Keywords
Hepatitis C Virus, Arteriovenous Fistula, Hemostatic Complications, Hemodialysis, End-Stage Renal Disease, Coagulopathy, Vascular Access, Direct-Acting Antivirals
This article is included in the Fallujah Multidisciplinary Science and Innovation gateway.
Hepatitis C virus (HCV) infection demonstrates significantly elevated prevalence among hemodialysis populations worldwide, with particularly high rates in Middle Eastern countries. Despite this epidemiological pattern, limited evidence exists regarding the impact of HCV infection on hemostatic complications following arteriovenous fistula (AVF) creation in patients with end-stage renal disease (ESRD).
This prospective cohort study enrolled 460 patients diagnosed with ESRD requiring AVF creation at three tertiary hospitals in Baghdad, Iraq (Al-Yarmouk Teaching Hospital, Ibn-Al-Nafees Teaching Hospital, and Al-Shaheed Ghazi Al-Hariri Surgical Hospital), between January 2017 and January 2024. Patients were stratified by HCV serological status using third-generation enzyme-linked immunosorbent assay. The primary outcome was hemostatic complications (bleeding requiring intervention or clinically significant hematoma) monitored for six weeks postoperatively. Secondary outcomes included coagulation parameters, AVF maturation rates, and other vascular complications. Multivariate logistic regression identified independent predictors of complications.
Among 460 patients, 151 (32.8%) tested positive for HCV. HCV-positive patients demonstrated significantly prolonged prothrombin time (19±5 versus 15±2.5 seconds, p<0.001), activated partial thromboplastin time (40±4 versus 32±3 seconds, p<0.001), and reduced platelet count (153±43 versus 215±72 ×103/mm3, p<0.001). Hemostatic complications occurred in 28.5% of HCV-positive patients compared to 5.5% of HCV-negative patients (crude OR: 6.835, 95% CI: 3.597-12.987, p<0.001). In multivariate analysis, HCV infection emerged as the strongest independent predictor of hemostatic complications (adjusted OR: 5.892, 95% CI: 3.102-11.196, p<0.001). AVF maturation rates were significantly lower in HCV-positive patients (74.2% versus 86.4%, p=0.002).
Hepatitis C virus infection increases hemostatic complications following AVF creation nearly six-fold and adversely affects fistula maturation rates. These findings emphasize the importance of incorporating HCV status into preoperative risk stratification and perioperative management protocols, particularly in high-prevalence regions.
Hepatitis C Virus, Arteriovenous Fistula, Hemostatic Complications, Hemodialysis, End-Stage Renal Disease, Coagulopathy, Vascular Access, Direct-Acting Antivirals
The global incidence of end-stage renal disease (ESRD) continues to rise, representing a significant public health challenge. Hemodialysis remains the predominant form of renal replacement therapy.1 The arteriovenous fistula (AVF) represents the gold standard for vascular access, demonstrating superior long-term patency rates and fewer infectious complications compared to alternatives.2 However, AVF success depends on numerous patient-specific factors, including advanced age, diabetes mellitus, cardiovascular disease, and poor vessel quality.2,3 Among these, hepatitis C virus (HCV) infection has emerged as a significant yet underrecognized factor influencing surgical outcomes.4
Hepatitis C virus is a single-stranded RNA virus that primarily targets hepatocytes, causing chronic inflammation and liver damage. HCV demonstrates significant genetic heterogeneity with seven major genotypes and a high mutation rate that contributes to viral persistence.5 Approximately 71 million individuals worldwide have chronic HCV infection, with disproportionately higher rates among hemodialysis populations.6 Globally, 8-10% of hemodialysis patients harbor HCV infection, with substantial geographical variation.6–8 The Middle East shows particularly elevated rates of 25-30%, with Iraq, Egypt, and Syria reporting prevalence exceeding 30-45% among dialysis patients8–10. This burden reflects nosocomial transmission, historical blood transfusions, shared dialysis equipment, and immunocompromised states in ESRD populations.8–10
The COVID-19 pandemic has strained dialysis healthcare systems and infection control protocols, potentially increasing nosocomial HCV transmission in resource-limited settings.11–14 Resource reallocation, reduced staffing, and challenges maintaining infection control may have exacerbated HCV transmission in dialysis facilities, particularly in high-prevalence regions.
HCV-related coagulopathy involves complex mechanisms. Chronic hepatic inflammation and fibrosis impair synthesis of vitamin K-dependent clotting factors, prolonging prothrombin and partial thromboplastin times.15 Thrombocytopenia arises through splenic sequestration, decreased thrombopoietin production, and potential viral suppression of megakaryopoiesis.16 HCV-associated cryoglobulinemia can induce vasculitis and endothelial dysfunction.17 The coexistence of uremia-associated platelet dysfunction in ESRD patients compounds these abnormalities, creating high risk for bleeding complications following vascular surgery.
Previous studies have established associations between HCV infection and coagulopathy.15,17 Northup et al. documented increased bleeding risk in HCV-infected patients undergoing invasive procedures,18 while Lisman and Porte demonstrated impaired synthesis of procoagulant and anticoagulant factors in chronic HCV-related liver disease.15 Zanetto et al. reported hypercoagulable profiles paradoxically coexisting with bleeding tendencies in cirrhotic patients.19 However, these investigations focused on general surgical populations or advanced cirrhosis, with limited evaluation of ESRD patients undergoing AVF surgery—a critical gap given ESRD patients’ unique hemostatic challenges.
In Iraq, HCV prevalence among hemodialysis patients ranges between 40% and 62%, among the highest rates globally.20 However, regional data on HCV’s impact on vascular access outcomes remain limited. Understanding this relationship in high-prevalence populations could inform risk stratification, perioperative management, AVF timing relative to HCV treatment, and infection control policies.
This study prospectively examined the association between HCV infection and hemostatic complications after AVF creation in Iraqi ESRD patients. Secondary objectives included comparing coagulation parameters between HCV-positive and HCV-negative patients, identifying predictors of complications, evaluating HCV’s impact on AVF maturation, and documenting vascular access complications by HCV status.
This prospective observational cohort study assessed hemostatic outcomes following AVF creation in ESRD patients, stratified by HCV status. The investigation was conducted at three tertiary referral hospitals in Baghdad, Iraq: Al-Yarmouk Teaching Hospital, Ibn-Al-Nafees Teaching Hospital, and Al-Shaheed Ghazi Al-Hariri Surgical Hospital, serving over eight million individuals. The study period extended from January 4, 2017, to January 31, 2024.
Inclusion criteria
Patients were eligible if they met the following criteria: (1) confirmed ESRD diagnosis (eGFR <15 mL/min/1.73 m2 for ≥3 months) by two nephrologists; (2) age ≥14 years; (3) clinical indication for AVF creation; (4) capacity for informed consent; and (5) availability for six-week follow-up.
Exclusion criteria
Patients were excluded if they had: (1) previous AVF creation with postoperative complications; (2) inherited or acquired bleeding disorders unrelated to uremia or HCV; (3) therapeutic anticoagulation beyond dialysis-related heparin; (4) thrombocytopenia (platelets <50,000/mm3) from non-HCV/ESRD causes; (5) active malignancy requiring systemic treatment; (6) pregnancy or breastfeeding; or (7) cognitive impairment precluding consent.
Sample size calculation used a two-proportion comparison formula based on anticipated hemostatic complication rates of 5% in HCV-negative and 15% in HCV-positive patients. Using α = 0.05 and 80% power, the minimum required sample was 420 patients, increased to 460 to accommodate an anticipated 10% dropout rate.
HCV serological status was determined using third-generation ELISA (Catalog number: ab108685, Abcam, Cambridge, United Kingdom) detecting anti-HCV IgG antibodies. All patients underwent preoperative evaluation including medical history, physical examination, and vascular mapping. Color Doppler ultrasonography assessed bilateral upper extremity arterial and venous anatomy, vessel diameter, flow, and patency. Baseline laboratory investigations included complete blood count, metabolic panel, coagulation profile (PT, aPTT, INR, fibrinogen), hepatic function tests (AST, ALT, alkaline phosphatase, bilirubin, albumin), and renal parameters.
All AVF procedures were performed by experienced vascular surgeons with over five years of training and more than 100 annual procedures. The primary approach was radiocephalic end-to-side anastomosis at the wrist (Brescia-Cimino fistula) when vessels were adequate. When distal vessels were inadequate (artery <2.0 mm or vein <2.5 mm), alternatives included brachiocephalic fistula or brachiobasilic transposition. All anastomoses used loupe magnification (3.5×) with 6-0 or 7-0 polypropylene continuous suture. Surgical duration, anastomotic size, and intraoperative complications were documented.
Primary outcome
The primary outcome was hemostatic complications within six weeks postoperatively, defined as: (1) clinically significant bleeding requiring intervention beyond standard pressure dressing (prolonged compression >30 minutes, additional suturing, surgical re-exploration, or transfusion); or (2) clinically significant hematoma causing pain, limiting range of motion, compromising fistula function, or requiring evacuation.
Secondary outcomes
Secondary outcomes included: (1) successful AVF maturation at six weeks (palpable thrill, audible bruit, venous diameter ≥4 mm, flow ≥500 mL/min by Doppler); (2) additional vascular complications (thrombosis, aneurysm/pseudoaneurysm, steal syndrome, infection, venous hypertension); and (3) evolution of coagulation parameters during follow-up.
Patients received systematic postoperative monitoring: hourly assessment for 24 hours, daily evaluation for seven days, and twice-weekly follow-up through six weeks. Each assessment included clinical examination, complication documentation, and Doppler ultrasound evaluation of fistula patency, flow, and maturation.
Statistical analyses used IBM SPSS Statistics version 24.0. Continuous variables were expressed as mean ± SD or median (IQR) and compared using Student’s t-test or Mann-Whitney U test. Categorical variables were presented as frequencies/percentages and compared using Chi-square or Fisher’s exact test.
Multivariable logistic regression with forward stepwise selection identified independent predictors, with forced entry of clinically relevant variables (age, sex, diabetes, platelet count, coagulation parameters, dialysis vintage). Model performance was assessed using AUC-ROC, Hosmer-Lemeshow test, and Nagelkerke R2. Significance was set at p < 0.05.
Maturation was defined by palpable thrill, audible bruit, venous diameter ≥4 mm, flow ≥500 mL/min, and ability to support hemodialysis with two needles at ≥300 mL/min for ≥8 of 12 sessions.
Between January 2017 and January 2024, 466 ESRD patients were screened; six were excluded (18 declined, eight on anticoagulation, five had previous AVF complications, two pregnant). The final cohort comprised 460 patients with 100% six-week follow-up completion.
The cohort included 460 patients (35.4% male, 64.6% female; mean age 44.39 ± 15.06 years). HCV seropositivity was 32.8% (151 patients; 95% CI: 28.6-37.3%). HCV-positive patients were significantly younger (37.2 ± 9.0 vs. 48.3 ± 16.2 years, p < 0.001), had longer dialysis vintage (24.2 ± 14.1 vs. 15.7 ± 10.2 months, p < 0.001), and lower diabetes prevalence (31.8% vs. 45.0%, p = 0.007). Gender, hypertension, and cardiovascular disease were similar between groups ( Table 1).
Hemostatic complications occurred in 60 patients (13.0%), varying significantly by HCV status ( Table 2). HCV-positive patients had 28.5% complications versus 5.5% in HCV-negative patients (OR: 6.835, 95% CI: 3.597-12.987, p < 0.001). Bleeding affected 6.5% overall: 15.2% HCV-positive versus 2.3% HCV-negative (OR: 7.506, 95% CI: 3.111-18.105, p < 0.001). Of 30 bleeding events, 50% were mild, 33.3% moderate, 16.7% severe. All moderate and severe bleeding occurred exclusively in HCV-positive patients, with 65.2% requiring intervention beyond compression.
Hematoma formation occurred in 6.5% overall, with higher rates in HCV-positive patients (13.2% vs. 3.2%; OR: 4.545, 95% CI: 2.062-10.018, p < 0.001). Large hematomas (≥5 cm) occurred almost exclusively in HCV-positive patients (7.3% vs. 0.6%; OR: 12.038, 95% CI: 2.625-55.192, p < 0.001), indicating that HCV infection increases both incidence and severity of hematoma formation.
HCV-positive patients demonstrated significantly abnormal coagulation ( Table 3): prolonged PT (19 ± 5 vs. 15 ± 2.5 seconds, p < 0.001), prolonged aPTT (40 ± 4 vs. 32 ± 3 seconds, p < 0.001), and elevated INR (1.6±0.4 vs. 1.2 ± 0.2, p < 0.001). Thrombocytopenia was marked (153 ± 43 vs. 215 ± 72 ×103/mm3, p < 0.001), with lower hemoglobin (7.0 ± 2.0 vs. 9.0 ± 2.0 g/dL, p < 0.001). Hepatic transaminases were elevated (AST: 59 ± 24 vs. 17±5 IU/L; ALT: 64 ± 17 vs. 9 ± 6 IU/L; both p < 0.001), and hypoalbuminemia present (3.2 ± 0.6 vs. 3.8 ± 0.5 g/dL, p < 0.001).
At six weeks, successful AVF maturation was 74.2% in HCV-positive versus 86.4% in HCV-negative patients (difference 12.2%, p = 0.002), representing 1.90-fold increased maturation failure risk (95% CI: 1.26-2.87). Multivariate analysis confirmed HCV as an independent predictor (adjusted OR: 2.18, 95% CI: 1.32-3.61, p = 0.002). Among patients with hemostatic complications, only 45.3% achieved maturation versus 82.5% without complications (p < 0.001).
HCV-positive patients had higher rates of additional vascular complications: ( Table 4) aneurysm/pseudoaneurysm (3.3% vs. 1.0%, p = 0.047), steal syndrome (6.0% vs. 2.9%, p = 0.019), and infection (4.0% vs. 1.0%, p = 0.032). Thrombosis rates were similar (4.0% vs. 2.9%, p = 0.558). Overall complications occurred in 50.3% of HCV-positive versus 15.9% of HCV-negative patients (OR: 5.543, 95% CI: 3.562-8.624, p < 0.001).
| Complication | HCV+ (n = 151) | HCV− (n = 309) | OR (95% CI) | P-value |
|---|---|---|---|---|
| Thrombosis | 6 (4.0%) | 9 (2.9%) | 1.381 (0.485–3.930) | 0.558 |
| Aneurysm/Pseudoaneurysm | 5 (3.3%) | 3 (1.0%) | 3.448 (0.819–14.518) | 0.047* |
| Steal syndrome | 9 (6.0%) | 9 (2.9%) | 2.128 (0.827–5.474) | 0.019* |
| Infection | 6 (4.0%) | 3 (1.0%) | 4.186 (1.045–16.761) | 0.032* |
| Venous hypertension | 7 (4.6%) | 8 (2.6%) | 1.823 (0.655–5.073) | 0.248 |
| Overall complications (any) | 76 (50.3%) | 49 (15.9%) | 5.543 (3.562–8.624) | <0.001 |
Multivariable logistic regression identified independent predictors of hemostatic complications ( Table 5). HCV-positive status was strongest (adjusted OR: 5.892, 95% CI: 3.102-11.196, p < 0.001). Other predictors included platelet count (adjusted OR: 1.133 per 10,000/mm3 decrease, p = 0.003), prothrombin time (adjusted OR: 1.103 per 1-second increase, p = 0.005), age (adjusted OR: 1.058 per 10-year increase, p = 0.046), and female gender (adjusted OR: 1.527, p = 0.032). The model showed excellent discrimination (AUC: 0.847, 95% CI: 0.802-0.892) and calibration (Hosmer-Lemeshow p = 0.412).
| Variable | Adjusted OR | 95% CI | P-value |
|---|---|---|---|
| HCV-positive status | 5.892 | 3.102–11.196 | <0.001** |
| Platelet count (per 10,000/mm3 decrease) | 1.133 | 1.043–1.231 | 0.003** |
| Prothrombin time (per 1 sec increase) | 1.103 | 1.030–1.181 | 0.005** |
| Age (per 10-year increase) | 1.058 | 1.001–1.118 | 0.046* |
| Female gender | 1.527 | 1.036–2.251 | 0.032* |
| Diabetes mellitus | 1.243 | 0.721–2.142 | 0.435 |
| Dialysis vintage (per year) | 1.015 | 0.982–1.049 | 0.372 |
Figure 1 illustrates AVF maturation outcomes stratified by HCV status and hemostatic complications. Panel A shows maturation rates of 74.2% in HCV-positive versus 86.4% in HCV-negative patients (12.2% difference). Panel B confirms HCV as an independent predictor of maturation failure (adjusted OR: 2.18, 95% CI: 1.32-3.61, p = 0.002). Panel C demonstrates reduced maturation in patients with hemostatic complications (45.3% vs. 82.5%).
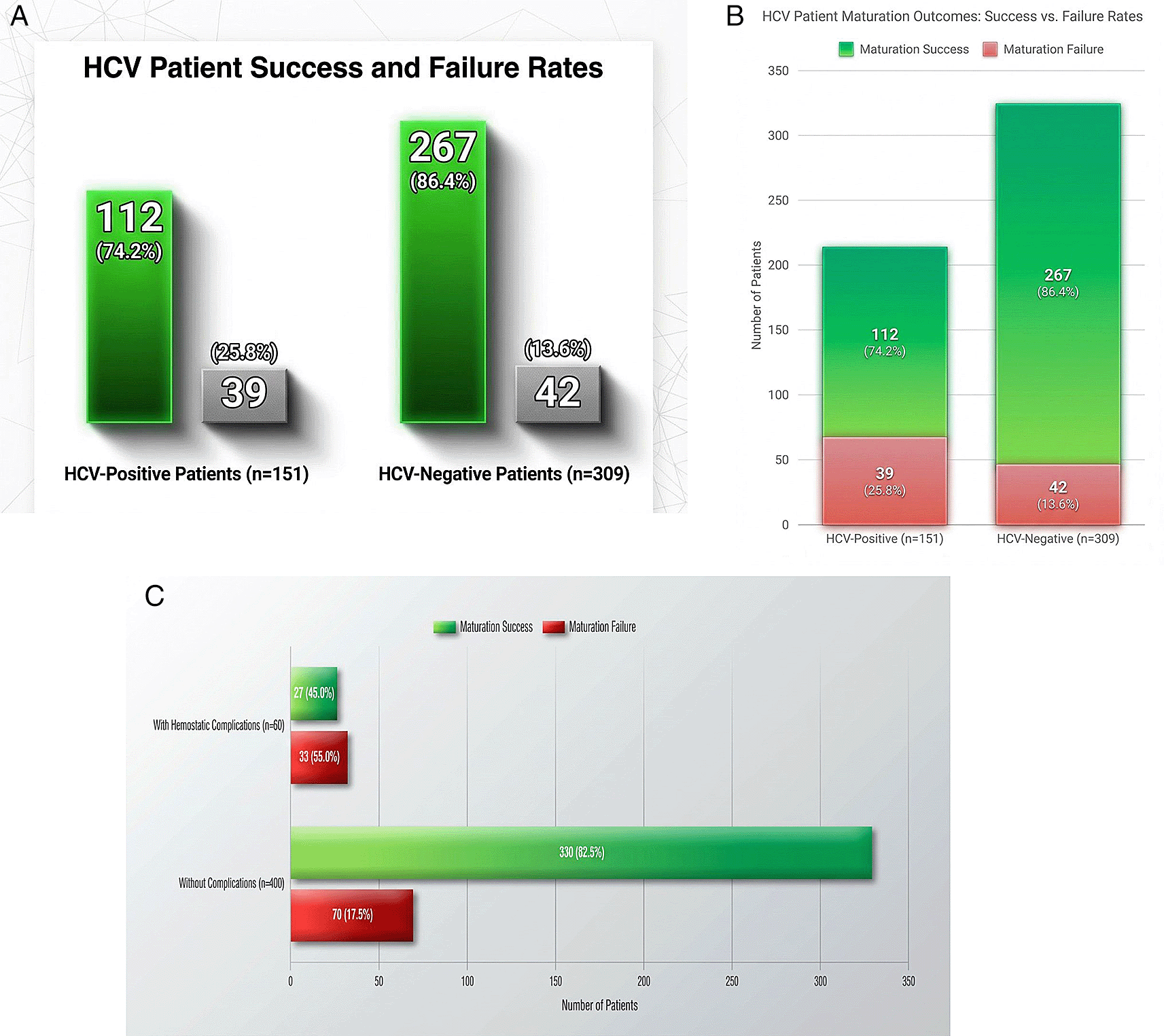
A. AVF Maturation Success by HCV Status.
B. Comparison of maturation of outcomes with adjusted analysis.
C. Impact of Hemostatic Complications on Maturation Success.
Panel A: Comparative pie charts illustrating AVF maturation outcomes at six weeks. Among HCV-positive patients (n = 151), successful maturation occurred in 74.2%. Among HCV-negative patients (n = 309), successful maturation occurred in 86.4% (p = 0.002).
Panel B: Visual representation of maturation success and failure rates by HCV status. Multivariate logistic regression confirmed HCV infection as an independent predictor of maturation failure (adjusted OR: 2.18, 95% CI: 1.32-3.61, p = 0.002).
Panel C: Bar chart demonstrating the mediating role of hemostatic complications in AVF maturation. Among patients with hemostatic complications (n = 60), successful maturation occurred in only 45.3% versus 82.5% in those without complications (p < 0.001).
Abbreviations: HCV, hepatitis C virus; AVF, arteriovenous fistula; RR, relative risk; aOR, adjusted odds ratio; CI, confidence interval.
This prospective cohort study demonstrates that HCV infection is a major independent risk factor for hemostatic complications following AVF creation in ESRD patients, with nearly six-fold increased risk after adjusting for coagulopathic parameters. HCV also significantly impairs AVF maturation, likely through hemostatic complications.
The 32.8% HCV prevalence aligns with regional data showing 22-35% prevalence in Middle Eastern and North African dialysis populations.8,9 These findings confirm persistent high HCV burden in Iraqi dialysis facilities. The 28.5% hemostatic complication rate among HCV-positive patients exceeds Western populations, where general AVF complication rates range from 10-20%.6
A multicenter European study reported 15% overall AVF complication rates in unselected dialysis populations, substantially lower than the HCV-positive subgroup in this study.21 This disparity likely reflects higher HCV prevalence, more advanced liver disease, limited access to direct-acting antivirals, and resource constraints. The 5.5% complication rate in HCV-negative patients approximates contemporary Western series, suggesting HCV infection primarily drives elevated complications.
Meta-regression showed decreasing HCV prevalence trends in Middle Eastern dialysis populations (OR 0.92 per year, 95% CI: 0.90-0.95),9 potentially reflecting improved infection control. However, absolute prevalence remains elevated compared to global averages of 8-10%,6,7 necessitating continued region-specific interventions.
The coagulation profiling elucidates the pathophysiological basis for increased bleeding risk. Chronic hepatic inflammation from HCV progressively impairs synthesis of vitamin K-dependent coagulation factors, manifested by 27% PT prolongation and 25% aPTT prolongation in HCV-positive patients.15 Chronic liver disease produces a “rebalanced” but fragile hemostatic system easily disrupted by surgical stress.17
The 29% platelet count reduction in HCV-positive patients reflects multiple mechanisms: splenic sequestration from portal hypertension, reduced hepatic thrombopoietin production, and possible direct viral suppression of megakaryocyte development.16,17,22 HCV’s independent predictive value (adjusted OR: 5.89) persisted after adjusting for platelet count and PT, suggesting additional mechanisms contribute to bleeding risk.
Plausible explanations include qualitative platelet dysfunction beyond quantitative deficits,23,24 HCV-associated cryoglobulinemia inducing vasculitis and endothelial dysfunction,17 disrupted coagulation-fibrinolysis balance through reduced α2-antiplasmin synthesis,18 and synergistic effects between uremia-induced platelet dysfunction and HCV-mediated abnormalities.15
HCV-positive patients undergoing AVF creation would benefit from enhanced perioperative management. Incorporating HCV status into preoperative risk stratification could identify high-risk patients requiring intensified surveillance. Validated risk prediction models exist but none currently incorporate HCV status despite its substantial predictive value.25,26
Integration of HCV serostatus into these models could enhance discrimination and enable more personalized risk prediction. Prophylactic platelet transfusion for thrombocytopenia (<100,000/mm3) may reduce bleeding risk.27 Hemostatic agents merit consideration, including desmopressin for platelet dysfunction28 and topical agents for local hemostasis.2
Desmopressin demonstrates effectiveness in reversing platelet dysfunction, particularly in uremic contexts, by releasing von Willebrand factor from endothelial storage sites to enhance platelet adhesion.28 Technical surgical modifications may prove beneficial, including smaller initial anastomoses with delayed cannulation (≥8 weeks rather than 4-6 weeks) to reduce early hemostatic stress and improve patency rates in high-risk patients.25 Intensified postoperative surveillance with earlier ultrasound evaluation could enable early detection of complications.26
Direct-acting antiviral (DAA) therapy has revolutionized HCV treatment, achieving sustained virological response rates exceeding 95% even in ESRD populations.29,30 Multiple DAA regimens demonstrate efficacy in ESRD. Successful viral eradication produces measurable improvements in coagulation parameters within 12-24 weeks, including platelet count recovery and prothrombin time normalization.31,32
Patients achieving sustained virological response experienced significant improvements in prothrombin time, platelet count, and albumin levels within six months.31,32 This raises an important question: could preoperative viral eradication before elective AVF creation normalize bleeding risk to levels comparable with HCV-negative patients? This hypothesis warrants evaluation through randomized controlled trials comparing preoperative DAA therapy versus standard care.
The 2018 KDIGO clinical practice guideline recommends treating all HCV-infected patients with chronic kidney disease, including those receiving dialysis.33 However, the guideline does not address optimal timing of treatment relative to planned vascular access procedures. Elucidating optimal treatment timing could substantially improve outcomes while maximizing cost-effectiveness of DAA therapy.
The persistently high HCV prevalence among hemodialysis patients in Iraq and the broader Middle East mandates comprehensive public health responses. Universal HCV screening at hemodialysis initiation with subsequent surveillance testing should become standard practice across all dialysis facilities.33 Stringent infection control measures require consistent implementation, including dedicated machines for HCV-positive patients, enhanced environmental cleaning protocols, and rigorous adherence to standard precautions.34
The CDC emphasizes that hemodialysis units should follow standard precautions for all patients, with additional measures including prohibiting sharing of medications, supplies, or equipment between patients.34 Expanded access to DAA therapy through national treatment programs and generic medication availability could dramatically reduce HCV burden in dialysis populations.35 Vascular access planning should systematically incorporate HCV status, potentially prioritizing earlier AVF creation in HCV-negative patients while optimizing HCV-positive patients through DAA treatment when feasible before elective access surgery.
Resource constraints continue to impede effective infection control despite awareness of transmission risks.8–10 Inadequate sterilization procedures, equipment sharing, and insufficient staff training remain ongoing challenges in dialysis facilities. The COVID-19 pandemic has imposed additional strain on infection control resources and delayed elective vascular access procedures.11,13,14,36 These challenges underscore the critical need for sustained investment in dialysis infrastructure, infection control programs, and access to antiviral medications.
This study has several strengths: prospective design with systematic six-week follow-up, substantial sample size (n = 460) enabling robust multivariable analysis, standardized surgical technique, complete follow-up, and multicenter design across three tertiary hospitals enhancing generalizability.
Several limitations warrant acknowledgment. HCV RNA quantification and genotyping were not performed. The single-country design may limit generalizability. Follow-up extended only six weeks, insufficient for assessing long-term patency. Unmeasured confounders including hepatic fibrosis staging were not assessed.
Future research should address several critical questions. Randomized controlled trials should evaluate whether DAA therapy before AVF creation improves outcomes. The optimal interval between viral eradication and surgery requires definition. Long-term studies examining fistula patency over 1-3 years are needed. Validation in diverse geographic regions would enhance generalizability. Enhanced risk prediction models incorporating viral and hepatic parameters could enable personalized management.
HCV infection independently increases hemostatic complications following arteriovenous fistula creation nearly six-fold (adjusted OR: 5.892, P < 0.001). HCV-positive patients demonstrated prolonged prothrombin time (27%), activated partial thromboplastin time (25%), and reduced platelet counts (29%). Consequently, fistula maturation rates were significantly lower in HCV-positive patients (74.2% vs 86.4%, P < 0.001).
HCV serostatus should be systematically incorporated into preoperative risk stratification for vascular access procedures. Comprehensive coagulation assessment should be mandatory for HCV-positive patients. Perioperative optimization may include platelet transfusion, hemostatic agents, and enhanced surveillance. Direct-acting antiviral therapy prior to fistula creation warrants consideration.
This prospective cohort study demonstrates that HCV infection increases hemostatic complications following AVF creation in ESRD patients nearly six-fold. HCV-positive patients exhibit prolonged coagulation times and thrombocytopenia, significantly impairing AVF maturation. The availability of direct-acting antiviral therapy raises the possibility that preoperative viral eradication could normalize bleeding risk.
The Ethics Committee of Anbar Medical College, Iraq (Reference: AMC/EC/2016-45) approved the study, which was conducted according to the Declaration of Helsinki. Written informed consent was obtained from all participants, with written assent and parental consent obtained for participants aged 14-17 years.
Repository Name: Hepatitis C virus infection as a six-fold risk factor for hemostatic complications following arteriovenous fistula creation in end-stage renal disease: A seven-year prospective study. https://doi.org/10.6084/m9.figshare.30719018.37
The project contains the following underlying data:
• HCV_AVF_Complete_Dataset.xlsx (Complete anonymized patient data including demographics, laboratory parameters, surgical details, and outcomes)
• Coagulation_Parameters_Raw.csv (Unmodified coagulation study results for all patients)
• Clinical_Outcomes_Six_Week.csv (Six-week follow-up data with maturation and complication outcomes)
Repository Name: Hepatitis C virus infection as a six-fold risk factor for hemostatic complications following arteriovenous fistula creation in end-stage renal disease: A seven-year prospective study. https://doi.org/10.6084/m9.figshare.30719018.37
This project contains the following extended data:
• Supplementary Table S1 (Detailed surgical procedure characteristics by HCV status)
• Supplementary Table S2 (Univariate analysis of all potential risk factors for hemostatic complications)
• Supplementary Figure S1 (Receiver operating characteristic curve for the multivariate prediction model)
• Study_Protocol.pdf (Complete study protocol with inclusion/exclusion criteria and assessment schedules)
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
The complete dataset supporting this study has been deposited in Figshare under a Creative Commons CC-BY 4.0 license (DOI: 10.6084/m9.figshare.30719018). The dataset includes Hepatitis C virus infection as a six-fold risk factor for hemostatic complications following arteriovenous fistula creation in end-stage renal disease: A seven-year prospective study. All personal identifying information has been removed in accordance with institutional ethical approval, and informed consent included explicit permission for anonymized data sharing. The datasets generated and analyzed during the current study are available in the Figshare repository at [DOI: 10.6084/m9.figshare.30719018. figshare. https://figshare.com/s/ea291b7e9a50870543cb].11
The authors thank all patients who participated and the clinical staff at Al-Yarmouk Teaching Hospital, Ibn-Al-Nafees Teaching Hospital, and Al-Shaheed Ghazi Al-Hariri Surgical Hospital for their support and collaboration throughout the study.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)