Keywords
Hemoglobin A1c, HPLC, β-thalassemia traits, Diabetes, NGSP
This article is included in the Fallujah Multidisciplinary Science and Innovation gateway.
Hemoglobin A1c is widely recognized as an indicator of long-term glucose control in patients with diabetes. Its measurement, however, can be influenced by several hemoglobin disorders, including the β-thalassemia trait, which is relatively common in the Middle East.
This work compared manual HbA1c testing procedures with an automated high-performance liquid chromatography (HPLC) technique in diabetic patients carrying the β-thalassemia trait. Sixty diagnosed diabetic cases with confirmed β-thalassemia were included from Fallujah Teaching Hospital. HbA1c was analyzed by three procedures: Variant II HPLC, ion-exchange resin, and Turbidimetric immunoassay. Data were processed using ANOVA, t-test, and Pearson correlation in SPSS, considering p < 0.05 significant.
Differences among the tested methods were clearly evident. Measurements obtained by the automated HPLC system were generally higher mean (9.2%) than those recorded with the Turbidimetric immunoassay (7.58%) and the ion-exchange resin technique (7.96%). The observed gap between automated and manual estimations, reaching 1.5% and 1.15% respectively, was found to be statistically significant. HbA1c values demonstrated a strong positive correlation with HbA values in all three analytical methods (r > 0.82, p < 0.001).
These results suggest that manual assays would be expected to erroneously underestimate HbA1c levels in β-thalassemia carriers, which could significantly affect its clinical interpretation. The NGSP-standardized HPLC method was the most reliable for the accurate measurement of HbA1c and reflects more precisely glycemic control in patients with variant haemoglobins.
Hemoglobin A1c, HPLC, β-thalassemia traits, Diabetes, NGSP
One of the main indicators used in diabetes care is the Hemoglobin A1c test, also known as glycated hemoglobin. It reflects the average blood glucose concentration over the last two to three months and hence is used to assess long-term glycemic control.1 The test is widely accepted as a reliable guide for making therapeutic decisions, estimating the risk of complications, and generally improving patient management. Obtaining accurate HbA1c values is critical because such results provide the basis on which treatment plans are modified to maintain effective metabolic control and prevent complications commonly associated with diabetes, such as cardiovascular disease, neuropathy, and nephropathy. For this reason, maintaining precision and consistency in the assessment of HbA1c is indispensable in both clinical practice and research environments.2
β-thalassemia is an inherited disorder of the blood in which mutations in the β-globin gene reduce or completely stop the production of β-globin chains in hemoglobin. The condition is common in regions where malaria has been endemic, including the Middle East, the Trans-Caucasus, Central and South Asia, Southeast Asia, and the Mediterranean.3 Thalassemia exists in two major forms: α-thalassemia and β-thalassemia. It upsets the normal balance of hemoglobin formation, with the production of microcytic anemia, and increases the percentage of various types of abnormal hemoglobin. β-thalassemia can be divided into three categories-major, intermedia, and trait-with the latter form of the disease normally mild and often presenting no symptoms.4
Individuals with the β-thalassemia trait frequently exhibit laboratory findings indicative of modifications in erythrocytes indices, including decreased the mean corpuscular volume (MCV) as well as the mean corpuscular hemoglobin (MCH).5 The hemoglobin A1c test can lead to false outcomes resulting in overtreatment or under-treatment of diabetes in people with inherited hemoglobin variants such as β-thalassemia a hemoglobin variant.6 This research evaluates the mini-column ion exchange resin method with National Glycohemoglobin Standardization Program (NGSP)- High Performance Liquid Chromatography HPLC method to assess the impact of β-thalassemia on gHb. Clinical laboratories can use more than 20 commercially available gHb measurement techniques.7 These techniques quantify gHb according to its known physicals, chemicals, and antigenic properties. Hemoglobin (Hb) composition variations as well as chemical compounds obstruct numerous techniques.8 The Bio-Rad Variant and the Tosoh A1C 2.21 are common cation-exchange and HPLC systems. systems for Diamat and Diastat. The National Glycohemoglobin Standardization Program (NGSP) certified these methods because they had minimal interference from hemoglobin variations and demonstrated traceability to the diabetes control and complications trial reference method.9 By hands Commercially accessible HbA1c testing methods use ion-exchange resin, which is negatively charged and packed in a minicolumn. This resin strongly attracts positively charged hemoglobin. When compared to a typical Glycohemoglobin preparation, the spectrophotometer will identify a specimen containing gHb (less fully positive charged) at 415 nm when it leaks first.10
The conversion equation was used to change the value from HbA1 to HbA1c. The third method, which used the Bio-Rad version I hemoglobin A1c system according to ion-exchange high performance liquid chromatography (HPLC), was used to compare the results from the first two procedures.11
These changes can interfere with diagnostic assays, including Hb A1C testing. Abnormal hemoglobin variants may lead to inaccurate measurements due to differences in hemoglobin glycation rates, structural alterations, or interference with the analytical methods employed.12
For this investigation, in this study, 60 diabetic patients with β-thalassemia trait were enrolled. Blood samples were obtained in EDTA tubes (whole blood) or fresh blood and in gel tubes (serum). EDTA tubes (whole blood) were used for the detection of β-thalassemia minor, hemoglobin variants, and glycated hemoglobin (A1c) by manual and automated methods, while gel tube samples (serum) were used for random blood glucose testing. Three widely used and commonly accessible techniques for ordinary investigation were utilized to measure hemoglobin A1c in blood samples:
• LINEAR CHEMICALS (Barcelona, Spain): Chromatographic determination in a tube with pre-weighted resin of Hemoglobin A1c in blood.
• BIOLABO Glycohemoglobin HbA1c Test (France): Turbidimetric Immunoassay Method.
• Each of these techniques relied on the same idea, which is that whole blood is combined with a lysing agent that contains borate ions and detergent. Thus, during hemolysis, the labile Schiff’s base is eliminated.
• Bio-Rad VARIANT II Hemoglobin A1c (USA): High-Performance Liquid Chromatography (HPLC) method.
• The subjects enrolled in this study were attending Fallujah Teaching Hospital on October 23, 2024. The diagnosis of patients was confirmed by specialized physicians at the mentioned hospital.
• This study was approved by the Department of Applied Chemistry, College of Applied Science, University of Fallujah.
Verbal informed consent was obtained from all subjects before taking the sample. This study involved taking blood samples, which was a normal part of a medical procedure. There were no invasive or experimental procedures involved in this study. Written consent was not considered necessary for this study, which was carried out in a public hospital setting. This is owing to literacy levels of subjects in a public setting, as verbal consent would be a better alternative in a public institution.
Random blood glucose levels were measured using a commercially available enzymatic glucose kit based on the glucose oxidase–peroxidase (GOD–PAP) reaction (Biolabo SA, Maizy, France; Catalogue No. 87409). For manual determination of glycated hemoglobin, two different commercial HbA1c kits were employed. The first was a glycated HbA1c kit based on weak cation-exchange chromatography (Linear Chemicals S.L., Barcelona, Spain; Catalogue No. 3155105). The second manual technique employed a turbidimetric immunoassay using the HbA1c Turbidimetric Immunoassay kit (Biolabo SA, Maizy, France; Catalogue No. 22010). The automated HbA1c analysis was conducted using the Bio-Rad D-10/VARIANT II instrument based on ion-exchange high-performance liquid chromatography (HPLC), with reagents supplied by the manufacturer. All analyses were carried out in accordance with the manufacturers’ instructions using whole blood samples collected in EDTA tubes and gel tubes (serum).
The used chemicals and their sources are given in Table 2.
Determination of Random Blood Glucose in blood serum
Principle of the Test
Glucose oxidase (GOD) breaks down glucose to create hydrogen peroxide and gluconate. The red quinoneimine dye, which is detected at 505 nm35, is the result of the hydrogen peroxide’s subsequent reaction with 4-aminoantipyrine (4-AAP) and phenol in the presence of peroxidase (POD).13 This method, commonly known as the Trinder reaction, was first described by P. Trinder in 1969 (Ann. Clin. Biochem.) and has become a standard enzymatic assay in clinical chemistry.
The concentration of glucose in the sample is directly correlated with the absorbance at 505 nm.
Procedure:
As directed by the kit, the sample is well combined with the reagents and then incubated for 10 minutes at 37°C or 20 minutes at room temperature. A spectrophotometer is then used to measure absorbance at 505 nm. The glucose concentration is determined based on a calibration curve and compared with reference values in Table 3.
| Test tube | Blank | Standard | Test |
|---|---|---|---|
| Reagent | 1 ml | 1 ml | 1 ml |
| Distilled water | 10 μl | ||
| Standard | 10 μl | ||
| Specimen | 10 μl |
Calculation:
Using reference values as a guide, a calibration curve is used to calculate the quantity of glucose.
Glycated hemoglobin (HbA1c) is quickly separated from other hemoglobin species using weak-cation-exchange chromatography in the Linear Chemicals HbA1c kit. The separated fractions are then measured spectrophotometrically (Linear Chemicals 2018), originally reported the fundamental technique for employing ion-exchange column separation of HbA1c in 1971.14
Procedure:
To enable HbA1c binding, blood obtained in an EDTA tube is hemolyzed and incubated with boronate affinity resin in accordance with the kit’s instructions. Absorbance is measured at 415 nm and compared to calibration standards following the centrifugation or use of the separator included in the kit to separate HbA1c from non-glycated hemoglobin.
Antibodies specifically bind to HbA1c to form antigen–antibody complexes in the Turbidimetric immunoassay that forms the basis of the Biolabo HbA1c assay. A spectrophotometer is used to measure the resulting turbidity, which is proportional to the HbA1c concentration.15 This immunoassay principle was first developed by Berson and Yalow in 1959.
Procedure:
After collecting and thoroughly mixing blood in an EDTA tube, use the hemolysis reagent included in the kit to lyse red blood cells. As directed by the kit, let hemolysis continue. To create antigen–antibody complexes, place the hemolyzed sample in a cuvette with HbA1c-specific antibodies and incubate. Using a spectrophotometer, determine the resultant absorbance at 540 or 630 nm in accordance with the kit protocol.
Calculation:
Determine HbA1c percentages by plotting a standard curve of HbA1c (%) versus Abs after calculating absorbance (Abs) for specimens, controls, and standards. Use the IFCC’s master equation to convert results to IFCC units (mmol/mol Hb).
The Bio-Rad VARIANT II analyzer separates hemoglobin components using ion-exchange HPLC, resulting in unique peaks for each variant. It measures HbA2 and HbF levels; carriers of β-thalassemia are indicated by HbA2 >3.5%.16 This system was developed by Bio-Rad Laboratories, Inc., founded by David Schwartz and Alice Schwartz in 1952.
Procedure:
Gather the whole blood that has been treated with EDTA and combine it with the diluent that the manufacturer has supplied. The prepared sample should be injected into the HPLC system so that the chromatographic column can separate the hemoglobin components. Software determines the percentage of each hemoglobin fraction by calculating the area under the peaks of each component, which is detected as a peak.
Calculation:
The percentage of each hemoglobin component is calculated by the software using the formula:
The percentage of glycated hemoglobin is measured manually using HbA1c determination techniques, which offer crucial insights into diabetic patients’ long-term glucose control.17 Even though automated systems are widely used, manual methods are still used in some labs because they are easy to use, inexpensive, and useful in situations where sophisticated analyzers are not available.18
1. Ion-Exchange Chromatography
As blood passes through a column filled with ion-exchange resin, hemoglobin variants are separated according to their charge differences using ion-exchange chromatography, as illustrated in Figure 1, a popular manual method for HbA1c testing.19 Because of its slightly higher positive charge, HbA1c binds to the resin more firmly, enabling it to separate from other types of hemoglobin.20 For determining HbA1c, this approach is tried-and-true, accurate, and dependable. However, atypical variants or hemoglobin disorders, like those in β-thalassemia trait, can cause interference with the measurement and lead to inaccurate results.
2. High-Performance Liquid Chromatography (HPLC)
Hemoglobin components are separated as the blood sample travels through a chromatographic column filled with a stationary phase, resolving molecules according to their interactions with the column matrix. This process, illustrated in Figure 2, is known as high-performance liquid chromatography (HPLC), and it is a manual method for determining HbA1c.21,22 To find the HbA1c concentration, the prepared sample is loaded into the HPLC system, where hemoglobin fractions are successively separated and measured. Patients with hemoglobin disorders like sickle cell disease benefit greatly from HPLC’s exceptional precision and ability to differentiate HbA1c from other variants.23 However, its viability in laboratories with limited resources is limited because it necessitates specialized equipment, takes longer, and occasionally interferes with accurate quantification due to aberrant hemoglobin variants.24
3. Turbidimetric immunoassay technique
Immunoturbidimetry (IT) utilization of formation of antigen-antibody complexes, but in solution rather than in agarose gel (as shown in Figure 3).25 With the with the proper proportion of antigen and antibody, the formation of antigen-antibody complexes can be monitored through as the process requires as little as a few minutes, this technique represents the method of choice for automation of analysis, but it is only suitable for protein concentrations above 0.5 to 1.0 mg/dl (5 to 10 mg/litter). This approach is widely used in human clinical biochemistry for determination of proteins such as CRP, but the limited accessibility of appropriate reagents has held back applications in veterinary medicine. Nonetheless, IT methods for canine CRP and feline AGP have been reported.26 Although commercial kits for human CRP based on IT have been verified for use in serum from some animal species, caution has to be exercised in their application, particularly since antiserum batches may exhibit varying cross-reactivity’s toward animal proteins, resulting in batch-to-batch variation that can arise.27 Immunonephelometry is a related method where reflected rather than absorbed light is measured, which aids in reducing interference.28 An alternative adaptation of the IT test is to employ antibody-coated latex particles, which may render assays more sensitive as well as stabilizing the antibodies. An approach employing latex particles coated with antibody to human serum amyloid A has been verified to detect this protein in horses.29
Table 4 displays the demographic information of 60 diabetes individuals who were confirmed to have the β-thalassemia trait after a normal check for diabetes management. The patients in our study had an average age group of 38.5 years, 204 mg/dl is the average random blood sugar levels. A mean hemoglobin A2 level of 4.8%, and an average Glycohemoglobin (Hb A1c) of 9.2%, in comparison with 7.58% with 7.96% using LINEAR CHEMICALS and the Biolabo Technique. Correspondingly, as shown in Table 4, that demonstrate a variation that is statistically significant (p<0.05). Additionally, it was discovered that the average variation among the automated approach (Bio-Rad version HPLC) as well as the manual procedures (Biolabo and LINEAR) was approximately (1.5, 1.15). With r = 0.856; p value <0.001, those variations demonstrate a substantial statistical association with hemoglobin A2 levels.30
The average glycated hemoglobin Hb A1c measured via Bio-Rad Variant II as well as the glycated hemoglobin measured via the human technique were found to differ significantly (p<0.05). Additionally, a substantial variance (a p value <0.05) were found among an average glycated hemoglobin Hb A1c via Bio-Rad Variant II while Hb A1c through LINEAR technique. However, for HbA1c, there were no appreciable variations between the LINEAR and Biolabo approaches Tables 5, 6.
| Bio-Rad Variant II (HPLC) | Biolabo (Turbidimetric immunoassay method) | LINEAR (Fast ion exchange resin) | P value | |
|---|---|---|---|---|
| HbA1c | 9.1 (±2.55) | 7.57% (± 1.5) | 7.95% (±1.66) | .001 |
| Biolabo (Turbidimetric immunoassay method) | LINEAR (Fast ion exchange resin) | P value | |
|---|---|---|---|
| HbA1c | 7.57% (± 1.5) | 7.95% (±1.66) | .205 |
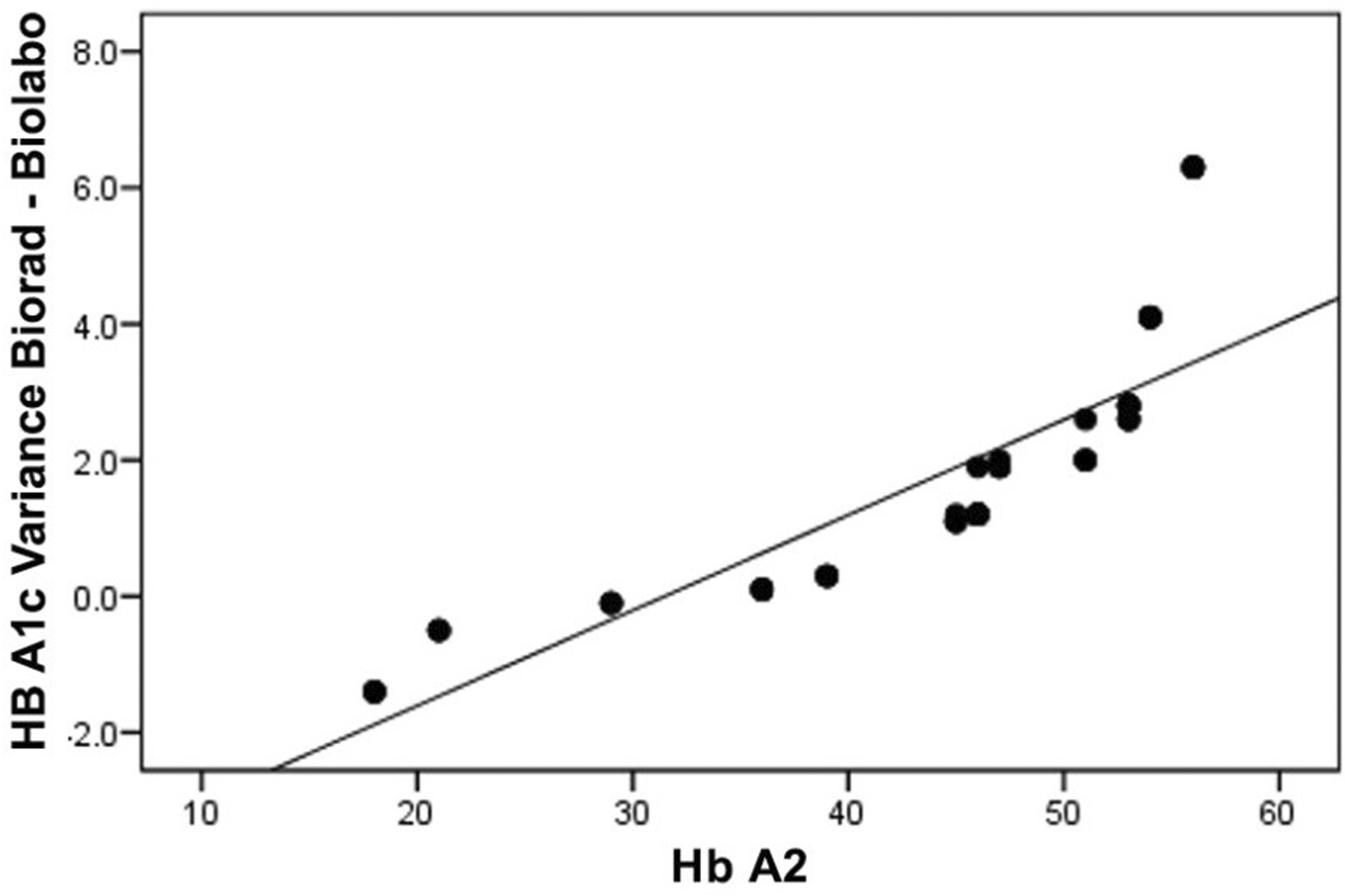
Patients Hb A2 levels and the difference in glycated hemoglobin within the Bio-Rad Variant I method as well The additional two techniques are displayed in Table 7. It indicates that the mean variances among Bio-Rad Variant I and the Biolabo method are approximately 1.4%, whereas the mean variations among Bio-Rad Variant I and the LINEAR method are 1.24 percent.
| HB A2 % | Bio-Rad Variant I – Biolabo method | Bio-Rad Variant I – LINEAR techniques |
|---|---|---|
| 6.3 (±1.16) | 1.4 (±1.8) | 1.24 (±1.9) |
A significant statistical association was discovered between this amount of difference and hemoglobin A levels, of r = 0.856; p value <0.001 for the Biolabo approach with r = 0.826, p value <0.001 for the LINEAR method Figures 4, 5.
The β-thalassemia trait is relatively common across many Middle Eastern populations, and this has important consequences for how HbA1c values are interpreted in diabetes diagnosis and follow-up.31,32 As in other hemoglobin variants, β-thalassemia can affect HbA1c both through biological mechanisms and laboratory limitations. From a biological point of view, this disorder is characterized by weak red-blood-cell production and faster breakdown of erythrocytes. Because the cells live for a shorter time, hemoglobin has less exposure to blood glucose. As a result, the HbA1c value can appear lower than it should be, even when the patient’s overall glucose level is high. The current observations are consistent with those of Mitchai M. et al.,33 who reported that hemoglobinopathies are common and that HbA1c results may vary considerably among different commercial testing systems. They also emphasized the need to identify hemoglobin types before interpreting HbA1c values. Tsilingiris D. et al.34 found that people with the β-thalassemia trait but normal hemoglobin levels showed HbA1c readings similar to healthy individuals. However, within the thalassemia group, a clear link between hemoglobin concentration and HbA1c was observed, showing how red-cell lifespan affects glycation. From the analytical side, higher fractions of HbF and HbA2 seen in β-thalassemia can disrupt some ion-exchange or immunoassay procedures that fail to separate these fractions from HbA0, leading to falsely low results. A review in Ref. 35, noted that tests unable to distinguish HbF from HbA1c often underestimate HbA1c in patients with thalassemia or related hemoglobin disorders. More recent research Ref. 36, suggests that the extent of interference depends largely on the disease severity and the analytical method used, as some studies report little or no difference between carriers and non-carriers. In real-world settings, the difference between manual and automated HbA1c testing in people with β-thalassemia mainly comes down to how precisely each method separates hemoglobin fractions. Manual techniques like ion-exchange resin or immunoturbidimetric assays often give lower readings because they can’t fully distinguish glycated hemoglobin from other forms.37
Automated HPLC analyzers, however, do a much better job at separating these fractions and reducing interference from HbA and HbF, hence making their results so much more reliable. This is not just a technical issue; it is clinically relevant as well. Underestimated HbA1c values can result in a misleading picture of blood glucose control in diabetic patients with hemoglobin variants. Thus, in regions with a common prevalence of β-thalassemia, using NGSP-certified automated systems still remains the most dependable method of obtaining correct and reproducible results.
Since the American Diabetes Association,38 continues to support HbA1c as a major diagnostic indicator of diabetes, the accuracy of this test needs to be maintained in populations where hemoglobin variants are common. The National Glycohemoglobin Standardization Program,39 points out that any condition influencing red cell lifespan or changing the structure of hemoglobin may mislead interpretation of HbA1c. For this reason, any assay being NGSP- or DCCT-traceable is recommended in order to avoid discrepancies in interlaboratory variability. In practice, if β-thalassemia or similar conditions are suspected family history and small MCV and/or raised HbA levels serve as pointers-alternative markers of glycaemic control, such as fructosamine measurement or CGM, can provide additional information.30 In summary, while in many cases the β-thalassemia trait does not cause an apparent derangement of HbA1c, standardized and accurate testing methods would be important. Especially in areas with high infection rates, such as the southern governorates (Basra, Maysan, Dhi Qar, Muthanna), according to data from the Iraqi Ministry of Health and the National Center for the Treatment of Hereditary Blood Diseases, the accuracy of the method used is essential for the correct assessment of diabetes control.
When the results of HbA1c obtained from local commercial methods were compared with the NGSP standardized methods, there was a definite discrepancy Some of the commonly employed methods tended to produce falsely low results in patients with β thalassemia trait This result suggests that in such patients, such methods should not be used for diagnosis or follow-up purposes Manual methods have tended to produce results that have been one to one and a half percent lower than those obtained form NGSP-certified HPLC methods This discrepancy can readily produce a misconstrued impression of glycemic control and misinformed treatment adjustments The correlation between increased HbA2 levels and discrepancies lends support to the postulate that hemoglobin variability does have a bearing on the validity of HbA1c results Although manual methods are less expensive and more readily available, they cannot be employed in regions with high hemoglobinopathy variability Automated HPLC is still the most reliable method to accurately determine HbA1c and can utilize other markers like fructosamine or glucose monitoring for more accurate assessment and care of patients with diabetes.
1. In practice, getting reliable HbA1c readings depends not only on using well-validated testing methods but also on having the awareness to question unexpected results, particularly when dealing with patients who have hemoglobin abnormalities that might distort the values.
2. More research is still needed on the different thalassemia genotypes to better understand how each may affect the accuracy of HbA1c in reflecting long-term glucose control.
3. When HbA1c values do not truly reflect glucose control, clinicians can rely on alternative indicators—such as fructosamine, glycated albumin, 1,5-anhydroglucitol, or continuous glucose monitoring—to obtain a clearer picture of glycemic status.
4. Ongoing clinical awareness and periodic revision of diabetes care guidelines will help improve disease management for patients living with thalassemia and related hematologic conditions.
The study was approved by the Ethical Approval Committee College of Applied Sciences, University of Fallujah, Iraq, under approval ID FAS/ETH/2024/013, dated October/22/2024, for the original protocol entitled (Challenges in Measuring Hemoglobin A1C in Diabetic Patients with Hemoglobin Beta-Thalassemia Trait Variants as a Model). An updated administrative amendment (approval ID FAS/ETH/2025/007) was subsequently issued on December/7/2025 reflecting an expanded study scope and a revised title, (Comparative Analysis of Manual and Automated (HbA1c) Methods in β-Thalassemia Carriers with Diabetes). This amendment was administrative in nature and did not constitute retrospective ethical approval. All data collected prior to this amendment were obtained strictly within the scope of the original ethical approval, and no additional risks, interventions, or procedures were introduced as part of the revised protocol. The study involved exclusively routine blood samples collected as part of standard medical care, with no invasive, experimental, or non-standard interventions. All data were anonymized and handled in full compliance with institutional privacy policies and informed consent requirements. Verbal informed consent was obtained individually from all participants prior to sample collection, as approved by the Ethical Approval Committee (Approval IDs: FAS/ETH/2024/013 and FAS/ETH/2025/007). Each consent was documented in the study log and witnessed by clinical staff present during collection. Participants were verbally informed about the study’s purpose, procedures, and voluntary participation, and consent was recorded only after confirming full understanding.
The dataset generated and analyzed during this study has been deposited in Figshare and is publicly available at https://doi.org/10.6084/m9.figshare.30945200.40
This project contains the following data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
For any inquiries or additional data requests, please contact the corresponding author (Mustafa M. Fahad, University of Anbar; mustafa.moh@uoanbar@edu.iq)
The authors would like to thank the volunteers who provided blood samples for this study, and the College of Applied Sciences, University of Fallujah for their support.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)