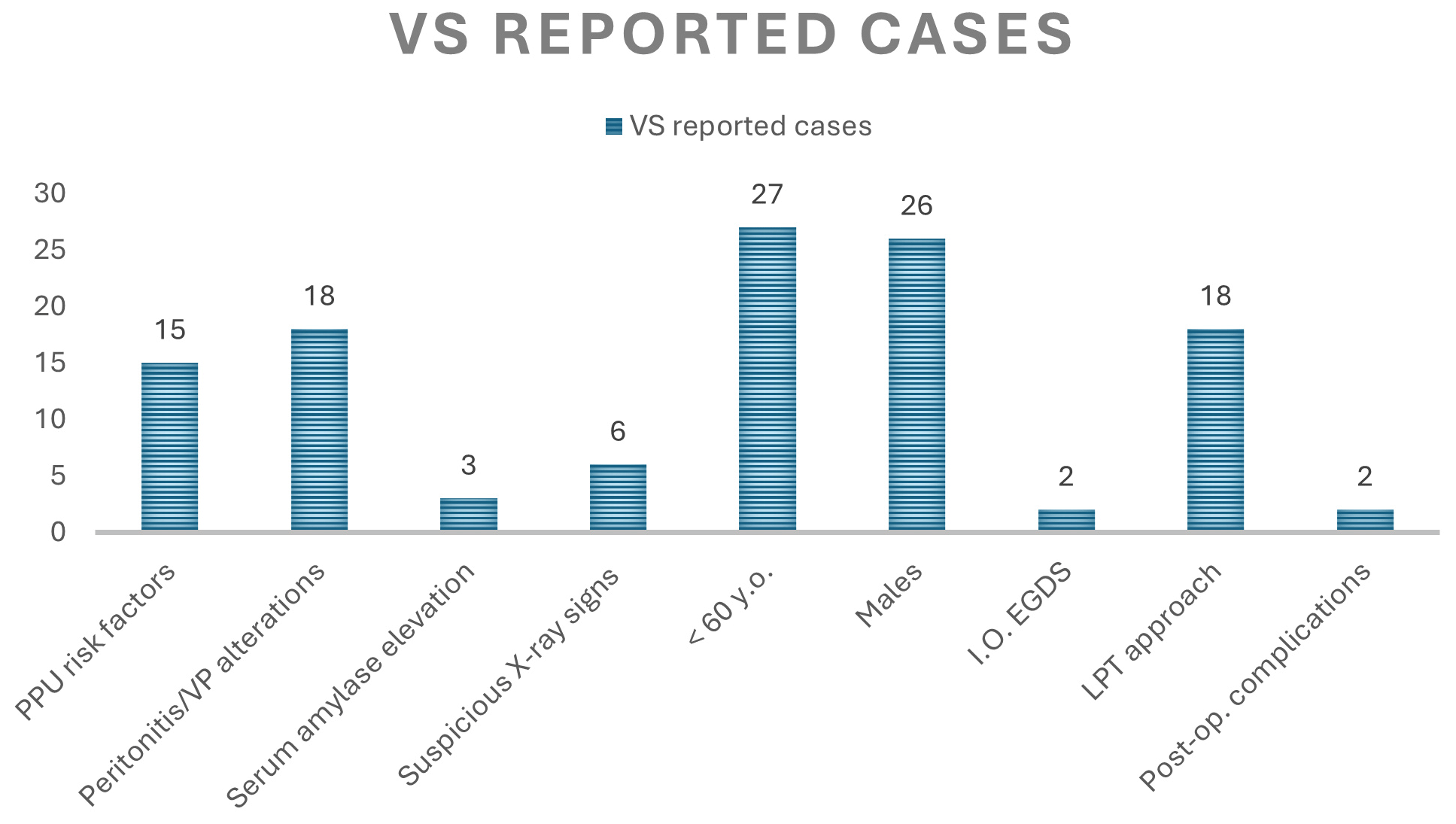
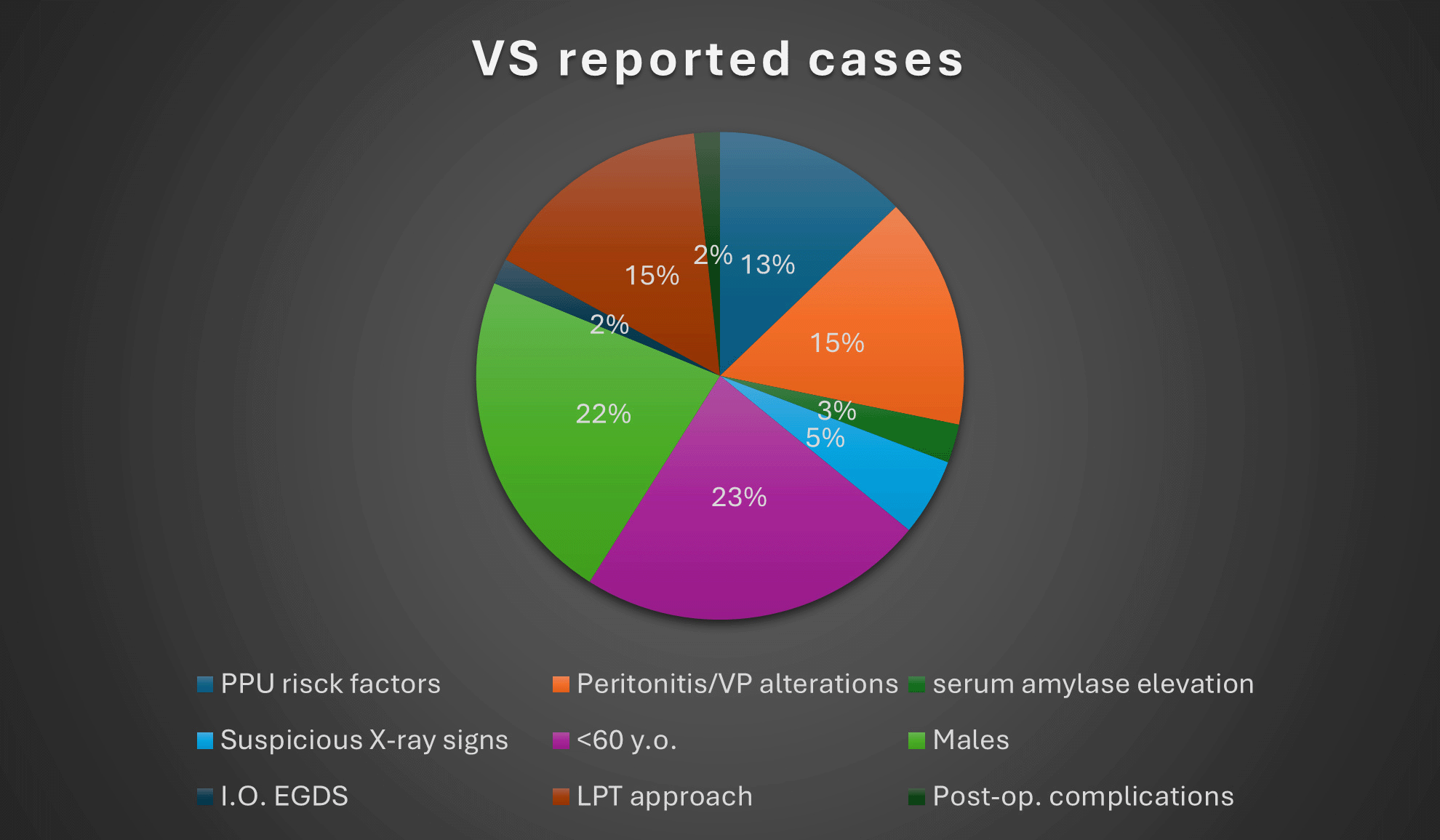
Valentino’s Syndrome, despite its rarity and the few cases described in the literature, should always be taken into consideration in the differential diagnosis in all cases of right abdominal pain with peritonism, especially in case of a medical past history of previous surgical interventions for perforated peptic ulcer or simply in case of the presence of risk factors for this pathology.
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Name of repository: Figshare. Checklist Title: CARE Checklist. DOI: 10.6084/m9.figshare.30879662. License: CC0.41
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)