Keywords
Twin pregnancies, acardiac fetus, ultrasound, monitoring, maternal and fetal prognosis.
The acardiac fetus is a rare malformation specific to multiple monozygotic pregnancies. Two main theories have been proposed regarding the etiology of this malformation: the vascular theory, and the second supposes a defect in the embryogenesis of the heart. Diagnosis is typically suspected and confirmed based on ultrasound findings.
We present the case of a 36-year-old woman, G3P4, with a bi-scarred uterus. The last pregnancy was a monochorionic monoamniotic twin pregnancy complicated by an acardiac fetus suspected in the first trimester and confirmed by ultrasound at 23 weeks of gestation. She gave birth by an emergency cesarean section at 34 weeks + 4 days to two babies: a normal female baby and an acardiac fetus.
Acardiac twin pregnancies present challenges and require careful management to optimize outcomes for both the acardiac and the healthy twins. Understanding the mechanisms underlying this condition is crucial to provide effective prenatal care and support.
Twin pregnancies, acardiac fetus, ultrasound, monitoring, maternal and fetal prognosis.
An acardiac fetus is a rare malformation specific to multiple monozygotic pregnancies. This involves a fetus deprived of a heart that develops under complete vascular dependence on a healthy second twin.
Its frequency is estimated to be 1/35,000 births, or 1% of monozygotic twin pregnancies(1,2). Its course is constantly lethal to the affected fetus.1,2
Currently, the contribution of Doppler ultrasound to antenatal diagnosis and monitoring of this pathology is essential. Different therapeutic methods have been proposed to preserve the prognosis of the healthy fetus.3,4
Herein, we report a case of an acardiac fetus diagnosed in the obstetrics and gynecology department at Ibn Jazzer University Hospital.
This is the case of a 36-year-old female patient with B-positive blood type, without medical history, Gravida 3, Para 4. Her first pregnancy was carried to term without incident with delivery by cesarean section of a female baby with a birth weight of 3000 g. The second pregnancy was also carried to term with delivery by cesarean section of a polymalformed male baby, complicated with neonatal death.
The current pregnancy was well-monitored, the serologies for toxoplasmosis, hepatitis, and syphilis were negative, and the serology for rubella showed a state of long-standing immunity.
The first ultrasound was performed at 11 weeks by her gynecologist and showed a monochorionic monoamniotic twin pregnancy. The first twin had a normal morphology and cardiac activity, while the second twin had no cardiac activity ( Figure 1)
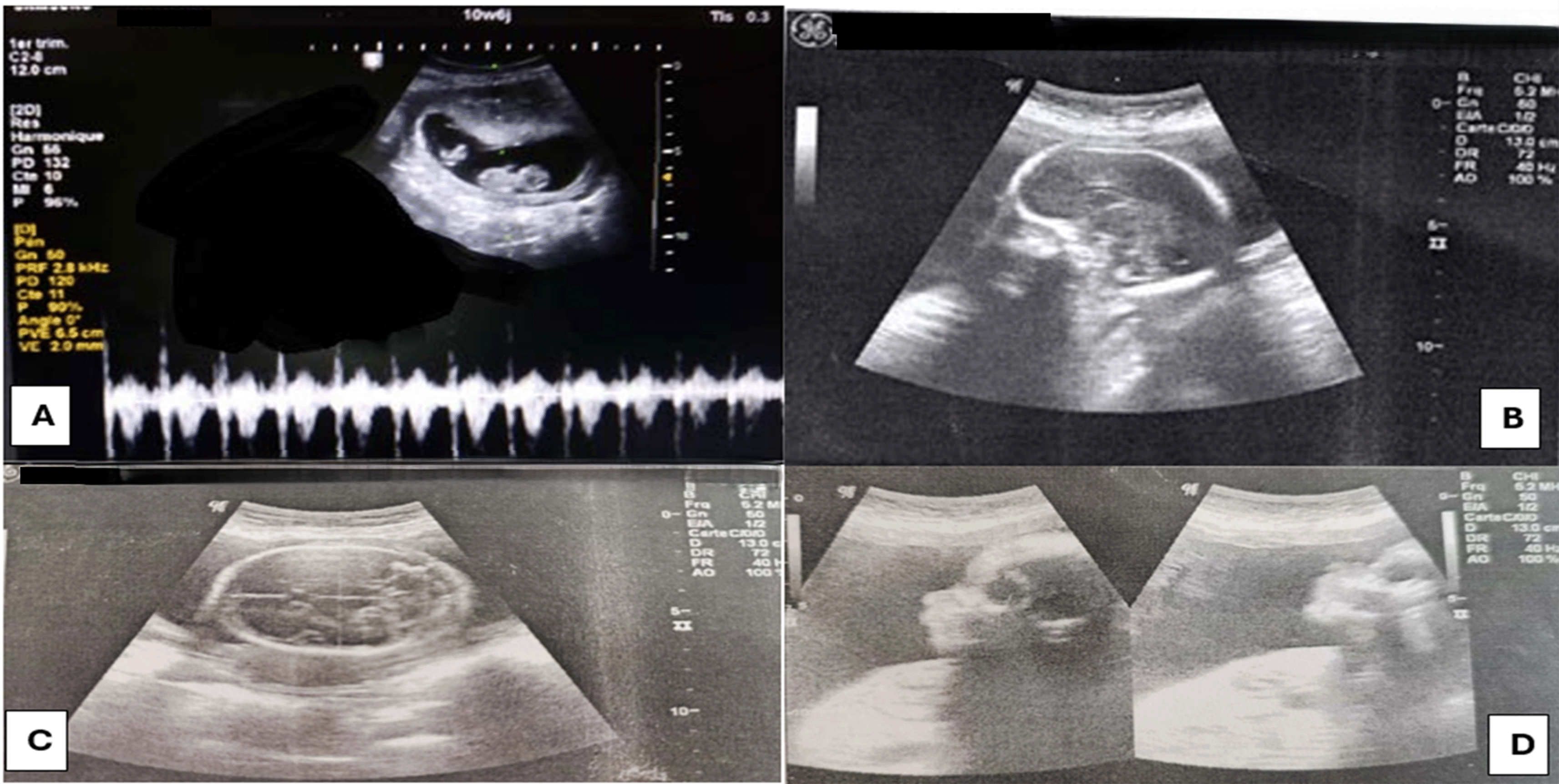
At 23 weeks an ultrasound check was carried out and showed:
➢ For the First twin: A healthy female fetus with measurements coherent with the presumed age of pregnancy without stunted growth. There were no visible malformations at this age ( Figure 1).
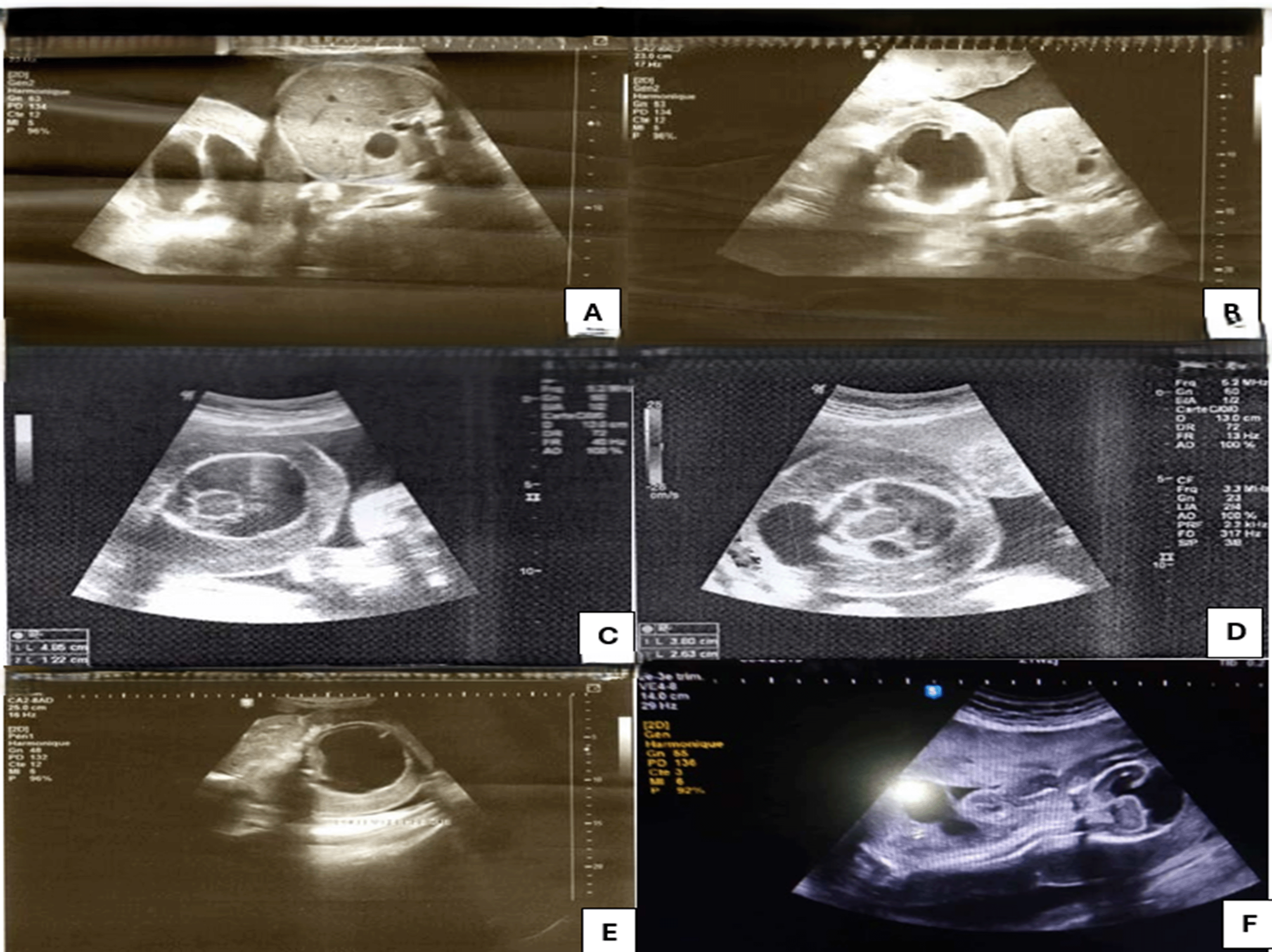
For the second twin: The acardiac monster shows: ( Figure 2)
• Alobar holoprosencephaly with thalamic fusion and a very large single ventricle without visible hemispherical parenchyma.
• Very large occipital meningocele.
• Absence of the upper limbs from the shoulder girdle.
• The lower limbs are present in their three segments.
• An important and diffuse thickening of the subcutaneous tissue.
• Very large cord cyst at the anterior wall of the trunk.
• The flow of the umbilical arteries appears reversed.
• The quantity of amniotic fluid is normal.
The patient was referred for follow-up and management after 24 weeks.
A weekly ultrasound check was carried out, showing: a favorable evolution of the healthy twin; on the other hand, for the acardiac twin, an increase in volume with obvious polyhydramnios was observed.
A diabetes screening was done, returned pathological.
The etiological investigation was completed with EBV and CMV serology, and control of toxoplasmosis serology with a negative result.
The evolution was marked by the appearance of uterine distension (uterine height = 50 cm) with uterine contractions (the cervical length = 2.6 cm) Hence tocolytic treatment was initiated as well as fetal lung maturation at 30 weeks.
At 34 weeks + 4 days, the patient presented with significant respiratory difficulty; hence, a cesarean section was decided in collaboration with the neonatologists and gave birth to two babies:
- The first was female, weight = 2150 g, size = 44 cm, cranial perimeter = 31 cm. Her Apgar scores at the first and fifth minutes were 9 and 10, respectively.
- The second twin was a female acardiac fetus weighting = 3700 and with a height of 30 cm. The fetus has two lower limbs, but no upper limbs ( Figure 3).
Acardiac fetuses were first cited in 1533 by Benedetti and were described in the 19th century by Geoffroy-Saint-Hilaire.5
In 1977, James estimated the incidence of acardiac fetuses at 1 in 35,000 births and 1% of monozygotic pregnancies.6
A literature review of 340 cases carried out by James revealed that the complication more frequently affects monozygotic monoamniotic pregnancies than diamniotic multiple pregnancies.6
The acardiac fetus results from an extreme form of twin-to-twin transfusion syndrome, described under the term “twin-reversed-arterial-perfusion-sequence”: TRAP sequence.3
This fetus, also called the “container twin”, is perfused in a retrograde manner by a viable twin called the “pump twin”. He receives blood from the normal fetus.7
The acardiac fetus is never viable, with a mortality rate of 100% intrauterine survival is only possible through the coexistence of a twin, which ensures its circulation and needs.8,9
This pathology has been described for monozygotic, monochorionic multiple pregnancies.6,10
Two main theories have been proposed regarding the etiology of this malformation.8
• Vascular theory: The primum movens of this pathology is the presence of arterio-arterial and veno-venous anastomoses in the placenta, with a reverse circulatory flow in the acardiac fetus responsible for dysmorphogenesis.8
Campebell and Shepherd (1905) evoked the notion of a strong fetus whose circulation would become dominant, causing a reversal of the circulatory current in the umbilical vessels of the other fetus.9
• The second suggests a defect in the embryogenesis of the heart. According to some authors, the initial anomaly of the acardiac fetus is the failure of development of the heart and not its degeneration secondary to placental anastomoses.1,8
The first antenatal diagnosis of the acardiac fetus by ultrasound was reported, in the literature, in 1978.11
The age of pregnancy at the time of diagnosis varies from 12 to 34 weeks; on average, the diagnosis is made at 23 weeks, the date of morphological ultrasound.12,13
For our case, the ultrasound diagnosis was made at 23 weeks.
In the first trimester, according to Baron, diagnosis can be suspected in the presence of the following:
• A monochorionic twin pregnancy.
• Single cardiac activity.
• A biometric discordance between the two twins: This is a constant and early sign visible from 9 weeks. This can be explained by the predominant morphological abnormalities at the cephalic pole, which is reduced or non-existent, and/or by the embryonic growth defect linked to the countercurrent perfusion of the acardiac twin.
• Diffuse subcutaneous edema for one of the fetuses, equivalent to anasarca.
• Morphological abnormalities in one of the two twins.
• The identification of early cardiac activity then its disappearance in one of the two fetuses.9,14,15
For Abboud, ultrasound and Doppler can guide diagnosis by showing the absence of cardiac activity, poor definition of the head and trunk, hypertrophy of soft tissues, and the existence of blood flow through the umbilical cord.
Ultrasound control 15 days after the first detection is of great diagnostic and prognostic value. It could specify the growth rate of the malformed fetus; if its dimensions have increased, the diagnosis will be strongly suspected.
In the second trimester, 30 of 35 acardiac fetuses were discovered in the second trimester. This was the case in our observation.3
Borrell proposed diagnostic criteria in case of the absence of cardiac activity:
• Limb movements.
• Progressive growth of the fetus (length of the femur, craniocaudal distance).
• Cystic hygromas.
• A single umbilical artery.
• Reverse circulation in the umbilical vessels on Doppler.
• Polyhydramnios, if the kidneys are present (50% of cases).
• An acardiac fetus is not very echogenic if the edema is significant and is more echogenic otherwise.
• Cardiomegaly, polyhydramnios and anasarca of the fetus pump with pleural and pericardial effusions can point towards the installation of complications.8
According to Monteaguado, the diagnosis will be made when an amorphous mass is visualized without proper movements, including cystic formations and major malformations of the spine and extremities.13
As for MRI’s contribution to diagnosis, an MRI was performed in 2005 by Hata for an acardiac fetus at 24 weeks whose Doppler ultrasound did not show umbilical blood flow. This made it possible to prove the existence of this flow. It shows better sensitivity to detect even minimal flow, which exceeds the capabilities of ultrasound detection.16
Our patient did not benefit from an MRI.
After delivery, examination of the acardiac fetus is mandatory and all anomalies must be noted. It is also advisable to perform karyotyping (on the blood or skin). A study published in 2003 on 18 cases of autopsies of acardiac fetuses showed that the heart is often rudimentary or absent; malformations are often encountered in the upper limbs and cephalic extremity.17
In our observation, the couple refused the fetopathological examination.
For the prognosis, the evolution is constantly lethal for the affected fetus.4
Healthy fetuses are exposed to a high risk of in utero and perinatal fetal death, heart failure, and complicated congenital malformations secondary to in utero fetal death of an acardiac fetus.9,18
Furthermore, obstetric complications such as polyhydramnios and the threat of premature delivery can punctuate the progression. Therefore, rigorous clinical and paraclinical monitoring must be established in order to detect these complications in a timely manner. During the second trimester, before viability, clinical examination and monthly ultrasound monitoring are necessary. Special attention should be paid to identifying signs of threatened late abortion, hypertension, and polyhydramnios. Ultrasound makes it possible to monitor the growth of the healthy twin and acardiac fetus and to screen for signs of heart failure.19
During the third trimester, clinical monitoring was maintained every two weeks. Fetal heart monitoring can be started several times a week from the 28th week. Ultrasound monitoring should be reinforced to look for signs of heart failure. The aim of managing twin pregnancies with an acardiac fetus is to protect the healthy fetus from complications.19
There are two therapeutic modalities:
Conservative attitude: Based on medical treatment: treatment of polyhydramnios, risk of premature birth, and heart failure. This attitude was selected in our case.20,21
An interventionist attitude consists of interrupting the vascularization between the two twins and causing the death of the acardiac fetus in utero.20,21
In summary, despite progress in diagnostic and therapeutic techniques, an acardiac fetus remains problematic. Ultrasonography prevails as the diagnostic technique of choice.
During pregnancy monitoring, ultrasound can detect the onset of complications affecting the fetus pump.
There is no consensus on therapeutic management. Therefore, a perfect collaboration between obstetricians and neonatologists would improve the prognosis of such pregnancies.
Written informed consent was obtained from the patient for the publication of this case report, including all clinical data and associated images. The patient was informed that all efforts would be made to ensure anonymity but that complete anonymity could not be guaranteed. The patient understood and agreed that the case report could be published in an open access journal.
All data underlying the findings of this case report are publicly available in the Zenodo repository under the Creative Commons Zero license (CC0 1.0), permitting unrestricted use and redistribution. The dataset, including the CARE checklist and patient consent forms in French, English, and Arabic, can be accessed at: https://doi.org/10.5281/zenodo.17634305.22
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)