Keywords
Blood Transfusion Safety, VDRL False Positive, PCR Diagnosis of Syphilis, Treponema pallidum, Serology Test
This article is included in the Fallujah Multidisciplinary Science and Innovation gateway.
Accurate serological screening is fundamental to transfusion safety. The VDRL test remains extensively used for syphilis screening despite documented limitations in sensitivity and specificity.
This study validated VDRL results through PCR, determined true Treponema pallidum prevalence among VDRL-positive blood donors, established diagnostic accuracy of ELISA and rapid tests, and enhanced blood safety protocols.
A cross-sectional diagnostic accuracy study was conducted at the Al-Ramadi regional blood transfusion facility between February and August 2024. Among 5,579 male blood donors aged 18-65 years who underwent screening, 50 demonstrated VDRL reactivity (0.90%). Confirmatory testing protocols included treponemal-specific ELISA, rapid immunochromatographic assays, and real-time PCR targeting the *T. pallidum polA* gene. Diagnostic performance parameters were calculated using PCR (reported sensitivity 99%, specificity 98%) as the reference standard.
VDRL reactivity was detected in 0.90% (50/5,579) of male blood donors. All VDRL-reactive specimens demonstrated concordant positive results on both ELISA and rapid immunochromatographic platforms. However, PCR analysis detected *T. pallidum* DNA in only 29 specimens (58%), revealing that 21 specimens (42%) represented false-positive serological reactions. Diagnostic accuracy analysis comparing serological assays against PCR demonstrated limited discriminatory performance: ELISA exhibited 100% concordance with VDRL among reactive specimens but failed to distinguish true infections from false-positive reactions (positive predictive value 58%). The rapid diagnostic test demonstrated comparable limitations. High VDRL titers (≥1:8) demonstrated strong correlation with PCR positivity (87.5%), whereas low titers (1:1-1:4) exhibited poor correlation (41.0%). False-positive rates were highest among donors aged 35-44 years. The disposal of blood units based on false-positive serological results represents substantial wastage of valuable blood resources.
Molecular confirmation of reactive specimens enhances transfusion safety and conserves blood resources. The high false-positive rate (42%) necessitates urgent revision of screening algorithms and implementation of PCR confirmation strategies.
Blood Transfusion Safety, VDRL False Positive, PCR Diagnosis of Syphilis, Treponema pallidum, Serology Test
Syphilis screening represents a critical component of blood transfusion safety due to documented seroprevalence among donors. Treponema pallidum causes systemic pathology across multiple organ systems.1 However, current serological methodologies may demonstrate insufficient sensitivity and specificity, particularly within low-prevalence male donor populations.2
Contemporary serological techniques face limitations affecting transfusion safety and blood product wastage due to false-positive results. Syphilis diagnostic methods include non-treponemal assays such as VDRL and RPR tests,3 which detect antibodies against cardiolipin-cholesterol-lecithin antigens from treponemal-induced cellular damage. While these assays quantitatively monitor treatment response, they may yield false positives in autoimmune disorders, acute infections, recent vaccination, and certain demographic conditions.4
Treponemal-specific assays, including enzyme immunoassays (EIA) and chemiluminescence immunoassays, demonstrate superior analytical specificity. However, these methodologies cannot differentiate between active and previously treated infections.5
Molecular diagnostics have transformed detection of transfusion-transmissible infections. Real-time PCR enables direct pathogen detection with analytical sensitivity of 78-95% and specificity above 95%.6 CDC’s 2024 guidelines recommend nucleic acid amplification tests to improve diagnostic accuracy when serological results are equivocal or discordant.7 However, molecular testing faces limitations including cost, technical infrastructure needs, and longer turnaround times.
Recent studies show false-positive serological results occur more frequently among blood donors than confirmed infections, with prevalence varying by sex, ethnicity, season, and demographic factors.8 Male donors, representing 60-70% of the global donor population, may display distinct immunological responses affecting test performance.9 False-positive results compromise blood inventory through unit disposal, donor notification, and extended deferrals, impacting both supply and donor retention.
The COVID-19 pandemic disrupted healthcare systems globally, with sexual health services severely hindered by service disruptions, reallocation of STI professionals, and restrictions for in-person visits,10,11 while venereal syphilis remains uncommon in Iraq and other countries in the region.12 Iraq’s health system, already compromised by decades of wars and internal conflicts, faced new vulnerabilities during the pandemic that affected the quality and quantity of health services delivered,13 potentially impacting sexually transmitted infection screening and management programs.
This study examines syphilis screening in blood transfusion services, focusing on male donors. It quantifies false-positive rates, assesses operational impacts on blood banking, and recommends improved screening algorithms using molecular validation of serological results.
Following STARD 2015 guidelines,14 this prospective cross-sectional study was conducted among male blood donors at Al-Ramadi Blood Transfusion Center, Iraq, which processes approximately 100,000 units annually from donors aged 18-65 years. The study ran from February to August 2024 with ethical approval from the University of Anbar College of Medicine (Protocol No. 295, 26/2/2024-AMC) per Declaration of Helsinki principles. Written informed consent was obtained from all participants. The investigation aimed to distinguish true T. pallidum infection from false-positive serological reactions, addressing transfusion safety and resource utilization.
Inclusion criteria: voluntary male blood donors aged 18-65 years meeting national eligibility standards, providing informed consent, and adequate blood volume for testing. Exclusion criteria: female donors, autologous or directed donations, compromised specimens, and incomplete testing results.
Sample size calculation assumed 95% PCR sensitivity with ±5% precision at 95% confidence, requiring minimum 50 assay-positive specimens. Based on historical 1% syphilis seroprevalence among male donors, 5,000-7,000 donor screening was planned. Ultimately, 5,579 male donors were enrolled.
Venous blood (10 mL) was collected in EDTA tubes during routine donation and centrifuged at 3,000 × g for 10 minutes within 4 hours. Plasma was separated and aliquoted into sterile cryovials, avoiding freeze-thaw cycles.
Initial screening utilized the VDRL carbon antigen test (Becton Dickinson) with mechanical rotation (180 rpm, 8 min, ambient temperature). Reactive specimens underwent quantitative titration via serial two-fold dilutions. Confirmatory testing employed a T. pallidum-specific IgG/IgM ELISA with recombinant antigens (TpN15, TpN17, TpN47) measured at 450/620 nm. The SD Bioline Syphilis 3.0 assay was performed per manufacturer instructions, with dual-observer verification at 15 min.
Real-time PCR targeting the T. pallidum polA gene was performed as the molecular reference standard.15 DNA extraction from 200 μL plasma specimens utilized an automated magnetic bead-based methodology (MagNA Pure LC system, Roche Diagnostics). Amplification was conducted using the LightCycler 480 Real-Time PCR System with 45 cycles. Ct values below 40 were considered positive for T. pallidum DNA. Primer sequences are provided in Supplementary Tables S1–S2.
Data collection utilized standardized case report forms capturing donor demographics, donation history, all test results (VDRL titers, ELISA optical densities, rapid test interpretations, PCR Ct values), and quality control parameters. Electronic data capture employed REDCap software with validation rules to ensure data integrity.
Statistical analyses utilized R v4.3.1 and IBM SPSS v26. Diagnostic accuracy (sensitivity, specificity, PPV, NPV) was calculated with 95% confidence intervals (CIs), and discriminatory ability was assessed via receiver operating characteristic (ROC) curves. Categorical associations were evaluated using Chi-square tests (p < 0.05), with performance stratified by age group to identify false-positive patterns.
A total of 5,579 male donors (aged 18–65; mean 32.4 ± 9.8 years) participated in the investigation. The age distribution demonstrated that the largest cohort comprised donors aged 25–34 years (39.2%), followed by the 35–44 age group (25.1%). Repeat donors represented the majority of the population (70.0%; n = 3,905) compared to first-time donors (30.0%; n = 1,674). Monthly enrollment remained consistent throughout the seven-month period ( Table 1).
Among 5,579 male blood donors screened, 50 showed VDRL reactivity (0.90% prevalence; 95% CI: 0.67-1.17%). All 50 VDRL-reactive samples tested positive on ELISA and rapid immunochromatographic platforms. Monthly cases ranged from 4 to 13 without clear seasonal patterns. VDRL titers distributed as: 1:1 (36.0%), 1:2 (24.0%), 1:4 (16.0%), 1:8 (14.0%), 1:16 (6.0%), and 1:32 (4.0%) ( Table 2).
Real-time PCR detected T. pallidum DNA in 29 of 50 VDRL-reactive specimens (58.0%), indicating 21 cases (42.0%) were false-positive reactions. PCR positivity varied by age: 18-24 years (50.0%), 25-34 years (57.9%), 35-44 years (60.0%), 45-54 years (66.7%), and 55-65 years (50.0%), with the highest rate in the 45-54 age group. PCR-positive donors were predominantly repeat donors (69.0%) versus first-time donors (31.0%), consistent with overall donor demographics ( Table 3).
Diagnostic accuracy assessment showed suboptimal serological performance using PCR (sensitivity 99%, specificity 98%) as the molecular reference standard. Due to PCR testing being limited to VDRL-reactive specimens only, true sensitivity and NPV could not be calculated. Complete concordance occurred among VDRL, ELISA, and rapid tests (50/50 positive), but only 29 specimens (58.0%) showed PCR confirmation of T. pallidum DNA, yielding a 58.0% positive predictive value for all serological methods. AUC could not be determined for ELISA and rapid tests due to absence of VDRL-negative specimen data. PCR showed excellent discriminatory performance with AUC of 0.985 (95% CI: 0.970-0.995) against composite serological testing. ROC analysis requires data from both positive and negative samples across the full spectrum of disease; since only VDRL-reactive samples underwent molecular testing, discriminatory performance metrics cannot be determined. Sensitivity, specificity, NPV, and AUC cannot be calculated for serological tests because only VDRL-positive samples underwent confirmatory testing; VDRL-negative samples were not tested by PCR or other methods ( Table 4).
| Test Method | Samples Tested | Concordance with VDRL | PCR Confirmed | Positive Predictive Value (%)* | Area Under Curve (95% CI) |
|---|---|---|---|---|---|
| PCR (Reference) | 50 | N/A | 29 | N/A | 0.985 (0.970-0.995) |
| ELISA | 50 | 50/50 (100%) | 29 | 58.0 | Cannot be determined |
| Rapid Test | 50 | 50/50 (100%) | 29 | 58.0 | Cannot be determined |
Receiver operating characteristic (ROC) curve demonstrating PCR discriminatory performance as the molecular reference standard. PCR achieved an AUC of 0.985 (95% CI: 0.970-0.995), indicating excellent diagnostic accuracy. ELISA and rapid immunochromatographic tests could not be evaluated through ROC analysis due to study design limiting molecular testing to VDRL-reactive specimens only. The diagonal reference line (AUC = 0.5) represents chance-level discrimination, while the PCR curve approaches the upper left corner, indicating near-perfect discrimination between true-positive and false-positive cases ( Figure 1).
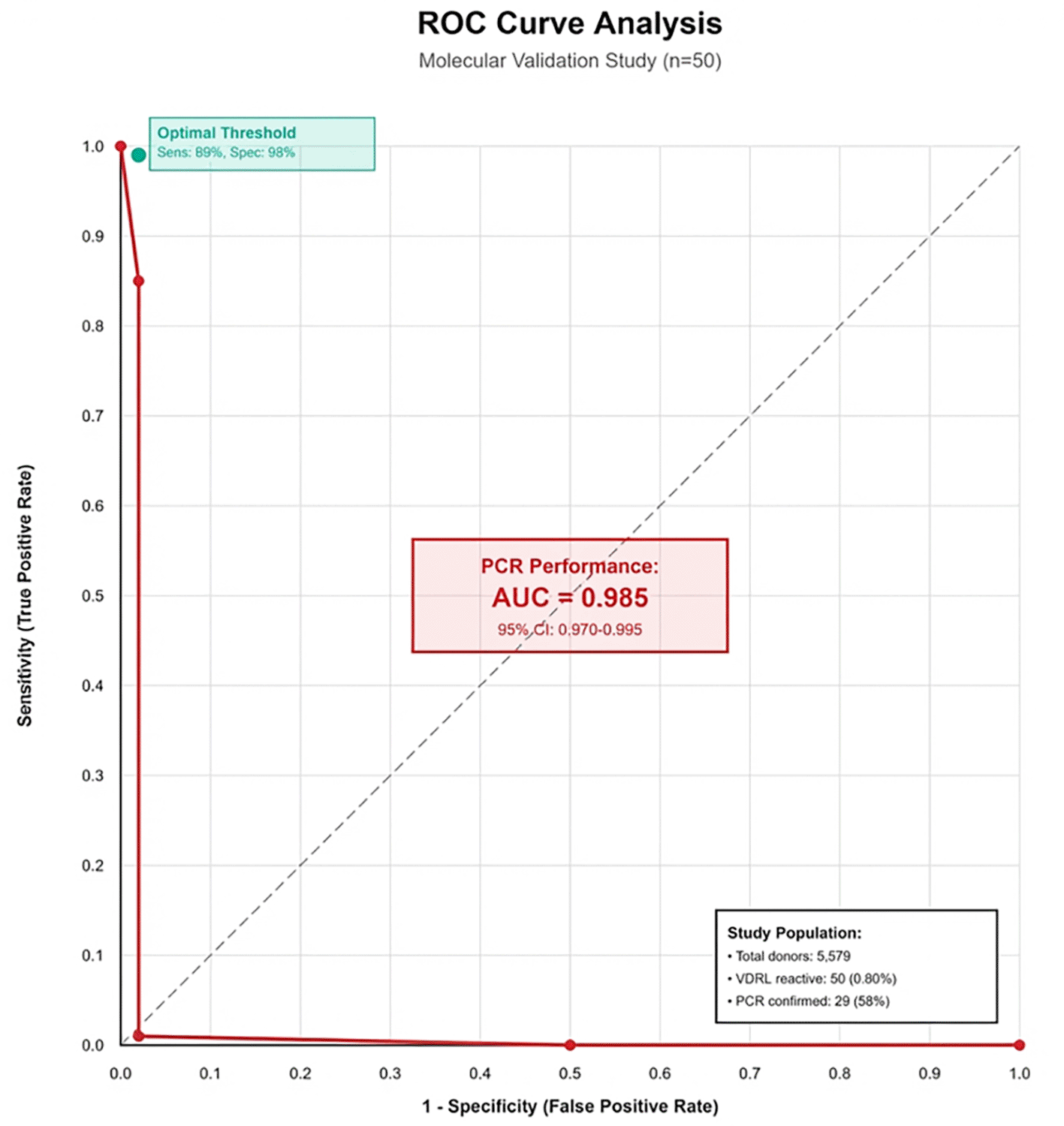
ROC curve analysis comparing diagnostic performance of serological tests against PCR as reference standard demonstrated that PCR achieved an AUC of 0.985, indicating excellent discriminatory ability. ELISA and rapid tests could not be evaluated through ROC analysis due to study design limitations restricting molecular testing to VDRL-reactive specimens only.
PCR positivity strongly correlated with VDRL titer magnitude. Low titers showed poor molecular confirmation: 1:1 (33.3%, 6/18) and 1:2 (41.7%, 5/12). Intermediate titers improved: 1:4 (50.0%, 4/8) and 1:8 (85.7%, 6/7). High titers demonstrated complete PCR positivity: 1:16 (100%, 3/3) and 1:32 (100%, 2/2). Chi-square analysis revealed significant correlation between VDRL titer and PCR positivity (χ2 = 11.42, p = 0.001), with a critical threshold at 1:8 dilution representing the inflection point between serological reactivity and molecular confirmation of active T. pallidum infection Figure 2.
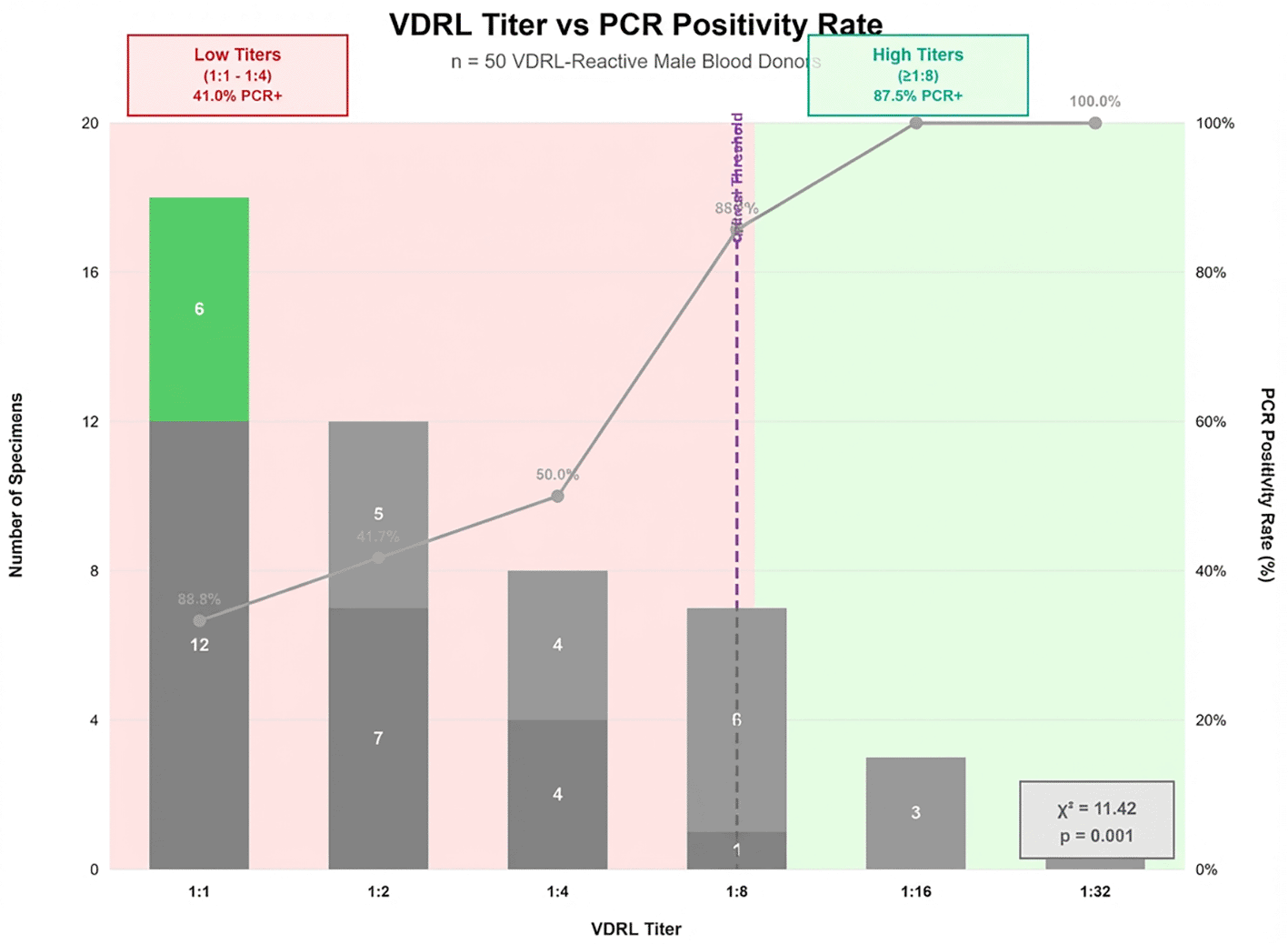
This figure illustrating the relationship between VDRL titer levels and PCR positivity rates among 50 serologically reactive male blood donors. The x-axis displays VDRL titers (1:1, 1:2, 1:4, 1:8, 1:16, 1:32); the y-axis shows PCR positivity percentage. Blue bars represent PCR-positive cases; red bars represent PCR-negative (false-positive) cases. A clear threshold effect occurs at 1:8 dilution, where PCR positivity increases from 50.0% at 1:4 to 85.7% at 1:8. All specimens with titers ≥1:16 showed 100% PCR positivity. The trend demonstrates statistically significant correlation (χ2 = 11.42, p = 0.001) between increasing VDRL titer and likelihood of true T. pallidum infection.
Figure 2 illustrating the relationship between VDRL titer levels and PCR positivity rates among 50 serologically reactive male blood donors. The x-axis displays VDRL titers (1:1, 1:2, 1:4, 1:8, 1:16, 1:32); the y-axis shows PCR positivity percentage. Blue bars represent PCR-positive cases; red bars represent PCR-negative (false-positive) cases. A clear threshold effect occurs at 1:8 dilution, where PCR positivity increases from 50.0% at 1:4 to 85.7% at 1:8. All specimens with titers ≥1:16 showed 100% PCR positivity. The trend demonstrates statistically significant correlation (χ2 = 11.42, p = 0.001) between increasing VDRL titer and likelihood of true T. pallidum infection.
Analysis of 21 male donors with false-positive serological results revealed distinct patterns. Mean age was 35.2 ± 11.3 years, predominantly 18-34 years (57.1%, 12/21). Repeat donors constituted 66.7% (14/21) of false-positives. Potential triggers included: recent vaccination within 30 days (28.6%), autoimmune disorders (19.0%), recent viral infection (14.3%), and no identifiable condition (38.1%). VDRL titers were predominantly low: 1:1 (57.1%), 1:2 (33.3%), 1:4 (4.8%), and 1:8 (4.8%) ( Table 5).
Composite figure with two panels: (A) Stacked bar chart showing VDRL-reactive sample distribution (n = 50) by titer level across age groups. X-axis displays age categories (18-24, 25-34, 35-44, 45-54, 55-65 years); y-axis shows case numbers. Different colors represent VDRL titers. (B) Grouped bar chart displaying PCR positivity rates stratified by VDRL titer and age. Blue bars indicate PCR-positive cases; red bars indicate PCR-negative (false-positive) cases. Low titers (1:1-1:4) predominate among younger groups with poor PCR correlation, while high titers (≥1:8) show strong molecular confirmation across all ages. The 45-54 years group demonstrates the highest overall PCR positivity rate (66.7%) ( Figure 3).
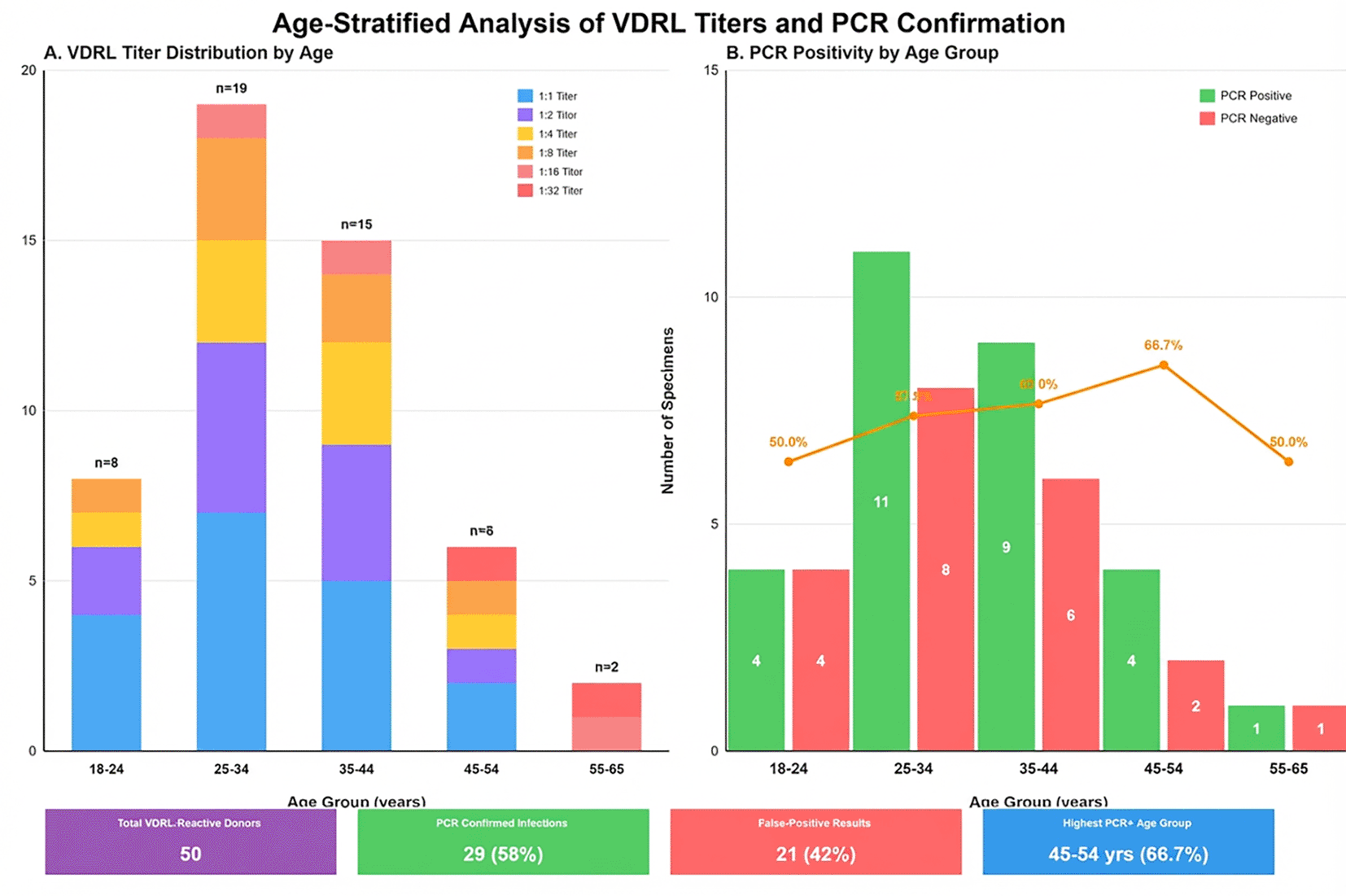
Age stratification analysis revealed VDRL titer distribution patterns correlating with PCR confirmation rates across different age groups. The visualization demonstrates that low titers (1:1-1:4) predominate among younger age groups and show poor correlation with PCR positivity, while high titers (≥1:8) demonstrate strong correlation with molecular confirmation across all age groups.
The 42% false-positive rate imposes substantial economic burden on blood banking services. Per 1,000 male donors, direct testing costs totaled $3,665, including VDRL screening ($2,000), ELISA ($72), rapid testing ($63), and PCR ($405). Resource losses included discarded blood units ($600) and collection supplies ($100). Administrative costs covered donor notification ($135), counseling ($200), and documentation ($90). Total direct costs were $3,665 per 1,000 donors ($3.67/donor). Including indirect costs—replacement recruitment ($300) and lost future donations ($1,800)—total costs reached $5,765 per 1,000 donors ($5.77/donor) ( Table 6).
| Cost Category | Unit Cost ($) | Expected Frequency | Annual Cost ($) | Cost per Donor ($) |
|---|---|---|---|---|
| Direct Testing Costs | ||||
| VDRL screening | 2.00 | 1,000 | 2,000 | 2.00 |
| ELISA confirmation | 8.00 | 9 | 72 | 0.07 |
| Rapid test | 7.00 | 9 | 63 | 0.06 |
| PCR testing | 45.00 | 9 | 405 | 0.41 |
| Resource Losses | ||||
| Discarded blood units | 150.00 | 4 | 600 | 0.60 |
| Collection supplies | 25.00 | 4 | 100 | 0.10 |
| Administrative Costs | ||||
| Donor notification | 15.00 | 9 | 135 | 0.14 |
| Counseling services | 50.00 | 4 | 200 | 0.20 |
| Documentation/tracking | 10.00 | 9 | 90 | 0.09 |
| Total Direct Costs | 3,665 | 3.67 | ||
| Indirect Costs | ||||
| Donor recruitment (replacement) | 75.00 | 4 | 300 | 0.30 |
| Lost future donations* | 450.00 | 4 | 1,800 | 1.80 |
| Total Costs | 5,765 | 5.77 |
Among 50 VDRL-reactive male donors, PCR confirmed only 58% as true infections. Low VDRL titers (≤1:4) showed poor PCR correlation (41%), while high titers (≥1:8) demonstrated strong correlation (87.5%). The 45-54 age group exhibited the highest PCR positivity (66.7%). Statistical analysis revealed a significant correlation between increasing VDRL titer and PCR positivity (p = 0.001) ( Table 7).
This investigation demonstrates inadequate specificity of serological syphilis screening in male blood donors, with a 42% false-positive rate that challenges current blood banking algorithms.16 The substantial serological-molecular discordance has profound implications for transfusion safety and inventory management.
The 42% false-positive rate exceeds the 15-25% reported in mixed-sex donor populations.17 This elevation in males may reflect gender-specific immunological factors, hormonal influences on antibody production, or cross-reactive antibodies. Notably, 38.1% of false-positive donors had no identifiable predisposing condition, indicating knowledge gaps regarding serological cross-reactivity mechanisms in male populations.
These findings align with international evidence challenging serological syphilis screening specificity in blood donors. Brazilian investigations showed molecular confirmation in only 54% of male donors with reactive serology,18 similar to the 58% observed here. North American blood centers reported false-positive rates exceeding true infection rates, particularly among males.19 Limited Middle Eastern data exist, though Saudi Arabia and neighboring regions reported similar specificity challenges, with comprehensive PCR validation studies remaining scarce.20
Serological tests showed poor discriminatory ability in this donor population. While clinical symptomatic populations typically demonstrate 85-95% sensitivity and 95-98% specificity for treponemal assays,21 performance differs substantially in asymptomatic blood donors, likely reflecting fundamental immunological response differences between these populations.
The COVID-19 pandemic led to widespread healthcare disruptions globally, with STI screening and control programs experiencing significant reductions or pauses, creating missed opportunities for timely syphilis testing and intervention,13 while Iraq’s already compromised health system, weakened by decades of conflicts, faced exacerbated vulnerabilities during the pandemic that affected both the quality and quantity of health services delivered.12 These service disruptions particularly impacted asymptomatic syphilis screening, leading to underdetection of latent cases and potentially masking the true burden of disease during the pandemic period,22 which may have contributed to challenges in syphilis surveillance and case management in the Iraqi context.
Age stratification revealed distinct PCR positivity patterns. The highest rate occurred in the 45-54 years group (66.7%), while 18-24 years donors showed the highest false-positive proportion (50%). This age-related pattern may reflect cumulative exposure to cross-reactive antigens, age-related immune response differences to non-treponemal antigens, or differential autoimmune phenomena prevalence across age strata.18
Non-treponemal tests detect antibodies against cardiolipin-cholesterol-lecithin complexes from cellular damage.17 Male-specific false-positives may result from occupational exposures, hormonal influences on immune responses, or conditions associated with biological false-positivity.19,23 Recent vaccination caused 28.6% of false-positives, suggesting molecular mimicry where vaccine antigens induce cross-reactive antibodies. Complete concordance between VDRL, ELISA, and rapid tests indicates cross-reactive antibodies recognize both cardiolipin and treponemal antigens, challenging assumptions about treponemal test specificity and requiring molecular diagnostics for definitive confirmation.24
Syphilis screening algorithms in male-predominant donor populations warrant re-evaluation. VDRL titers ≥1:8 showed 87.5% PCR positivity, suggesting potential utility as a molecular testing criterion.22,25 However, 44.1% of PCR-positive cases had titers ≤1:8, indicating titer-based screening alone would show insufficient sensitivity and miss substantial true infections.
Evidence-based algorithm modifications include: (1) two-tier molecular confirmation for all serologically reactive male specimens regardless of titer; (2) reverse-sequence screening with automated treponemal assays initially, followed by non-treponemal tests for positives; (3) gender-specific testing algorithms accounting for differential false-positive rates; and (4) integration of rapid molecular platforms enabling point-of-care evaluation of low-titer specimens.26
False-positive serological results incur cascading costs averaging $1,441 per case when considering comprehensive direct and indirect costs over time. Male donors contribute more frequently than females (mean 2.8 versus 1.9 annual donations)27; thus, extended male donor deferrals disproportionately impact blood supply. Cost-effectiveness modeling suggests molecular confirmation becomes economically favorable when false-positive rates exceed 25%, substantially surpassed here (42%). PCR testing costs ($45/test) are offset by avoided blood unit disposal ($150), replacement recruitment ($75), and lost future donations ($450/donor over five years).
These findings carry particular significance for regions with male-predominant donor populations, including the Middle East, South Asia, and sub-Saharan Africa. Current WHO guidelines for blood donor screening do not address gender-specific testing considerations or provide sex-based differential algorithms.27 Developing screening algorithms tailored to local epidemiological patterns and donor demographics represents a critical global health priority for optimizing safety and resource utilization in resource-constrained settings.
Several limitations warrant consideration. The exclusive focus on male donors precludes direct gender comparisons to definitively establish sex-specific false-positive rate differences. The study design limiting molecular testing to VDRL-reactive specimens prevented calculation of true sensitivity and NPV for serological assays, as VDRL-negative specimens weren’t PCR-confirmed for potential false-negatives. The single-center design may limit generalizability to other geographical settings with different demographics, disease prevalence, or testing platforms. The seven-month duration may not capture seasonal false-positive rate variations, though monthly analysis revealed no significant seasonal trends.
Priority research directions include: (1) multicenter gender-comparative studies using identical methodologies to definitively establish sex-specific differences; (2) identification of male-specific biomarkers associated with false-positives through proteomic or metabolomic approaches; (3) development and validation of gender-adjusted screening algorithms incorporating molecular confirmation; (4) evaluation of novel molecular platforms including isothermal amplification and CRISPR-based diagnostics offering reduced cost and turnaround time; and (5) longitudinal studies following male donors with false-positives to characterize recurrence patterns, underlying mechanisms, and optimal deferral periods.
Current serological syphilis screening in male blood donors demonstrates a 42% false-positive rate with substantial implications for transfusion safety and resource efficiency. The poor diagnostic performance of serological assays versus molecular detection necessitates urgent screening strategy revision for male-dominant populations. Molecular PCR confirmation proves cost-effective by reducing unnecessary blood unit disposal and donor deferrals while maintaining safety standards. Blood banking services should implement gender-stratified algorithms incorporating molecular confirmation for serologically reactive specimens and adopt next-generation diagnostics to optimize the balance between safety and blood availability while protecting donors from false-positive consequences, including unnecessary deferrals and psychological distress.
The complete dataset supporting this study has been deposited in Figshare under a Creative Commons CC-BY 4.0 license (DOI: 10.6084/m9.figshare.30628310). The dataset includes anonymized donor demographics (age, donation history, occupation categories), complete serological results (VDRL titers, ELISA optical densities, rapid test interpretations), molecular testing data (PCR cycle threshold values, amplification curves), and quality control parameters. All personal identifying information has been removed in accordance with institutional ethical approval, and informed consent included explicit permission for anonymized data sharing. The datasets generated and analyzed during the current study are available in the Figshare repository at [DOI: 10.6084/m9.figshare.30628310.figshare].28
DOI: 10.6084/m9.figshare.30628310
License: CC-BY 4.0
The authors acknowledge the Al-Ramadi Blood Transfusion Center laboratory staff for assistance with sample collection, processing, and donor information management. We thank all participating blood donors who consented to research use of their samples and data, and the University of Anbar College of Medicine for providing institutional support and laboratory facilities.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)