Keywords
ISNAG-Fluorimeter, Flow injection analysis, Etoricoxib, Fluorescence, Fluorescein Sodium Salt.
This article is included in the Fallujah Multidisciplinary Science and Innovation gateway.
The developed ISNAG-spectrofluorometric method of Etoricoxib determination is a novel approach, based on quenching of fluorescein fluorescence under interaction with the analyzed compound. In order to stabilize the emitted signal, low-pressure mercury lamp (LPML) as an excitation source and eight solar cells arranged at a 90° interval were used. As a result, the correlated signal is more sensitive and stable. The linear nature of the method in the 0.08-12 mmol/L range reflects a correlation coefficient of 0.9905 & R2 %= 98.10 which is a perfect linearity. The limit of detection was calculated as 0.61 μg/170μL while the limit of quantification as 1.83 μg/sample ensuring excellent trace detection of etoricoxib. The precision on RSD (relative percent standard deviation) scale was determined to be less than 0.5 % which reflects a high level of repeatability and reproducibility of the method. The proposed method was applied to commercial pharmaceutical formulation. In the following statistical experiment, both paired two-sided t-tests were employed. The strength of the proposed method in relation to the official references was an object for one test whereas the speed of the routine one as well as its decrease in reagents usage proportions was an object for another test. There was no significant difference in those tests which proves the applicability of the proposed method. Moreover, the precision of the novel method utilized less reagents, and a significant variation, forming a confidence level utilization was observed. In conclusion, the developed ISNAG-spectrofluorometric method of Etoricoxib determination proved to be a rapid, stable, accurate, and validated method suitable for routine investigation of commercial pharmaceutical formulations.
ISNAG-Fluorimeter, Flow injection analysis, Etoricoxib, Fluorescence, Fluorescein Sodium Salt.
Etoricoxib, Arcoxia in its trade name, is a dipyridinyl derivative with a molecular weight of 358.8 g/mol and a molecular formula: C 18H 15ClN 2O 2 S.1 It is a second-generation selective cyclooxygenase-2 (COX-2) inhibitor that has a relatively higher COX-1 to COX-2 selectivity ratio than other COX-2-selective NSAIDs, such as rofecoxib; valdecoxib; and celecoxib.2 Etoricoxib is commonly used to treat moderate dental pain following surgery, arthritis, osteoporosis, and gout.3,4 The drug has a moderate absorption when administered orally, and notification of peak plasma concentrations is achieved approximately one-hour post-dose, with bioavailability being the same for both oral and intravenous pathways.5
Despite its positive effects, Etoricoxib has been reported to cause side effects such as severe skin reactions, gastrointestinal issues, headache, dizziness, and cardiovascular disorders.6,7 Like other COX-2 inhibitors, this drug contraindicates with ischemic heart disease. Additionally, it should be cautiously administered to patients with a history of stroke or those prone to cardiovascular risk.8
The assay of Etoricoxib has been reported to be performed using various methods such as high-performance liquid chromatography, HPLC, Reverse-Phase-HPLC, solid-phase extraction, ion –pair HPLC, anion exchange chromatography, spectrophotometry and liquid chromatography.9–22 Fluorescence is the phenomenon in which a molecule is excited to a higher energy level when a photon of incoming radiation is absorbed by an orbital electron in it. The electron then de-exites to its original ground state and emits a photon of lower energy, which is called fluorescence.23 This phenomenon has been extensively used in analytical chemistry, most commonly in the pharmacological field, due to its high sensitivity and selectivity.24,25
Fluorescence quenching:defined as the reduction in fluorescence intensity due to specific molecular interactions, serves as an effective strategy for quantifying pharmaceutical compounds. Through this mechanism, analytes can be measured indirectly by observing their influence on the fluorescence behavior of a chosen reagent or probe. This method has proven useful in analyzing a wide range of drugs, offering improved sensitivity and selectivity in detection.26
Flow Injection Analysis (FIA) is a flexible and automated technique in which the sample is injected into a continuously moving carrier stream, promoting rapid interaction with reagents and enabling efficient signal acquisition. Its application in pharmaceutical analysis is well-established, thanks to its high sample throughput, low reagent consumption, and consistent performance.27–34 When combined with fluorescence-based detection, FIA provides a reliable and streamlined approach for determining compounds such as Etoricoxib, particularly when fluorescence quenching plays a central role in the measurement process.
Recent developments in FIA-fluorescence systems have demonstrated their applicability in determining drugs with intrinsic fluorescence or those forming fluorescent complexes. For example, determination of vilazodone HCl was successfully quantified using a direct fluorometric- FIA method, revealing the technique’s robustness and simplicity.35
In this study, the etoricoxib determination was done by measuring its quenching effect on fluorescein fluorescence. A low-pressure mercury lamp serves as an excitation source, which was included in a flow injection system as a standalone module. The detection unit consisted of a quartz flow tube fixed along the irradiation axis and was surrounded by a pair of symmetrical groups of solar cells: four on each side, located at ±90° to the flow axis. This module allowed optimal light acquisition from two opposed sides of the tube as well as a signal enhancement and linear voltage output. The underlying analytical concept is based on the continuous excitation of fluorescein and its fluorescence reduction due to the interaction with etoricoxib, which is directly proportionate to the substance’s concentration. Etoricoxib is non-fluorescent under the principal wavelengths of the low-pressure mercury lamp. Thus, the signal reading came exclusively from fluorescein.
All solutions were prepared using distilled deionized water to ensure purity and minimize ionic interference. To prepare a standard solution of 1 mmol/L fluorescein sodium salt (FSS) (376.275 g/mol, BDH), weigh 0.18814 g/500 ml of distilled water. To prepare a 50 mmol/L solution of etoricoxib((C17H14ClF3NO2S), M.Wt = 358.81 g/mol, - Sigma-Aldrich (Germany): 1.7941 g in 100.0 mL of absolute ethanol. The solution was sonicated briefly to ensure complete dissolution and stored in a tightly sealed amber vial at room temperature until use. According to the MacCulloch citric acid and phosphate buffer system, a series of buffer solutions covering the pH range from 5 to 8.0 were prepared as described in reference [36] by mixing appropriate amounts of 0.1 M citric acid monohydrate and 0.2 M disodium hydrogen phosphate (Na2HPO4), both of analytical grade and purchased from BDH. Each buffer solution was prepared to a final volume of 100 ml using deionized water. The pH was adjusted by varying the proportion of components according to the MacIlvaine protocol. All solutions were freshly prepared and stored at room temperature in Airtight containers maintain stability and prevent microbial contamination. This buffer system was chosen for its broad pH coverage, chemical compatibility, and suitability for fluorescence-based analytical applications. A series of sodium hydroxide solutions were prepared by diluting a standard solution (0.1 mol/L) with distilled water after titration against hydrochloric acid.
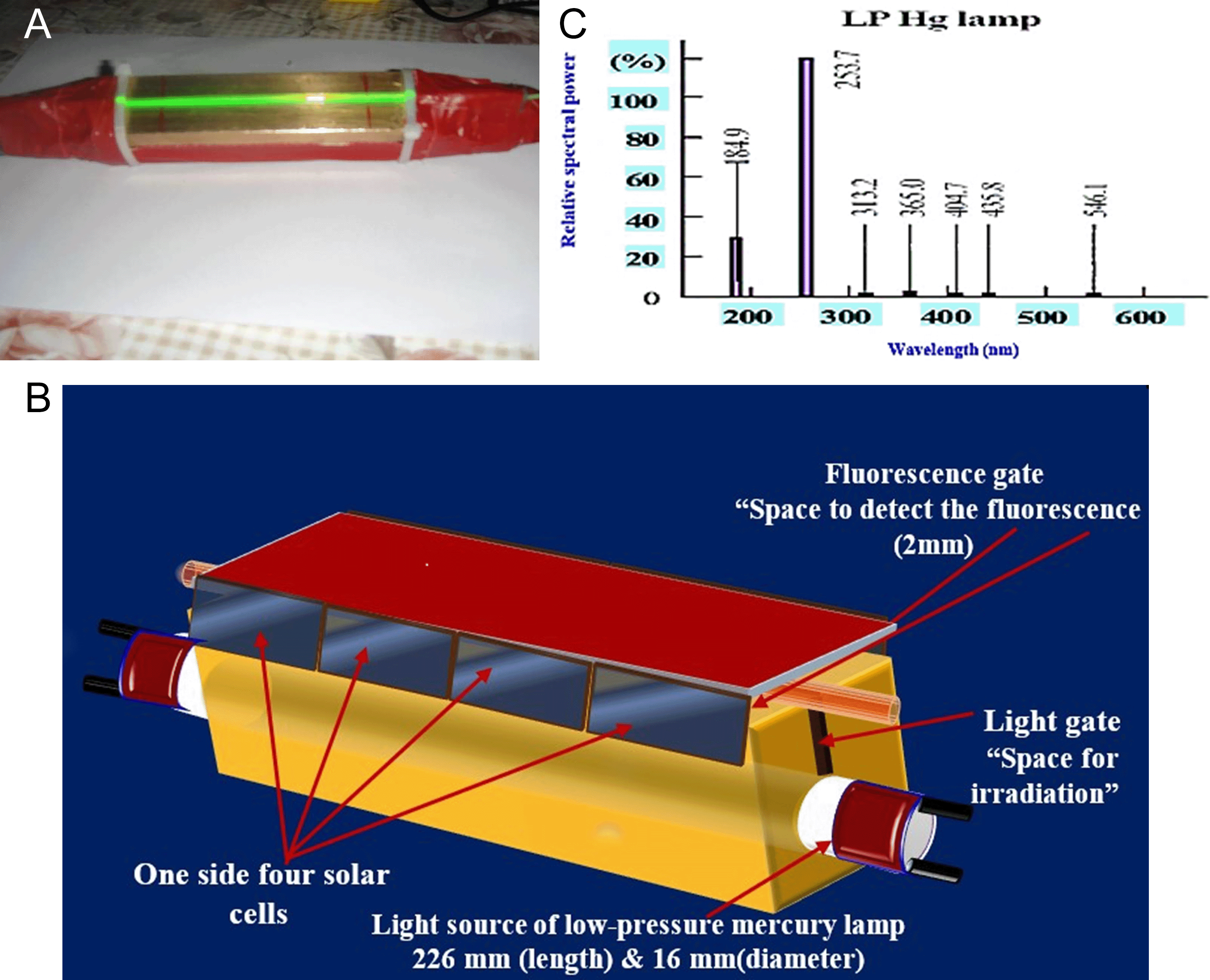
The response was measured using a home-made ISNAG fluorometer37(Figure 1). The locally designed instrument includes a brass incubator with dimensions of 80 mm in length, 30 mm in width and 30 mm in depth to contain the radiation source of low-pressure mercury lamp. A longitudinal slit is made on one side along the incubator, i.e. 80 mm in length and 2 mm in diameter (which is equal to the inner diameter of the flow cell used) and extends to a depth of 7 mm, which in turn leads to a 16 mm diameter opening that touches the outer wall of the irradiation tube and along the length of the incubator to house the mercury irradiation tube and to pass a specific and selected portion of the ultraviolet rays of the mercury lamp. In addition, another opening 1 mm away from the surface and 4 mm in diameter along the longitudinal slit is used to house the flow tube with a length of 100 mm (made of Pyrex (quartz) glass with a length of 100 mm (80 mm of which is placed inside the first opening within the metal incubator with a diameter of 4 mm) and close to the surface to be as close as possible to the radiation source). The flow tube is then confined. And it is fixed in the first hole which is 1 mm away from the surface and covered with a solid sheet of reflective aluminum with a length of 100 mm, a width of 30 mm, and a thickness of 1 mm, and covered with black adhesive skin which does not allow the passage of penetrating rays or reflect them due to the flexibility of the adhesive skin used to only allow the passage of rays from the sides of the transient flow cell to an array of solar cells located at an angle of ± 90° (i.e. opposite the flow cell from the sides) and it is eight solar cells, four on each side (the dimensions of one solar cell are 14 mm wide, 1.2 mm thick, and 2.5 mm long) (Figure 1). The ISNAG fluorometer uses a low-pressure mercury lamp, which features two lambdas (184.9 and 253.7) nm. The detector used in this locally designed system is eight solar cells arranged four on each side with a length of 2.5 cm and symbolized by 2 [4 × 2.5 cm] and at an angle of ±90 degrees with respect to both the radiation source and the flow tube. In addition, the cells are connected in series to collect the output of the solar cells together in a cumulative manner so that the result of the mixture is collected in the visible region. The peristaltic pump used is single-line and variable-speed type (Ismatec, Switzerland) and a six-port medium-pressure injection valve (IDEX Company, USA) with a sample loop (1 mm inner diameter Teflon, variable length). Two sides each one consists of 4-solar cells [4 × 2.5 cm (length)] are used as detectors to collect the signal as the sample travels through a 2 mm optical aperture line extending 80 mm. The output signals (continuous fluorescence signal or quenching signal by etoricoxib) were recorded by a voltage recorder (Siemens, Germany) (1–5 V i.e.; 1000–5000 mV). A spectrophotometer (UV-1800, Shimadzu, Japan) was used for classical spectrometry. Conventional fluorescence measurements were performed using an F-7000 fluorescence spectrophotometer manufactured by Hitachi High-Tech, Japan. The instrument is equipped with a high-intensity continuous xenon lamp as the excitation source, providing stable and broadband illumination suitable for a wide range of fluorophores. Detection was achieved using a photomultiplier tube (PMT), offering high sensitivity and rapid response. In this system, the signal-to-noise ratio was 800 RMS, and the scanning speed 60,000 nm/min, so the spectrum is recorded accurately. All measurements were taken into the quartz cuvette of 10 mm optical path length because this material has good transparency in the visible and UV spectra. Therefore, this system is stable and can be used in pharmaceutical and biochemical fields for the conventional fluorescence analysis.
Electronic Transitions and UV - Absorbance of Etoricoxib drug
The maximum absorption of etoricoxib, a selective COX-2 inhibitor, is determined in the UV range due to the presence of a conjugated aromatic system. The data of validated spectroscopic studies indicate that the drug is characterized by the highest absorption at a wavelength of about 277 nm,38 especially in ethanol solutions or acidic media. This is due to π→π* transitions that occur when the excitation of electrons in the aromatic ring’s π-bonding orbital to a higher anti-π* orbital after UV exposure. Such transitions are specific to compounds with extended conjugation and are very dependent on the solvent’s polarity and the molecule’s structure. Etoricoxib-containing electron-withdrawing groups of fluoro and chloro substituents stabilize the π* orbital, making the absorption maximum slightly below the high end of the UV range. This is a confirmation of the molecule’s photophysical behavior and a rationale for quantifying etoricoxib using UV-Vis spectroscopy. Absorption at 277 nm under different conditions was used in many standard ways for etoricoxib determination and provided an effective and reproducible signal for the calibration and quantification of samples (Figure 2). Meanwhile, while optimal for direct absorption, this wavelength does not interfere with the fluorescence detection of fluorescein by ISNAG-Fluorimeter, which confirms the selectivity of the analytical system. Therefore, the UV absorption peak at 277 nm is directly related to the molecule’s π-aromaticity and is a critical indicator of classical methods. Its electronic transitions can serve as a reliable reference point for comparing innovations and promoting system expansion.
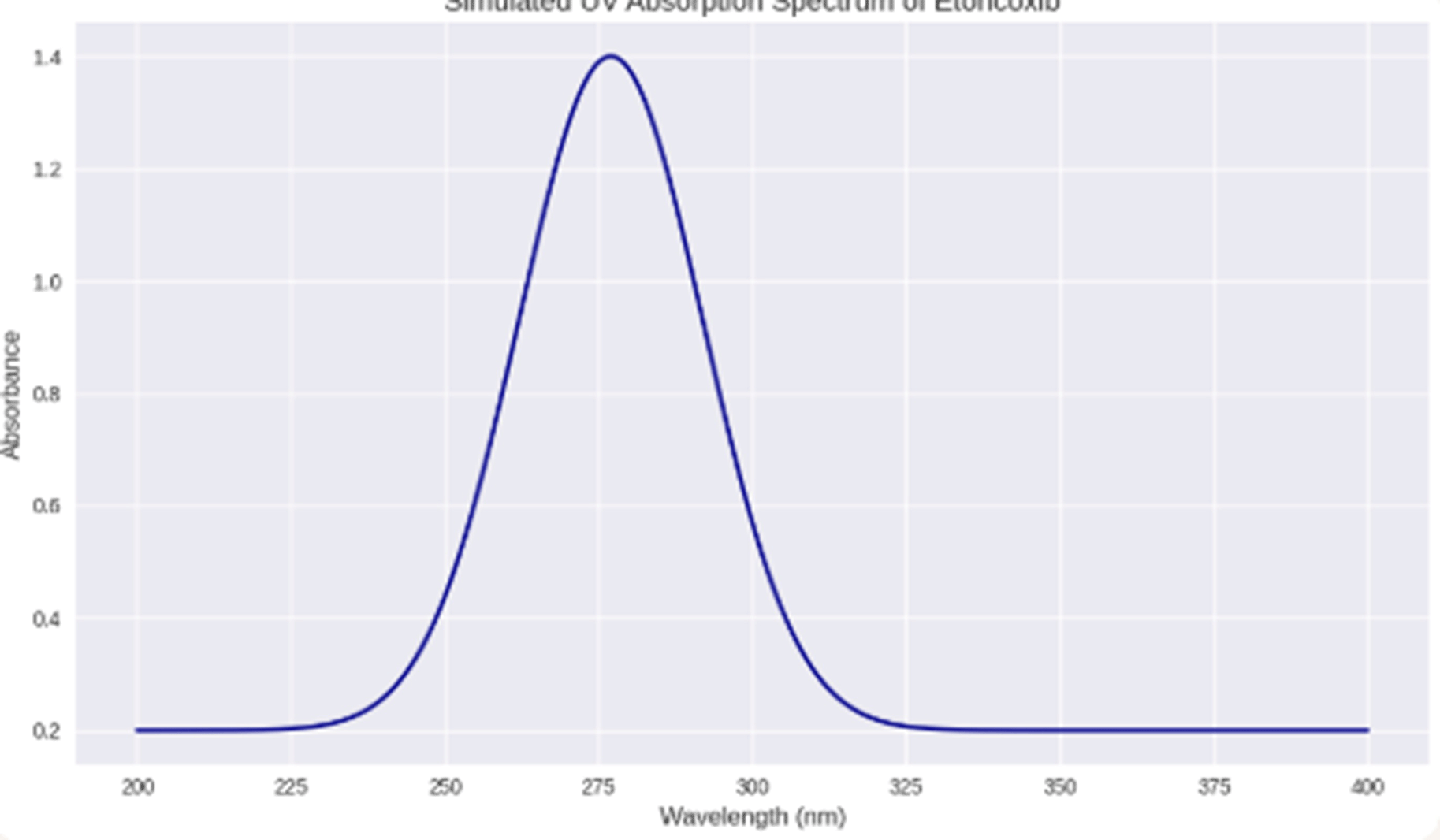
Photo physical Behavior of Sodium Fluorescein in the Presence of Etoricoxib: Excitation, Emission, and Quenching Dynamics.
The spectrum presented in Figure 3 shows the excitation and emission spectra of the sodium fluorescein, a widely-used fluorescent dye characterized by a high signal and photostability. The molecule is usually excited at 490 nm, which belongs to the blue region of the visible electromagnetic spectrum, and emitted at 515 nm, green radiation, which is consistent with the compound’s photophysical properties. The addition of etoricoxib results in a substantial decrement in the fluorescent intensity, indicating efficient quenching.
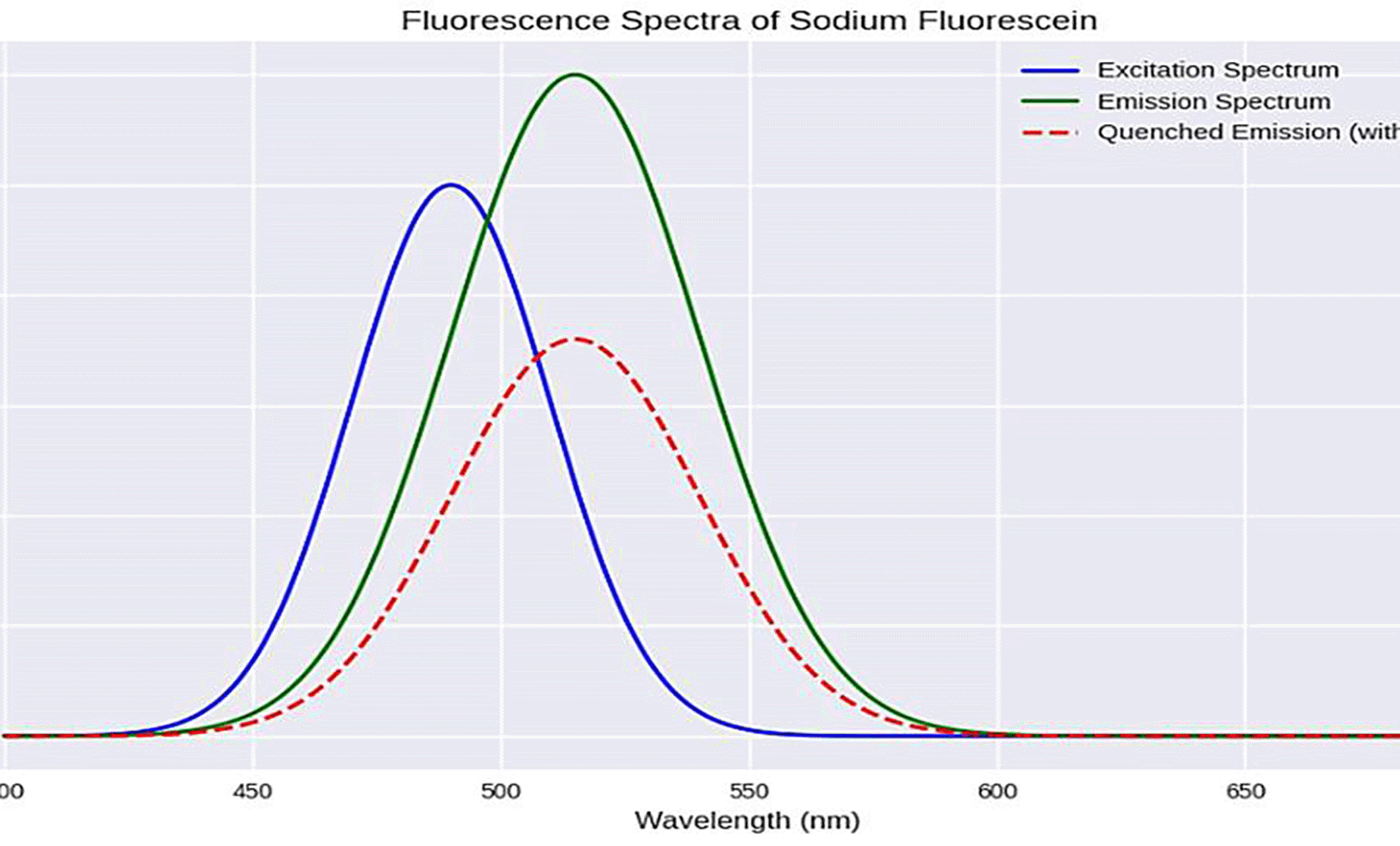
This decrease in signal is attributed to molecular interactions between fluorescein and etoricoxib, which may involve static quenching through complex formation or dynamic quenching via collision processes. Another plausible explanation is orbital overlap between the drug molecule and fluorescein, which can facilitate non-radiative energy dissipation in the form of heat, rather than photon emission. Since etoricoxib does not fluoresce under the emission wavelengths of a low-pressure mercury lamp, the observed changes can be confidently attributed to its effect on fluorescein. The degree of quenching is related to the drug concentration, making this approach suitable for indirect quantitative measurement in pharmaceutical analysis.
The analytical system was designed as a single-flow system (Figure 4), where a fluorescein solution was continuously passed through a quartz flow cell under optimal fluorescence conditions (0.07 mmol/L of FSS as a carrier stream (1.2 ml/min flow rate)). 0.003 mmol/L of sodium hydroxide was used to promote stable and sustained fluorescence, facilitating the deprotonation of fluorescein molecules and improving their quantitative yield. Once a stable fluorescence baseline was established, the drug sample of etoricoxib (170 μL) was injected into the flow stream, resulting in a marked decrease in fluorescence intensity. This quenching response was consistently negative and directly proportional to the drug concentration, in contrast to the small and nonspecific quenching effect observed when distilled water was injected. The latter served as a control, and its small effect was subtracted from the drug-induced quenching signal to ensure analytical accuracy.
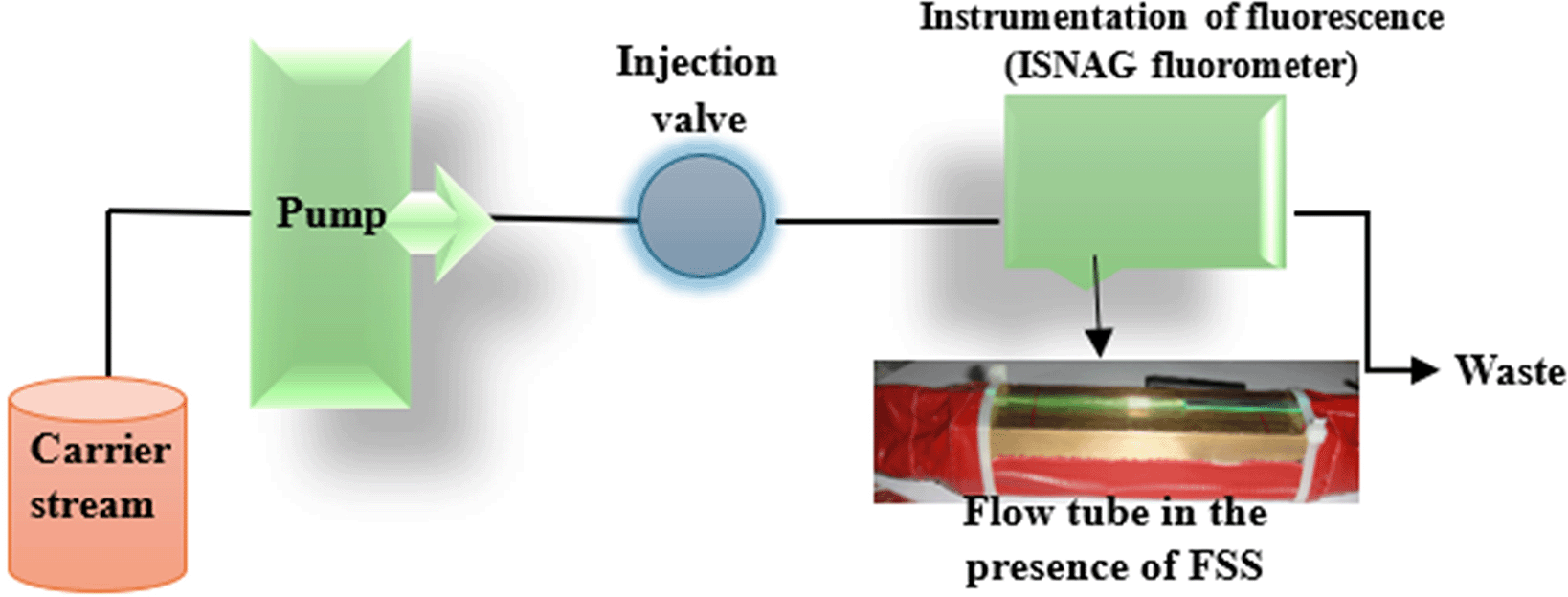
Excitation is provided by a low-pressure mercury lamp (LPML).
During excitation, fluorescein molecules were excited by fluorescein subsequently. Excitation was stimulated using radiation emitted from a low-pressure mercury lamp whose wavelengths were 184.9 and 254 nm. These radiations provided enough energy to elevate the electrons to a higher energy level. Subsequently, the electrons relaxed back to their ground state levels emitting photons during relaxation generating a fluorescent. A magnet of 8 symmetrically placed solar cells on each side, 90 degrees to the radiation’s axis hunted the photons in all the angle and safeguarded moderate integration strengthened the stability and sensitivity. At every instant of fluorescence, long multiplication of increase and quenching responses was videotaped on SSE 04 chart recorder tape program.
The observed quenching effect is attributed to molecular interactions between the excited fluorescein molecules and etoricoxib. These interactions may involve dynamic quenching mechanisms, such as collisional deactivation, or static quenching, where complex formation leads to non-fluorescent aggregates. In addition, self-quenching or delayed relaxation may occur at higher drug concentrations, further contributing to the decreased fluorescence intensity. Together, these mechanisms validate the use of fluorescence quenching as a sensitive and selective approach for drug quantification in flow-based analytical systems (Scheme 1).
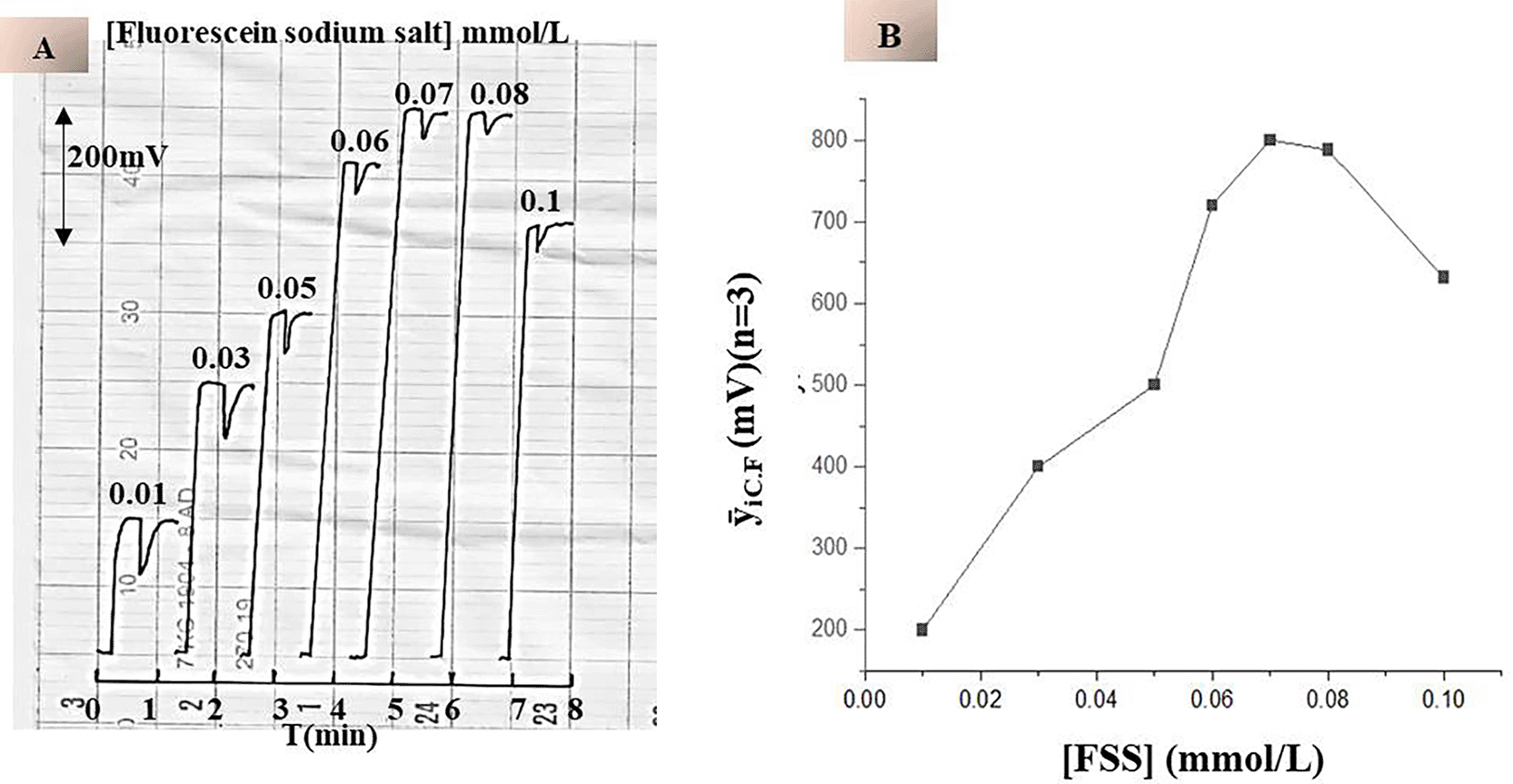
To study the effect of chemical variables on the fluorescence response, a series of fluorescein sodium salt (FSS) solutions were prepared at concentrations ranging from 0.01 to 0.1 mmol/L as the carrier current. All experiments were performed under controlled flow conditions (0.7 mL/min) with a constant sample volume of 75 μL. Distilled water was used as the injected sample in the absence of etoricoxib. The study focused on assessing the efficiency of a low-pressure mercury lamp in excitation of fluorescein molecules. Each measurement was repeated three times to ensure reproducibility.
As shown in Table 1 and Figure 5-A & B, increasing the concentration of fluorescein sodium salt resulted in a gradual increase in the sustained fluorescence intensity. This trend is justified by a larger amount of fluorescein molecules capable of absorbing excitation energy and generating fluorescence. Thus, the fluorescence signal at concentrations above 0.07 mmol/L was achieved in a steady state, which suggests the saturation of the optical response. This behavior was determined by the dynamic photophysical properties of the fluorescein molecules. At high FSS concentrations, the system is nearing an equivalent fluorometric reading, and further fluorescein did not contribute much to signal boosting because of the limitation of excitation sources and potential inner filter effects. In addition, very high concentrations might promote self-quenching phenomena or reabsorption, reducing the overall efficiency of fluorescence. Finally, regarding these outcomes, 0.07 mmol/L was determined as the ideal FSS concentration. This level provided maximum fluorescence intensity combined with minimal consumption of reagents and a stable signal, crucial for the flow-based system to ensure analytic selectivity.
| FSS Concentration (mmol/L) | Continuous Fluorescence Response (mV) | Blank Response (mV) |
|---|---|---|
| 0.01 | 200 | 80 |
| 0.03 | 400 | 76 |
| 0.05 | 500 | 58 |
| 0.06 | 720 | 40 |
| 0.07 | 800 | 20 |
| 0.08 | 788 | 20 |
| 0.10 | 632 | 20 |
To determine the optimal medium for continuous fluorescence and subsequent quenching with etoricoxib, the carrier stream consisted of fluorescein disodium salt (FSS) at its optimal concentration of 0.07 mmol/L, dissolved in various chemical media. These media included distilled water (D.W.), buffer solutions of disodium hydrogen phosphate and citric acid (DSHP+C.A) at different pH values, and sodium hydroxide (NaOH) at different concentrations. All experiments were performed under constant flow conditions (0.7 mL/min, 4 mmol/L (75 μL) of drug). The fluorescence response of each medium was recorded by injecting etoricoxib into the flowing FSS solution and monitoring the resulting quenching. In Figure 6A & B1, the fluorescence intensity of DSHP+C.A buffer solution decreased gradually with an increase in pH. This is because the fluorescent molecule fluorescein is deprotonated at higher pH levels and changes its electronic configuration hence reduction in quantum yield. FSS in distilled water had the highest fluorescence then in buffer media at pH 5 and 6, and the fluorescence decreased significantly above pH 7. Preparing FSS in NaOH fluorescence buffers gave more stable and reproducible fluorescence signals shown in Figure 6A & B2. FSS in 0.003 mmol/L NaOH quenched etoricoxib and had the highest fluorescence intensity of 888 mV. This concentration probably ensures that fluorescein is in its emissive ionic form and the slightest background interference and photo degradation.
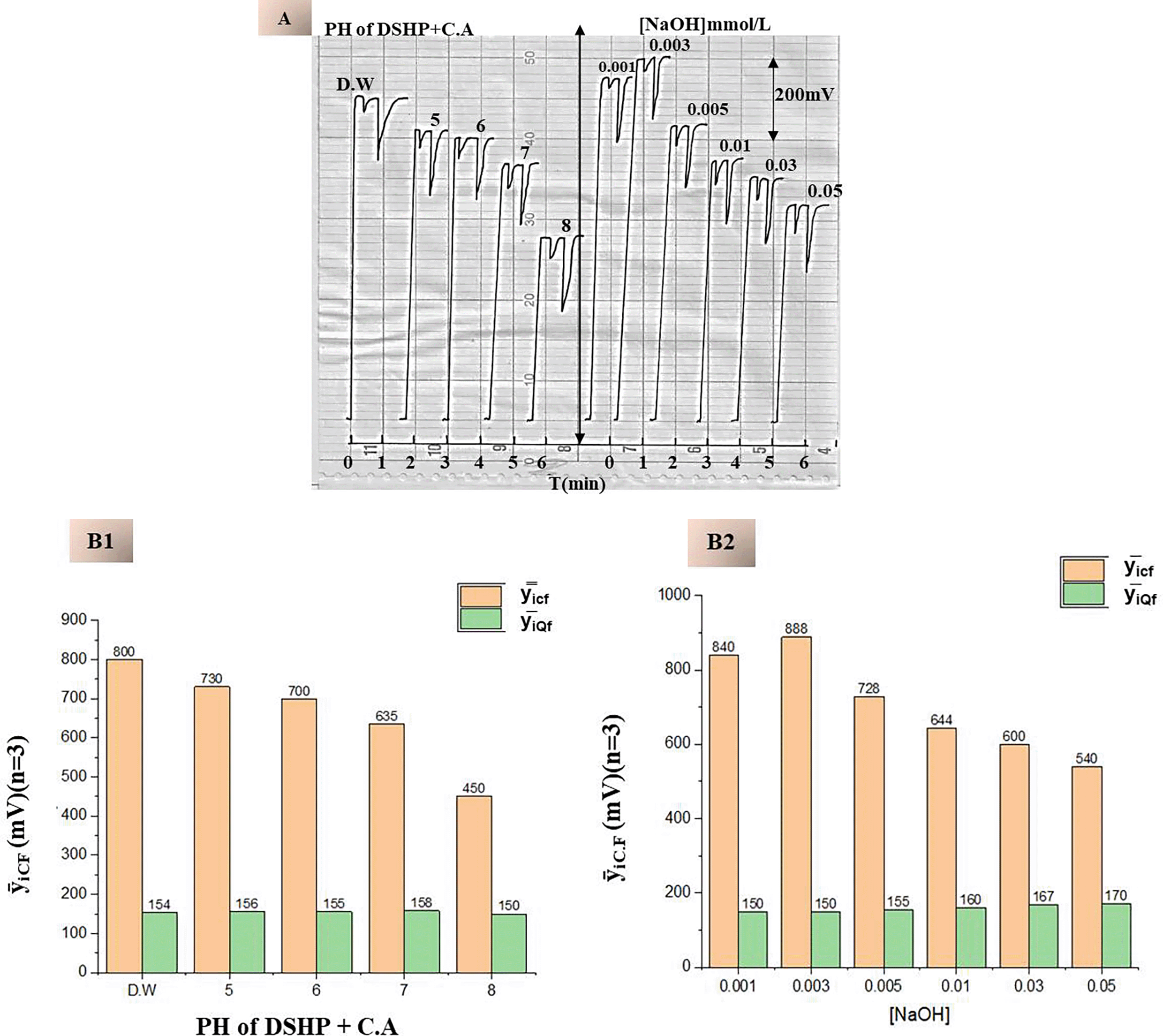
A. Temporal Profile of Continuous Fluorescence Response at Varying Medium.
B1. Effect of pH Variation Using DSHP+C.A Buffer.
B2. Effect of NaOH Concentration, Optimal Quenching of Response at 0.003 mmol/L of NaOH.
Table 2 summarizes the continuous fluorescence yield and quenching efficiency across different media. Suppression values were calculated based on the difference between the total quenching (QT), resulting from etoricoxib injection, and the blank quenching (QB), resulting from distilled water injection. This allows for precise differentiation of the drug’s specific quenching effect from the background signal:
The results confirm that while both distilled water and low-concentration of sodium hydroxide (NaOH) support strong fluorescence, 0.003 mmol/L NaOH offers superior sensitivity and stability (Table 2), making it the preferred medium for inducing the highest continuous fluorescence intensity of the fluorescein solution as the carrier current in subsequent analytical procedures.
Influence of Flow Rate and Sample Volume on the Quenching Efficiency of Etoricoxib
To evaluate the effect of physical variables on the drug’s ability to quench the continuous fluorescence of fluorescein disodium salt (FSS), two key variables were studied: flow rate and sample volume. The former two factors affect the dynamics of interaction between etoricoxib with the probe, while the other does, expressed in the terms of the quenching of the crashing fluorescence used to define the analytical signal. Flow Rate Effect: varying the flow rate from 0.5 to 2.0 mL/min with the 75 μL etoricoxib sample of 4 mmol/L and the 0.07 mmol/L final sample solution. The influence of this factor is demonstrated in Table 3 and Figure 7A & B; on the one hand, low flow rates exhibited a broader zone of sample quenching depth because they caused it to over-disperse, increasing the residence time within the detector cell. A longer exposure time improves the quenching of the drug; however, over-dispersion would likely cause peak broadening, when flow rates are elevated, more than 1.2 mL/min. On the other hand, sample zones of etoricoxib traverse the cell rapidly, yielding too small a zone to interact. Thus, more as 1.2 mL/min gives less quenching efficiency. Moreover, the drug’s ability is further reduced by dilution and axial dispersion. Therefore, 1.2 mL/min is an optimal rate due to the sufficient interaction time combined with a negligible impact on the signal’s distortion. A sample Volume Effect: 25-200 μL injection of etoricoxib sample o.4 mmol/L using an optimal flow rate of 1.2 mL/min and a 0.07 mmol/L FFS.
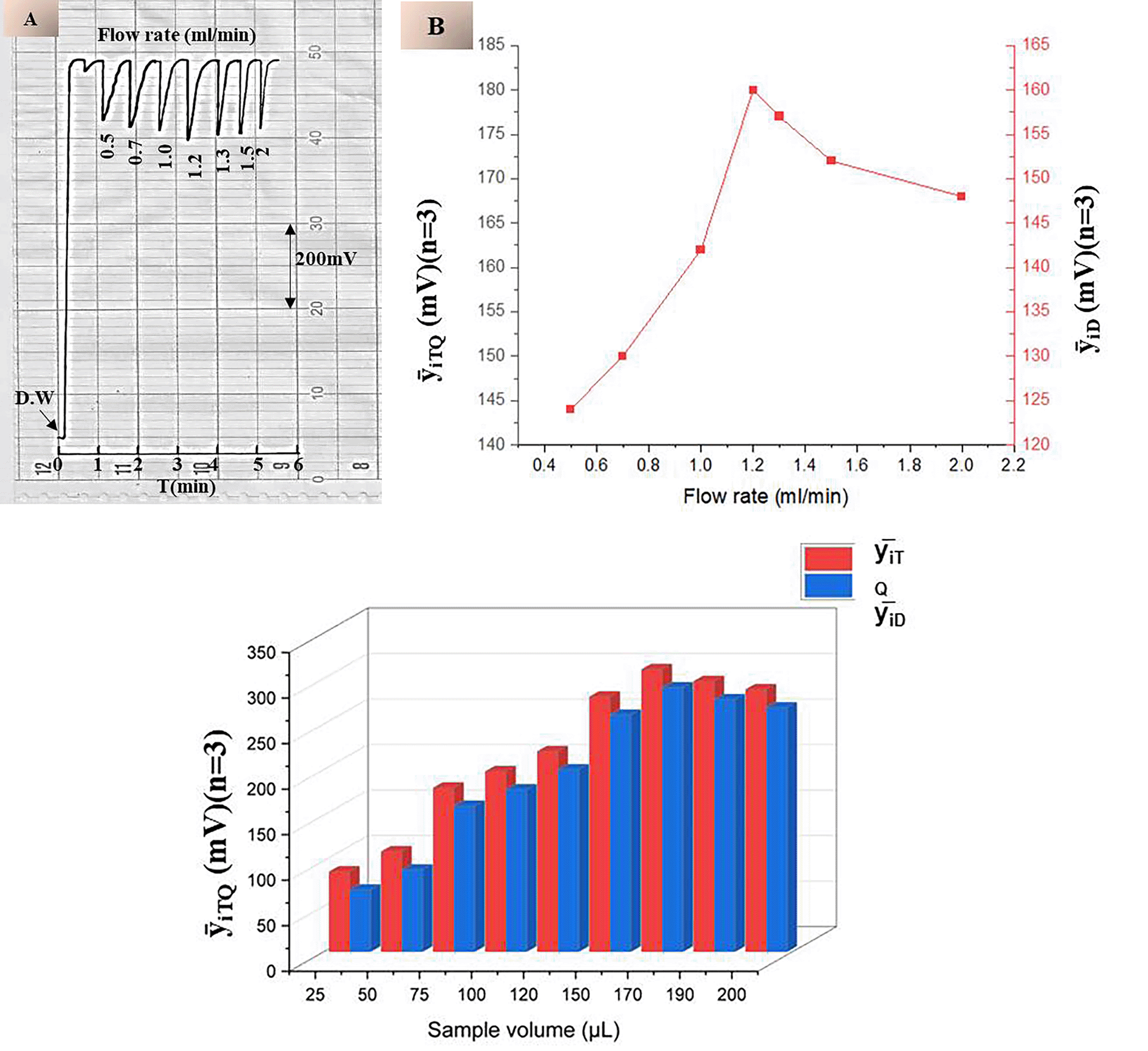
Table 3 and Figure 7-C show that increasing the sample volume improves the quenching response due to greater drug availability. However, volumes exceeding 170 μL resulted in a decreased response, attributed to excessive dispersion and a longer passage time through the reagent, which reduces the effective concentration at any given moment. A volume of 170 μL was determined to be optimal, providing the highest quenching efficiency with an acceptable signal profile and reproducibility.
The effect of etoricoxib concentration on the quenching efficiency of continuous fluorescence emitted by disodium fluorescein salt (FSS) was thoroughly investigated under optimized analytical conditions. The fluorescence intensity of FSS was recorded at 888 mV using a low-pressure mercury lamp emitting at wavelengths of 184.9 nm and 254 nm, which served as the excitation source. The system head of the fluorometric detector was composed of eight symmetrically arranged twin solar cells, each pair at an angle ±90° to the radiation axis, and an 80 mm quartz flow tube with a 2 mm radiation path. It was structured this way to facilitate fish photon capture and maintain a stable conserved fluorescence signal. Subsequently, a series of etoricoxib solutions were injected, ranging from 0.08 to 12 mmol/L, which quenched fluorescence progressively as the drug’s concentration increased.
At the highest tested concentration (12 mmol/L), quenching reached 712 mV (Figure 8-A), leaving a residual fluorescence of 176 mV. The linearity of the calibration curve was confirmed up to this concentration, with a coefficient of determination (r2) of 0.9810 (Figure 8-B). However, slight deviations from linearity at higher concentrations may be attributed to fluorophore surface saturation, limited diffusion within the flow cell, or imperfect mixing, collectively reducing the efficient interaction between the drug molecules and the fluorescent probe. The Repeatability and reproducibility of the method were evaluated at fixed concentrations of 5 and 10 mmol/L with five replicates (n = 5). Intra-day precision (repeatability) refers to the consistency of measurements under identical conditions within a short time frame, while inter-day precision (reproducibility) assesses the method’s stability across different days or analysts. The relative standard deviation was calculated as less than 0.5%; thus, the analytical reliability was declared excellent. To prepare the calibration curve, it was necessary to carry out to achieve minimum concentration 10 μmol/L a few times successively diluted portions. Thus, the limit of detection was determined as 0.61 μg in 170 μL of sample volume, and on the basis of the empirical formula, the limit of quantitation is 1.83 μg per sample. The functional capacity of the system is set at about 50 samples per some hours and can be used for high-efficiency screening. The developed method was compared with UV spectroscopy Shimadzu UV-1800 spectrometer, Japan, with the use of a quartz cuvette with a path of 10 mm.
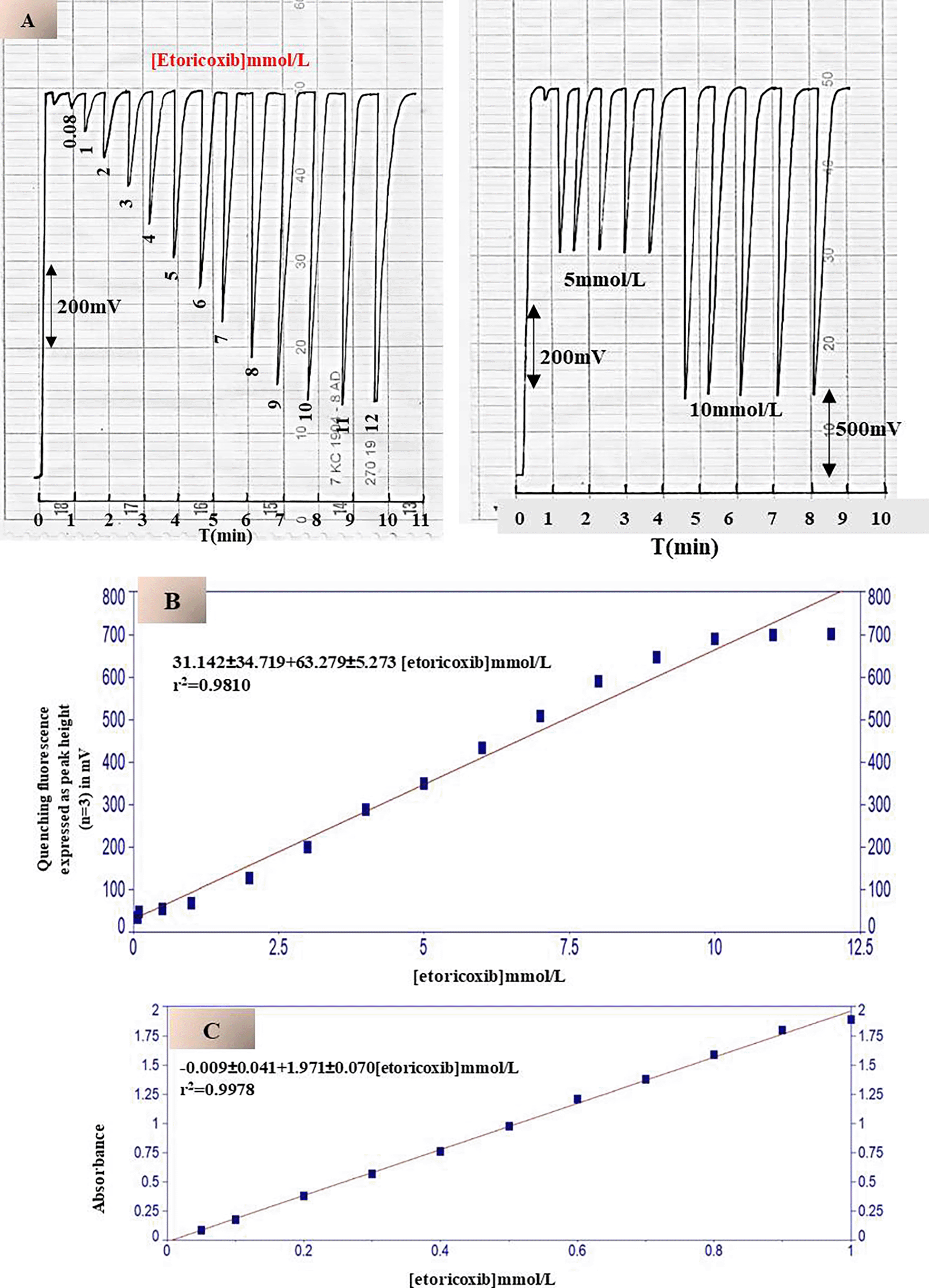
Measurements were performed at the maximum drug absorption wavelength (λmax = 277 nm). The conventional method showed a narrow linear range (0.05–1 mmol/L) (Figure 8-C) and a limit of detection of 8 μmol/L, equivalent to 10.047 μg/sample (3.5 mL). Linear regression equations were developed for both methods: for the improved fluorescent method:
To evaluate the selectivity of the developed fluorescence spectroscopy method, a series of potential interfering substances, commonly found in pharmaceuticals, were tested at two concentration levels: 1 mmol/L and 10 mmol/L. The selected interfering substances included ascorbic acid, glucose, citric acid, lactose, magnesium stearate, sodium chloride, and talc. Each compound was individually introduced into the fluorescein system under identical experimental conditions to assess its ability to quench the continuous fluorescein signal.
The results have demonstrated that none of the tested interfering substances possessed any significant quenching effect on the fluorescence of fluorescein. Moreover, this effect was not only insignificant but has several reasons to be so. The first, as none of the declared structures of the phenolic form of parsidol occurs: structures with π conjugated systems or electron-withdrawing terminal groups that can ensure non-radiative energy transfer take place. The second reason lies within the size of molecules and their affinity to the fluorophore of fluorescein. While those substances are relatively small enough to form a temporary complex with it, their affinity to the fluorophore is too low to block it permanently, thus no dynamic or static quenching takes place. Thus, the interference ratio was close to zero, affirming the method as selective. This means that the effect of these reagents is statistically and practically irrelevant, no matter their concentration. This fact further confirms the method’s applicability to practical pharmacy, where various excipients and fillers are employed. These compounds were unable to influence stable fluorescence of fluorescein, hence, they will not influence the selectivity of the method and it may be used in practical analysis. Due to this fact, the method may be considered chemical-structural specific, operational and reagent-resistant, adding on its practical value for pharmacy.
The developed method in this work was used to analyze etoricoxib in three different drugs (Orotix, Arcoxia and Atoxia 90 mg to each one) and was compared with the classical method using spectrophotometry at λmax = 277 nm. A standard addition was prepared by gradually adding volumes ranging from 0.0 to 0.5 mL of a 50 mmol/L etoricoxib solution, corresponding to final concentrations up to 2.5 mmol/L. Each addition was made to a fixed volume of 5.0 mL from each sample, which had been previously prepared at a concentration of 1 mmol/L. Subsequently, the quenching in continuous fluorescence was measured for each sample after injecting a volume of 170 μL into the flow system.
The results obtained are summed up in Table 4, which includes the fluorescence quenching responses for each sample. A paired t-test was performed to compare the experimentally measured values ( ) obtained using the developed spectrofluorometric method with the official reference values (μ). The statistical analysis revealed that no significant difference between the mean values, confirming the accuracy and validity of the developed method for pharmaceutical analysis. The system demonstrated high sensitivity and reproducibility in detecting fluorescence quenching. It can be reliably used as an alternative method for the quantitative determination of various chemical species capable of quenching the fluorescence of fluorescein solution, or any fluorophore that responds to excitation by a low-pressure mercury lamp. Its design allows for precise detection of fluorescence changes, making it suitable for compounds that inhibit or enhance fluorescence under controlled excitation conditions.
To further assess analytical performance, by another paired t-test was conducted to compare between the developed method with the conventional -UV spectrophotometric technique.
Therefore, the following assumptions were made at a confidence level of 95%:
Alternative hypothesis: H1≠ μUV-spectrophotometer≠ μISNAG-Fluorimeter, thus clearly indicates that there is a significant difference between the mean obtained from two different methods.
At a confidence level 95% in addition to a significant level of α = 0.05, this means any value less than 0.05 will accept the alternative hypothesis and reject Null hypothesis. Will any value of significant more than 0.05 accept Null and refuse alternative hypothesis. Therefore, although the statistical results showed no significant difference between the two methods due to tcal (0.256) << ttab (4.303), and thus clearly indicates that both methods are suitable for quantitative estimation, but the developed system exhibited a greater precision, especially in handling microliter volumes and achieving low detection limits using minimal reagent quantities. Indeed, the developed method provides for rapid analysis, efficacious and cost-effective procedures, and an improved sensitivity that renders this method appropriate for utilization as a routine procedure. This efficacy is facilitated by the utilization of a low-pressure mercury lamp as an excitation source, which virtually guarantees stable and effective photon emission. Eight solar cells spaced at 90° intervals produce detection, which results in cumulative photon capture and augments the signal. Thus, the unique system design ensures the highest capacity for the fluorescence response, minimizes the background interference, and provides efficacious and robust analytical performance. This fact means that the developing method may demonstrate not only a full-fledged competing but even outperform traditional technique.
This study presents a novel fluorescence-based injection method for quantifying etoricoxib in commercial pharmaceuticals, using a locally designed ISNAG-Fluorimeter. The analytical principle relies on the continuous fluorescence stimulation of disodium fluorescein by a low-pressure mercury lamp as the excitation source, followed by quenching of the fluorescence in the presence of etoricoxib. This quenching behavior was exploited to develop a sensitive and selective quantification protocol for the drug.
The method demonstrated excellent accuracy and clarity, with the results statistically validated by paired t-tests. No significant difference was observed between the values obtained using the developed method and those reported by official reference procedures, confirming the method’s validity and reliability for routine pharmacologic analysis. Furthermore, a standard addition technique was employed to avoid interference effects, ensuring robustness across a diverse range of samples.
In addition to statistical equivalence, the developed method demonstrated superior analytical performance in several key areas. Quantitative measurement using microliter-scale sample sizes was enabled, achieving low detection limits down to the microgram level while maintaining high reproducibility. The system design—comprising a low-pressure mercury lamp as the excitation source and eight solar cells arranged in a bifacial 90° array to capture cumulative photons—provided improved signal stability, reduced background interference, and clean, noise-free responses. This configuration significantly improved the signal-to-noise ratio, enabling accurate monitoring of drug concentrations even at trace levels.
Compared to conventional UV-Vis spectroscopy, the ISNAG-Fluorimeter method offered higher sensitivity, faster throughput, and lower operating costs, making it particularly useful for monitoring the quality of high-throughput drugs. Its ability to detect fluorescence quenching with minimal reagent consumption and short sample preparation time underscores its environmentally friendly and cost-effective nature.
Furthermore, the modular, locally manufactured system design is characterized by its accessibility and scalability, particularly in resource-constrained environments. The integration of solar cell-based detection not only enhances analytical performance but also aligns with sustainable instrumentation practices. The method’s adaptability to other fluorescently modulating chemicals opens up broader applications in drug screening, environmental monitoring, and biochemical analysis.
In short, the developed fluorescence injection method represents a technologically innovative, analytically robust, and environmentally friendly alternative to conventional techniques (Table 5). Its successful application in etoricoxib detection, along with its ability to detect multiple analytes, constitutes a valuable contribution to modern analytical chemistry.
| Method Type | Brief Description | Linear Range (μg/mL) | LOD (μg/mL) | Sample Type | Reference |
|---|---|---|---|---|---|
| UV Spectrophotometry | Direct absorbance at 284 nm in methanol; validated for tablet dosage forms | 2–20 | 0.5 | Tablets | 39 |
| UV Spectrophotometry | Absorbance in phosphate buffer (pH 7.4) at 284 nm; simple and accurate | 1–25 | 0.3 | Tablets and bulk drug | 40 |
| Fluorescence Quenching | Indirect method via quenching of fluorescein fluorescence in flow system | 0.1–10 | 0.05 | Tablets and urine | Present study (developed method) |
| Derivative Spectroscopy | First derivative UV method using 0.1 N HCl; enhanced selectivity and sensitivity | 2–24 | 0.4 | Capsules | 38 |
| Ion-Pair UV Spectroscopy | Complex formation with bromothymol blue; absorbance at 420 nm | 5–30 | 0.6 | Bulk and formulations | 41 |
“The data supporting the findings of this study are included within the article. No additional datasets were generated or analyzed during the current study.”
The authors are grateful for Prof. Dr. Issam Al-Hashimi for his efforts with the researchers.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)