Keywords
Anxiety, Depression, Gastric cancer, Survival, Systematic review
Gastric cancer is associated with a high mortality and morbidity rate. Patients often experience anxiety and depression, which are associated with a poor health-related quality of life. Furthermore, anxiety and depression have been associated with worse survival in patients with cancer. Therefore, this systematic review aims to identify the risk factors and prognostic impacts of anxiety and depression in gastric cancer.
We searched for relevant literature published up to April 7, 2025 from the MEDLINE, EBSCOhost, and ProQuest databases. We included studies that evaluated the risk factors and prognostic impact of anxiety and depression in gastric cancer. Studies that also included other types of cancers or only analyzed risk factors of psychological distress were excluded. Quality of studies was analyzed using the Newcastle-Ottawa Scale. Meta-analysis was performed if the adjusted odds ratio for a specific risk factor was evaluated in ≥ 3 studies. Otherwise, only qualitative analysis was performed.
Fourteen studies were included in the review with a total number of 31,660 patients. From the included studies, we identified various risk factors that were significantly associated with anxiety or depression. We classified those risk factors based on Engel’s biopsychosocial model. Meta-analysis for eligible risk factors showed that female sex (adjusted OR = 3.04; 95% CI 2.04–4.54) and diabetes mellitus (adjusted OR = 4.78; 95% CI 2.46–9.28) were independently associated with higher odds of depression in gastric cancer. Furthermore, most studies showed that anxiety or depression was associated with worse survival in patients with gastric cancer.
We identified various risk factors for anxiety and depression in gastric cancer based on the available literature. It is important to screen for anxiety and depression in gastric cancer because of their negative prognostic impact on the patients.
Anxiety, Depression, Gastric cancer, Survival, Systematic review
Gastric cancer is commonly encountered in daily practice. In 2022, gastric cancer had the 5th highest incidence rate among all cancers. The highest prevalence of gastric cancer is in Eastern Asia and Eastern Europe. Furthermore, gastric cancer also had the 5th highest mortality rate of all cancers.1 Advancements in treatment modalities have resulted in improved patient survival.2 However, the morbidity rate among patients with gastric cancer remain high. Gastric cancer survivors also experienced various health-related quality of life problems (QoL). Studies have shown that gastric cancer patients have lower QoL scores than the general population.3
Patients with cancer often have depression and anxiety symptoms, which contribute to the poor QoL. The spectrum of anxiety and depressive symptoms can range from temporary and nonpathological to full-blown psychiatric syndromes.4 A meta-analysis of 18 studies conducted from various countries found that the pooled prevalence of depression in gastric cancer was 37% (95% CI 26%–48%). They also found that the highest prevalence of depression was in the Eastern Mediterranean region, followed by the Western Pacific region.5 The prevalence of depression was also higher in patients with gastric cancer than in the non-gastric cancer matched cohort.6 Meanwhile, the prevalence of anxiety in a study involving patients with gastric cancer who underwent surgery was 34.1%, which increased to 45.6% at 3 years follow-up.7
Besides a reduction in the QoL, anxiety and depression can also negatively affect a patient’s survival. A recent meta-analysis showed that depression significantly increased cancer-specific mortality in various cancer sites.8 This phenomenon can be owing to reduced adherence to cancer treatment and increased risk of suicide in patients with psychiatric syndromes. Anxiety and depression may also directly promote cancer progression through activation of the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis, releasing catecholamines that interact with various downstream pathways.9
To detect these often-neglected complications, it is important to identify the risk factors of anxiety and depression in gastric cancer. Although there had been systematic reviews about the risk factors of anxiety and depression in patients with cancer, none had analyzed those in gastric cancer specifically. Therefore, this systematic review aimed to identify the risk factors of anxiety and depression, along with their prognostic impact in patients with gastric cancer.
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) checklist.10 We also adapted some of the study methods from Ikhile et al.11 and Riedl et al.12 for our review.
The inclusion criteria include: 1) published observational studies that evaluated risk factors for anxiety and depression in patients with gastric adenocarcinoma. The risk factors included sociodemographic factors and clinicopathologic characteristics of the cancer; 2) studies that evaluated the association between anxiety and depression with survival in gastric cancer; 3) studies in which anxiety and depression symptoms were evaluated using validated questionnaire (e.g. Hospital Anxiety and Depression Scale13 (HADS) or medical records with ICD codes; 4) studies with full-text available. To maximize the number of studies included, we also included studies that included cases with either depression or anxiety instead of both.
The exclusion criteria include: 1) studies that also included other types of cancer and did not have a separate analysis for gastric cancer; 2) randomized-controlled trials that evaluated the effect of a specific intervention (e.g., types of gastrectomy or prehabilitation) on patients’ QoL. We decided to exclude this type of study because it did not analyze other baseline risk factors for anxiety and depression. In addition, these types of studies usually use a general quality-of-life questionnaire with only a single question to evaluate for anxiety (e.g., EORTC QLQ-C3014); 3) studies that only investigated risk factors for suicidal ideation. Even though suicidal ideation can be a part of depressive symptoms, it can also be caused by other factors; 4) studies that evaluated the risk factors for psychological distress and not specifically for anxiety or depression; 5) studies that used individuals without gastric cancer as controls; 6) studies that analyzed factors that were more likely to be the consequences of depression and anxiety, rather than risk factors of depression and anxiety; 7) studies with no English language publications.
Two independent reviewers searched through relevant articles from the MEDLINE, EBSCOhost, and ProQuest databases. We searched articles published up to 7 April 2025. Table S1 showed the search terms for each database. We also searched for relevant articles from the reference lists of included studies. Filters included the following key words: English language and full text available.
Two reviewers independently extracted the relevant data from each article using a standardized table. The relevant data include: authors, study design, disease setting, number of patients, country where the study was conducted, period of patient recruitment, prevalence of anxiety and depression, risk factors for anxiety or depression, and the survival outcomes (e.g., overall survival [OS], cancer-specific survival, disease-free survival, and progression-free survival) in patients with anxiety or depression.
Two reviewers also independently assessed the quality of each study. Quality of studies is assessed using the Newcastle-Ottawa Scale (NOS).15 For a cross-sectional study, we used the modified NOS used in a study by Herzog et al.16
Given that each study evaluated different risk factors for anxiety or depression in gastric cancer, we only performed meta-analysis if: a) the adjusted odds ratio (for risk factor analysis) or hazard ratio (for survival analysis) could be obtained from the study, and b) the specific risk factor or survival outcome had been assessed in over three studies. Otherwise, the risk factor and survival outcome were only analyzed qualitatively. If follow-up data were available, only the risk factors associated with baseline anxiety or depression were included in the meta-analysis. We used the Review Manager (RevMan) 5.3 program (The Nordic Cochrane Center, Copenhagen, Denmark)17 to conduct the meta-analysis. We used random-effects analysis if there was significant heterogeneity between studies. Otherwise, fixed-effect analysis was used. If there were an adequate number of studies (≥ 10), we assessed publication bias using a funnel plot. Whenever possible, we also used the ProMeta 3 (Internovi, Cesena, Italy)18 program to perform Egger’s test.
Figure 1 shows the PRISMA study flow chart. Initially, 870 records were obtained from the database searches. After deduplication, 611 articles were obtained. Two independent reviewers performed screening of the titles and abstracts, resulting in 27 articles for a full-text review. After applying the exclusion criteria, 14 studies were included for the systematic review. The total number of patients was 31,660. All articles evaluated risk factors of anxiety or depression, while five articles analyzed the impact of anxiety or depression on patient survival. Table S2 showed the excluded studies and the reasons for their exclusions.
Table 1 shows the characteristics of studies included in this systematic review. Fourteen studies were included in the systematic review. Some studies only analyzed risk factors for anxiety or depression (n = 9), and the rest of the studies evaluated both the survival impact and risk factors for anxiety or depression in patients with gastric cancer (n = 5). Some studies only analyzed for depression in gastric cancer (n = 5), and the rest of the studies analyzed for both anxiety and depression in gastric cancer (n = 9).
| No | Study, country | Design | No. of patients | Disease setting | Psychiatric symptom assessed & definition | Survival outcome assessed & follow-up information | Prevalence of anxiety/depression | Risk factors assessed | Significant results | Survival outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Li J 2023, China20 | Cohort | 110 | Postsurgical gastric cancer patients |
|
|
|
|
|
|
| 2 | Liu P 2022, China7 | Cohort | 226 | Postsurgical gastric cancer patients |
|
|
|
|
|
|
| 3 | Maeda T 2006, Japan44 | Cross-sectional | 82 | Postsurgical gastric cancer patients (>3 yrs) |
| - | - |
|
| - |
| 4. | Palgi Y 2011, Israel45 | Cross-sectional | 123 | Posttreatment gastric cancer outpatient |
| - |
|
|
| - |
| 5. | Han KH 2013, South Korea43 | Cross-sectional | 391 | Gastric cancer survivor (at least 1 year) |
| - |
|
|
| - |
| 6. | Zhang JK 2016, China50 | Cohort | 830 | Gastric cancer survivor |
| - |
|
|
| - |
| 7. | Kang JI 2012, South Korea34 | Cohort | 93 | Newly diagnosed gastric cancer |
| - |
|
|
| - |
| 8. | Hu LY 2018, Taiwan6 | Retrospective cohort | 28753 | Newly diagnosed gastric cancer |
| - |
|
|
| - |
| 9. | Yu H 2012, China56 | Cohort | 300 | Newly diagnosed gastric cancer |
|
|
|
| - |
|
| 10. | Zhao H 2024, China49 | Cross-sectional | 239 | Gastric cancer patient |
| - | - |
|
| - |
| 11. | Xu L 2016, China37 | Cross-sectional | 53 | Preoperative gastric cancer |
| - |
|
|
| - |
| 12. | Han L 2020, China24 | Cohort | 200 | Surgical gastric cancer patients |
|
|
|
|
|
|
| 13. | Tao F 2023, China55 | Cohort | 178 | Advanced gastric cancer patient receiving systemic chemotherapy |
|
|
|
|
|
|
| 14. | Zhang L 2021, China35 | Cross-sectional | 82 | Recurrent gastric cancer |
| - |
|
|
| - |
Tables 2 and 3 showed the quality of cross-sectional and cohort studies, respectively, based on the NOS. Based on the NOS, all studies were considered to be of high quality. However, some studies had a limited sample size (<100 patients).
| No | Study | Representativeness of sample | Sample size | Non-respondents comparability & response rate | Ascertainment of the exposure | Comparability between subjects in different outcome groups | Assessment of the outcome | Statistical Test | Total |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Zhang L 202135 | * | - | * | ** | ** | * | * | 8 |
| 2 | Xu L 201637 | * | - | * | ** | ** | * | * | 8 |
| 3 | Han KH 201343 | * | * | * | ** | ** | * | * | 9 |
| 4 | Maeda T 200644 | * | - | - | ** | ** | * | * | 7 |
| 5 | Palgi Y 201145 | * | * | - | ** | ** | * | * | 8 |
| 6 | Zhao H 202449 | * | * | - | ** | * | * | * | 7 |
| No | Study | Representativeness of the exposed cohort | Selection of the non exposed cohort | Ascertainment of exposure | Demonstration that outcome is not present at start of study | Comparability of cohorts | Assessment of outcome | Follow-up long enough for outcomes to occur | Adequacy of follow-up of cohorts | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Hu LY 20186 | * | * | * | * | * | * | * | * | 8 |
| 2 | Han L 202024 | * | * | * | * | * | * | * | * | 8 |
| 3 | Li J 202320 | * | * | * | * | * | * | * | * | 8 |
| 4 | Liu P 20227 | * | * | * | * | * | * | * | * | 8 |
| 5 | Kang JI 201234 | * | * | * | * | ** | * | * | - | 8 |
| 6 | Tao F 202355 | * | * | * | * | ** | * | * | - | 8 |
| 7 | Yu H 201256 | * | * | * | * | ** | * | * | * | 9 |
| 8 | Zhang JK 201650 | * | * | * | * | - | * | * | * | 7 |
We divided the risk factors assessed in each study based on Engel’s biopsychosocial model.19 Based on the framework, we classified the risk factors into biological, cancer-specific, psychological, and social risk factors. Table S3 presents all of the risk factors for anxiety or depression assessed in each included study, along with the number of studies that found the particular risk factor to be significantly associated with anxiety or depression. If a particular study included multivariate analysis, then only risk factors that were found to be independently associated with anxiety or depression were considered to be statistically significant. Eleven studies included multivariate analysis in the results.
We also performed meta-analysis for risk factors that were analyzed in ≥ 3 studies and had a reported adjusted odds ratio value. Based on those criteria, we performed five meta-analyses (one for anxiety and four for depression). For the meta-analysis, we only used random-effect analysis owing to the low number of qualified studies. Figure 2 shows the forest plot for the association between female sex and anxiety in gastric cancer. The result showed that female sex was independently associated with increased odds of anxiety in gastric cancer (aOR = 2.91; 95% CI 1.91–4.44).
Figures 3–6 show the forest plot for the association between female sex, older age, diabetes mellitus, and higher cancer stage, respectively, with depression in gastric cancer. The result showed that female sex (aOR = 3.04; 95% CI 2.04–4.54) and diabetes mellitus (aOR = 4.78; 95% CI 2.46–9.28) were independently associated with higher odds of depression in gastric cancer. Meanwhile, older age (aOR = 1.01; 95% CI 0.46–2.20) and higher cancer stage (aOR = 1.86; 95% CI 0.99–3.49) were not independently associated with higher odds of depression in gastric cancer. Funnel plot and Egger’s test were not performed owing to the low number of studies in each forest plot.
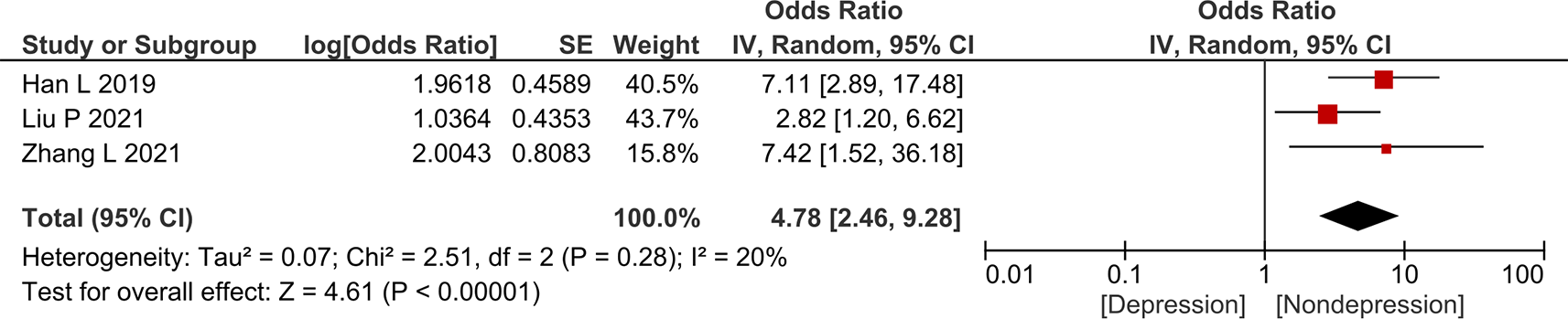
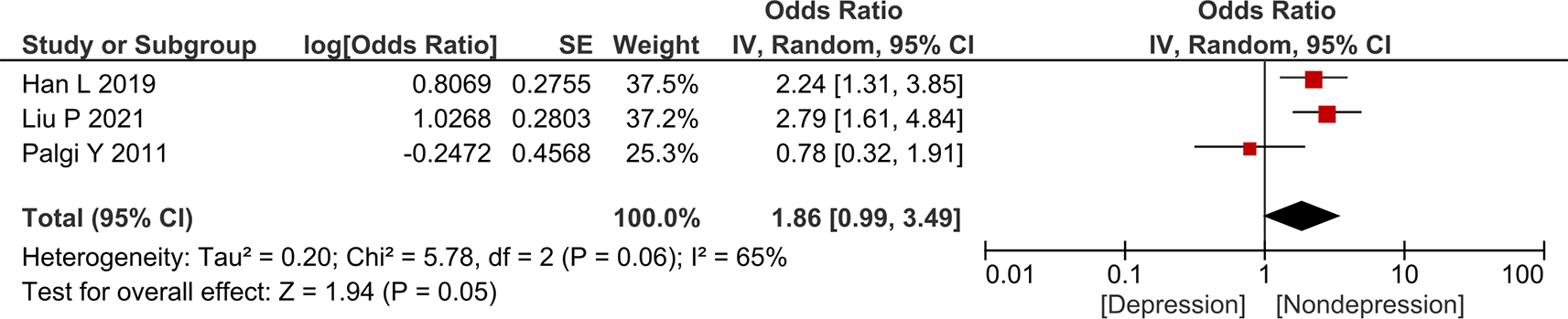
Figure 7 shows the biopsychosocial framework for risk factors that were found to be associated with anxiety or depression in gastric cancer. A risk factor was listed if ≥1 of the included studies showed a statistically significant association with anxiety or depression in gastric cancer.
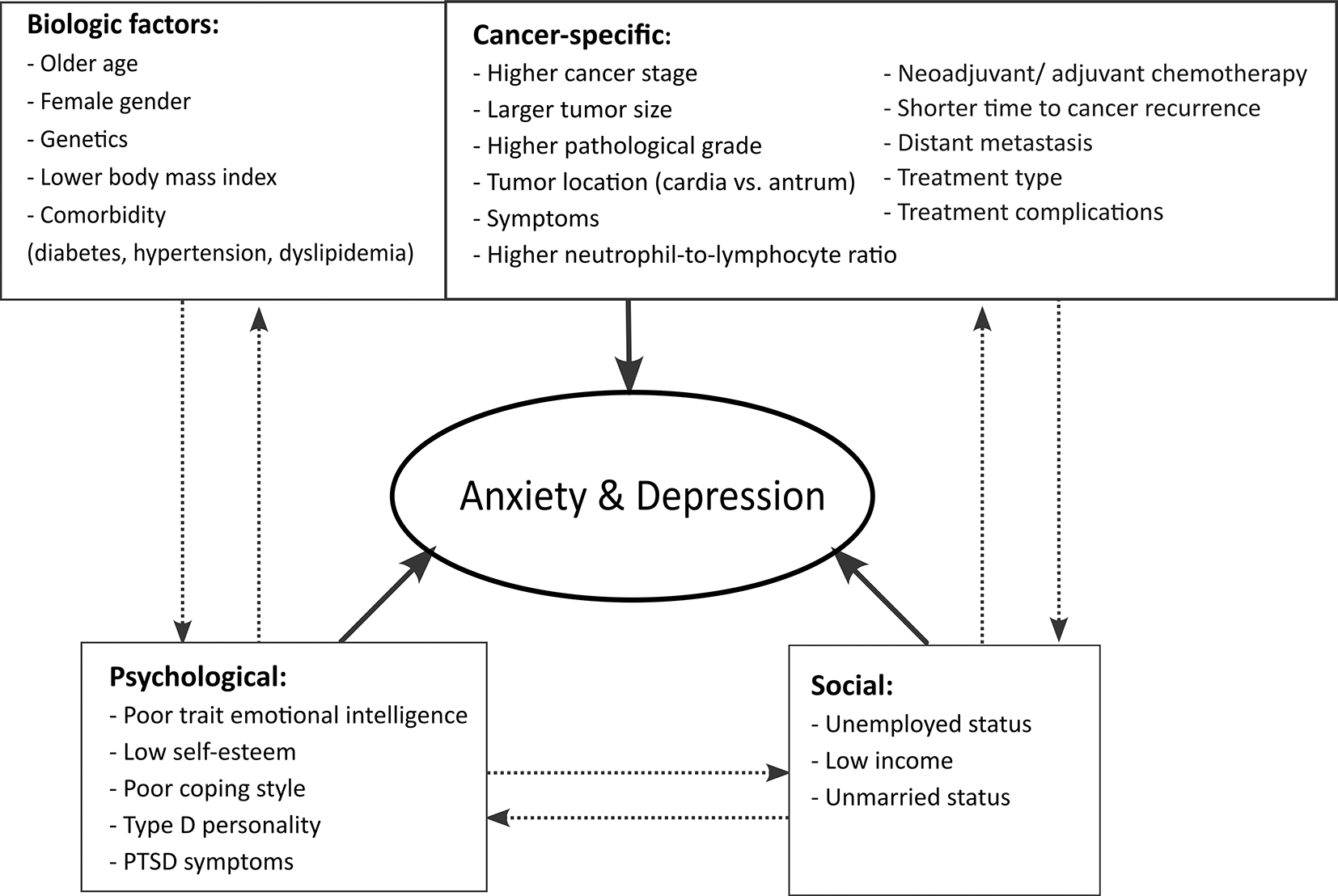
Risk factors were classified into biological, cancer-specific, psychological, and social factors (bold arrows). Listed below each category were the significant risk factors from our systematic review. The dashed arrows showed that each category was interconnected to each other.
Table 4 shows the risk factors of anxiety or depression based on the phases of care in patients with gastric cancer (diagnosis and treatment, survivorship, and cancer recurrence). The highest prevalence of anxiety or depression was found in the cancer recurrence phase, although it was only based on one study.
Table 1 shows the results of the survival analysis of the included study. Some studies evaluated the association between anxiety alone with survival (n = 3), depression alone with survival (n = 4), and both depression and anxiety with survival (n = 1). Some of the studies measured OS (n = 4), cancer-specific survival (CSS; n = 1), progression-free survival (PFS; n = 1), or disease-free survival (DFS; n = 2). Most studies found that anxiety or depression was associated with worse survival rates in patients with gastric cancer. Only one study found that anxiety or depression was not correlated with OS in gastric cancer.20 Meta-analysis was not performed owing to the lack of data.
The results of our systematic review revealed the various risk factors of anxiety and depression in gastric cancer, which we categorized based on Engel’s biopsychosocial model of disease.19 In 1977, Engel19 proposed the biopsychosocial model as a more holistic approach to view a disease. He argued that the biomedical model alone is not sufficient to explain and treat a disease, suggesting that psychosocial factors also play a significant role. This is also true for psychiatric diseases such as depression and anxiety. Although the biopsychosocial model has flaws, it is still useful as a conceptual framework.21 This framework is not only useful as a way to understand the development of a disease, but can also be used as a guide to treat the disease.
Below, we discussed each significant risk factor of anxiety and depression in gastric cancer based on Engel’s biopsychosocial model. For biological risk factors, we decided to separate the cancer-specific risk factors into their own category.
Older age: The relationship between age and risk of anxiety or depression in patients with cancer is complex. In general, younger age at diagnosis was associated with a higher risk of anxiety or depression in most cancers. This may be owing to a higher tendency to have intrusive thoughts and worry about their future. They may also have less social support and less skill to adapt to stressful events than older patients.22 However, our review found that older age at diagnosis may also be associated with a higher risk of anxiety or depression than younger patients with gastric cancer.7 This may be owing to a higher rate of treatment complications in geriatric patients, which might be related to frailty or immunosenescence.23 Older patients may also have a more difficult physical recovery process and adaptation than younger patients.24
Female sex: In the general population, female sex is associated with a higher prevalence of anxiety and depression than male sex. This may be related to sex differences in brain structure, hormonal fluctuation, environmental stress, cultural sex stereotypes, and coping styles.25 In patients with cancer, Parás-Bravo et al.26 reported that female oncology patients had a higher risk of anxiety symptoms than male patients. Meanwhile, a meta-analysis by Vitale et al.27 showed that female patients with cancer had a higher risk of depression than male patients. However, male patients had a higher risk of anxiety than female patients. They hypothesized that this may be because of the reluctance of male patients to express their emotions or request help from others. Our review showed that female patients with gastric cancer had a higher risk of anxiety or depression than male patients. Possible reasons include hormonal fluctuation20 and less social support than male patients.28 However, studies performed in different countries may have shown different trends owing to the different cultural contexts.
Low body mass index (BMI): Our review found that patients with lower BMI had a higher risk of anxiety or depression, which may be related to malnutrition. Lim et al.29 reported that patients with gastric cancer along with malnutrition (assessed by Subjective Global Assessment) had a higher rate of anxiety and a lower QoL than patients with normal nutritional status. Specific nutrients have been linked to anxiety and depression in gastric cancer. Vahid et al.30 showed that patients with gastric cancer having anxiety or depression had higher consumption of salt and sugar than those without anxiety or depression. Furthermore, higher consumptions of vitamin A, vitamin B6, beta-carotene, and black tea were associated with lower odds of depression in gastric cancer. They hypothesized that consumption of certain nutrients could affect the brain neurotransmitters, thus affecting the risk of anxiety or depression. For instance, vitamin B6 is needed for serotonin and norepinephrine in the brain. However, further studies are required to clarify the causal links.
Comorbidity: The presence of comorbidities can also increase the risk of anxiety or depression in gastric cancer owing to the increased health-related burden. Certain comorbidities may also directly increase the risk of anxiety or depression. Most of the studies included in our review did not state whether the comorbidities were controlled or not. Our meta-analysis found that the odds of anxiety or depression were four times higher in patients with gastric cancer having diabetes mellitus than those without diabetes mellitus. Diabetes mellitus itself can cause a condition called diabetes distress. Diabetes distress is defined as the emotional consequence of living with diabetes in the form of guilt, anxiety, and concerns about self-management. While diabetes distress is not the same as depression and is not considered to be a psychiatric disease, it can increase the risk of depression.31 Data from the Asia Cohort Consortium reported that diabetes was associated with increased risk of gastric cancer (HR 1.15; 95% CI 1.06–1.25). Diabetes also increased the mortality rate of patients with gastric cancer (HR 1.15, 95% CI 1.03–1.28). Possible explanations include hyperglycemia and insulin-like growth factor-1-stimulating oncogenic pathways such as AKT/mTOR, Ras/RAF/MEK/ERK, and JAK1/STAT3.32 Symptoms and complications from diabetes could also complicate the clinical manifestations and treatment complications of gastric cancer (e.g., gastroparesis and increased risk of infection from diabetes). One study in our review showed that hypertension was associated with increased risk of depression in gastric cancer, whereas the rest showed no significant association.6 The association of hypertension and depression can be complex. A study using data from the National Health and Nutrition Examination Survey showed a significant association between diastolic blood pressure and depression, especially in patients with cancer. Meanwhile, there was a U-shaped relationship between systolic blood pressure (SBP) and depression. SBP <129.7 mmHg showed a negative association between SBP and depression, while SBP above it showed no significant association.33
Genetics: Certain genes may influence stress response in patients with cancer, predisposing them to depression and anxiety. One example is the FK506 binding protein 5 (FKBP5) gene, which is a major regulatory protein for the hypothalamic-pituitary-adrenal (HPA) axis. The HPA axis plays a key role in regulating stress response. FKBP5 modulates the gene by binding to the glucocorticoid receptor and influencing its sensitivity. Kang et al.34 showed that certain single-nucleotide polymorphisms of the FKBP5 gene were associated with increased risk of depression and anxiety in gastric cancer.
Advanced cancer: More advanced gastric cancer (higher TNM stage or pathological grade) was associated with anxiety or depression. This may be owing to a higher symptom burden and stronger fear of the disease.24 Higher cancer stage and larger tumor size were also associated with a more intensive treatment regimen.
Tumor location: A tumor located in the cardia could also increase the risk of anxiety or depression than a tumor located in the body of the stomach.35 Cardiac gastric cancer, which is a more aggressive cancer phenotype than distal cancer,36 may lead to a higher rate of anxiety or depression.
High neutrophil-to-lymphocyte (NLR) ratio: Xu et al.37 showed that high NLR is independently associated with a higher rate of anxiety and depression. NLR is a nonspecific marker of inflammation. High neutrophil count has been associated with oncogenic effects (e.g., promoting angiogenesis, suppression of T-cells against cancer cells, facilitating metastasis), while lymphocytosis is associated with antitumor effects (e.g., recognition of antigens in cancer cells). A meta-analysis showed that high NLR is associated with worse OS in resected gastric cancer.38 Besides gastric cancer, NLR was correlated with depression and survival in lung cancer.39
Treatment complications: Complications from chemotherapy (neoadjuvant or adjuvant) and surgery are associated with anxiety or depression in gastric cancer. Gastrectomy can cause postsurgical complications such as anastomotic leak, dumping syndrome, gastric stasis, bile reflux gastritis, afferent and efferent loop syndromes, and recurrent ulcerations, which can manifest as epigastric pain, fullness, fatigue, nausea, and nocturnal awakening.40 Certain surgical approaches may be associated with a better QoL. Several studies showed QoL benefits in patients undergoing subtotal distal gastrectomy than total gastrectomy. Similarly, minimally invasive laparoscopic gastrectomy is generally preferred over open gastrectomy.41 However, achieving a proper oncologic margin should remain the priority in choosing the appropriate techniques.
Symptoms: Patients with gastric cancer often experience multiple symptoms, such as gastrointestinal symptoms (nausea and vomiting, bloating, abdominal pain, loss of appetite), fatigue, sleep disturbance, and weight loss.42 The frequency, severity, and control of those symptoms contribute greatly to the patient’s QoL. This holds true in gastric cancer survivors, where lingering symptoms such as fatigue, insomnia, dyspnea, body image change, and digestive symptoms (postsurgical weight loss, loss of appetite, postprandial symptoms) are associated with an increased risk of depression.43
Low self-esteem: Maeda et al.44 showed that low self-esteem was independently correlated with depression in postgastrectomy patients. Low self-esteem was influenced by unmarried status, low level of emotional support, and high interpersonal dependency. This suggested that interventions to enhance patients’ self-esteem may be beneficial (e.g., educating patients with self-care guidance after gastrectomy to increase self-reliance and involvement of caregivers for emotional support).
Posttraumatic stress disorder (PTSD): Depression in gastric cancer could be associated with other psychiatric comorbidities, such as PTSD. Cancer diagnosis is a highly stressful life event; thus, PTSD is often observed in patients with cancer. Palgi et al.45 reported a 15-fold increase in the risk of depression in patients with gastric cancer owing to PTSD.
Coping style: A certain coping style (defined as a person’s cognitive and behavioral response to manage internal and external demands from a stressful situation46) is also associated with increased risk of depression or anxiety in gastric cancer. For instance, “anxious preoccupation” and “helplessness-hopelessness” coping styles (as assessed by mini-Mental Adjustment to Cancer/mini-MAC scale)47 were independently associated with higher HADS-A and HADS-D scores.34 Resigned coping style (assessed by Medical Coping Modes Questionnaire48) was also associated with a higher risk of anxiety or depression in gastric cancer.37
Low trait emotional intelligence: A low-trait emotional intelligence (a person’s ability to realize and manage their own and others’ emotions) can also affect a patient’s quality of life by increasing the risk of anxiety or depression. In contrast, a high-trait emotional intelligence is related to a better ability to self-manage negative emotions, better coping skills, and a strong social support network, leading to a lower risk of anxiety or depression.49
Type D personality: Type D personality (characterized by a high tendency for negative affectivity and social inhibition) was also associated with an increased risk of anxiety or depression in gastric cancer. A patient with gastric cancer with a type D personality may be more reluctant to seek healthcare, leading to delayed presentation to the hospital. Zhang et al.50 showed that patients with gastric cancer with type D personality also exhibited a deterioration in QoL on follow-up, as well as worse three-year OS than patients with non-type-D personality.
Socioeconomic factor: Social determinants of health are key factors in the development of anxiety and depression. The most important is socioeconomic disadvantage, which consists of various dimensions, including education level, employment status, financial condition, and standard of living.51 Cancer patients often experience severe financial burden (financial toxicity) during their care. A meta-analysis by Kitaw et al.52 reported that the prevalence of catastrophic health expenditure (defined as out-of-pocket expense that significantly exceeds a person’s financial capability) in cancer patients was 56.1%. The risk factors of catastrophic health expenditure were unemployment, large family size, low income, lack of health insurance, longer duration of cancer, older age, lower education level, and a larger number of treatments. The relationship between socioeconomic factors and mental health can be bidirectional. For instance, patients with anxiety and depression are more likely to experience challenges in their occupational work, thereby leading to lower income and unemployment.
Walker et al.53 also showed that the rates of depression and anxiety in patients with cancer may be higher if they are from low and lower-middle-income countries than in upper-income countries. This may be related to a lower education level and a more advanced stage of cancer at diagnosis.53 In our review, we also found that lower monthly income was associated with an increased risk of depression in gastric cancer.43 Unemployment was also associated with higher risks of anxiety and depression in gastric cancer.7
Unmarried status: Liu et al. showed that unmarried status was associated with a higher rate of anxiety and depression at follow-up visits.7 The potential reason may be lower social support in unmarried patients. Social support is defined as the support that includes the resources and relationships required to meet an individual’s needs through emotional support, instrumental support, appraisal, and informational assistance. Social support level has been linked to psychological disorder, quality of life, and survival in patients with cancer.54 Family members can fulfill the emotional needs of patients, and therefore, unmarried patients may have less social support than married patients.
Our review also found that there may be different risk factors of anxiety or depression in different phases of care. Broadly, we divided the phases of care into “diagnosis and treatment,” “post-treatment & survivorship,” and “recurrence.”
Gastric cancer recurrence is a unique setting that is associated with a high rate of anxiety or depression. Zhang et al.35 reported that the prevalence of anxiety and depression was higher in patients with recurrent gastric cancer than in those with newly diagnosed gastric cancer or in healthy controls. Anxiety or depression in those with recurrent gastric cancer also had unique associated risk factors. Patients with a shorter time to recurrence had a higher prevalence of anxiety or depression, probably because it may be more difficult for patients to accept, and they may lose confidence in the medical treatment.
Most studies in our review reported that cases with anxiety or depression exhibited a worse OS than those without anxiety or depression.24,55,56 Anxiety and depression were also associated with poor PFS and DFS in gastric cancer.7,24,55 This finding is also true after adjusting for cancer stage. There are several possible explanations. Patients with anxiety or depression may be less compliant with therapy. They may also have higher symptom burden, such as fatigue, insomnia, and pain, which could be associated with a difficult recovery process.24
The relationship between depression and gastric cancer can be bidirectional. Gastric cancer is a stressful event that predisposes patients to anxiety and depression. However, depression itself may be a risk factor for the progression of gastric cancer. A meta-analysis of 24 studies showed that the attributable risk percentage and population attributable risk percentage of depression with gastric cancer were 45.65% and 5.86% respectively.57 Depression can increase the activation of the HPA axis, which produces catecholamines. The catecholamines bind to β-2 adrenergic receptors and upregulate MACC1 expression. MACC1 controls c-MET transcription, which can enhance epithelial-to-mesenchymal transition and promote tumor invasion and metastasis.9 Depression in gastric cancer also causes oxidative stress by increasing the levels of reactive oxygen species (ROS) and decreasing the levels of antioxidants. ROS can increase the activity and expression of proto-oncogenes, such as ABL1.58 These proto-oncogenes can promote the progression of gastric cancer through activation of their downstream pathways, which can affect patient survival.
This review had a few limitations. First, the number of studies included in this review was small. Second, the wide range of reported prevalence of depression and anxiety in the included studies may be because of the different tools used to define anxiety and depression. Most studies used a self-reported questionnaire to detect anxiety and depression as opposed to a structured interview by an expert clinician. Most of the studies used HADS-D as a screening tool for depression. However, European Society for Medical Oncology (ESMO)4 and American Society of Clinical Oncology (ASCO)59 recommend the use of Patient Health Questionnaire-9 or Beck Depression Inventory-II instead because the cut-off score for HADS-D may need to be increased in patients with cancer. One of the items in HADS-D is anhedonia, which may not necessarily be a pathological symptom in patients with advanced cancer. For anxiety, the ESMO4 guideline recommended the use of HADS-A or GAD-7. Third, the included studies were not PROSPERO- registered. Fourth, most studies were conducted in China, thereby limiting the generalizability of the findings to other regions or populations with different healthcare systems, cultural contexts, or demographic characteristics. Fifth, only a limited number of meta-analyses could be performed. Sixth, substantial heterogeneity was observed across the included studies. The sources of heterogeneity came from the different treatment setting, types of measurement tools for anxiety and depression, the number of risk factors analyzed, and the presence of multivariate analysis. In response, we had classified those risk factors based on each phase of care and performed random effect meta-analysis for eligible risk factors.
Despite these limitations, our systematic review comprehensively discussed the risk factors of anxiety and depression in patients with gastric cancer. To our knowledge, this is the first systematic review investigating the risk factors and prognostic impacts of anxiety and depression in gastric cancer specifically. We also provided categorizations of those risk factors based on Engel’s biopsychosocial model and phases of cancer care. These frameworks are useful for risk stratification and can be used as a guide for the treatment of anxiety and depression in gastric cancer. For example, based on the significant risk factors of anxiety and depression in our review, screening for anxiety and depression in gastric cancer should be highly considered for female patients, older patients, patients with lower socioeconomic levels, those with multiple comorbidities, and those with advanced cancer. Certain treatment types (subtotal gastrectomy vs. total gastrectomy, minimally invasive laparoscopic gastrectomy vs. open gastrectomy) are preferred whenever possible to increase patients’ postsurgical quality of life. Treatment of anxiety and depression in patients with cancer involves psychotherapy and medications.4,59 Our review has shown that various psychological risk factors could significantly increase the risk of anxiety and depression in gastric cancer. A recent network meta-analysis showed that psychotherapy interventions such as cognitive behavioral therapy, reminiscence therapy, and narrative nursing had substantial positive effects in decreasing anxiety and depressive symptoms in patients with gastrointestinal cancers.60
Our systematic review identified various risk factors for anxiety and depression in gastric cancer, which we categorized into biological, cancer-specific, psychological, and social risk factors. We also classified those risk factors based on each phase of patient care in gastric cancer. These findings could aid clinicians in risk-stratifying patients with gastric cancer based on their risk of anxiety and depression. In addition, clinicians could create a holistic framework for managing anxiety and depression in these patients. Further research is needed to investigate other risk factors for anxiety and depression in gastric cancer. Randomized controlled trials are needed to evaluate a multimodal treatment approach to anxiety and depression in gastric cancer.
Figshare: Supplementary Tables and Figures. http://dx.doi.org/10.6084/m9.figshare.30220882.61
Data is available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Figshare: PRISMA checklist and flowchart for ‘Risk Factors and Prognostic Impact of Depression and Anxiety in Gastric Cancer: A Systematic Review’. http://dx.doi.org/10.6084/m9.figshare.30220348.62
Data is available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)