Keywords
Antioxidants, Brain, Cerebral Cortex, Cyclooxygenase, Hippocampus, Nanoparticles, Neuroinflammation, Neurotransmitters
This article is included in the Fallujah Multidisciplinary Science and Innovation gateway.
This article is included in the Nanoscience & Nanotechnology gateway.
This study aimed to evaluate the protective role of piperine and piperine-loaded titanium dioxide nanoparticles on oxidative and biochemical parameters, antioxidant levels, and cytogenetic parameters in the brain.
forty rats were randomly divided into four equal groups for 35 days as follow: the control group was administered DW/IP; the G1 group received 40 mg/kg B.W. of piperine orally; The G2 group was given TiO2NPs (50 mg/kg B.W.)/i.p. received; and the G3 group received 75 mg/kg B.W. /I. P. of PIP-TiO2NPs.
A significant increase in serum TAO-C and a significant decrease in ROS were observed in G1 and G3 compared to G2 and the control groups. A significant reduction in serum neurotransmitter concentration was found in the G2 group compared to the G1, control, and G3 groups, while a significant increase was observed in the G1 group. Gene expression data for cyclooxygenase showed upregulation in the G2 group and was significantly higher than in the G3, G1, and control groups. Histopathological examination showed normal brain layers, hippocampus, and thalamus appearance in G1, G2, G3, and the control groups. The G2 group showed mild vascular congestion, hyperplasia, and mild disruption in the molecular cell layer of the cerebral cortex. The hippocampus of the G3 group showed moderate vacuolation with glial reduction.
These findings indicate that piperine, combined with TiO2NPs (PIP-TiO2NPs), provides neuroprotection by reducing oxidative stress and inflammatory responses induced by TiO2NPs in the rat brain.
Antioxidants, Brain, Cerebral Cortex, Cyclooxygenase, Hippocampus, Nanoparticles, Neuroinflammation, Neurotransmitters
Exposure to titanium dioxide nanoparticles (TiO2NPs) is associated with several systemic toxic effects. Titanium is extensively used in biomedical applications owing to its high biocompatibility. Titanium implants may release titanium dioxide (TiO2) nanoparticles (NP), which can enter the bloodstream and reach different organs including the brain.1,2 Despite the precise processes and particle quantities that penetrate the blood-brain barrier being unclear, TiO2NPs can enter the brain after ingestion. According to Ref. 3, exposure to titanium dioxide nanoparticles induces oxidative stress, neuroinflammation, brain chemistry alterations, and impairment of neuronal structure and functionality. Neural damage may result in behavioral disturbances and contribute to the development of neurodevelopmental and neurodegenerative diseases. Such neuronal injury and the neurodevelopment or neurodegenerative disorders that follow, along with any behavioral disorders that may be present, can be partially attributed to oxidative stress and inflammation.4 Although Ref. 5 noted that inflammation levels in mineral miners exposed to titanium dioxide nanoparticles were significantly higher, the specific details surrounding the neurotoxicity of such exposure remain nebulous. The central nervous system responds to TiO2NPs exposure by activating the neuroinflammatory pathway, as reported by Ref. 6. TiO2NPs can induce genotoxicity through both direct and indirect mechanisms. Direct genotoxicity occurs when nanoparticles penetrate the nucleus, interact with the DNA, and inhibit replication. Indirect genotoxicity arises from ROS generation, suppression of antioxidant defenses, and releasing toxic ions from NPs.7,8 Following cellular uptake, TiO2NPs accumulate in lysosomes, leading to lysosomal damage and cytosolic enzyme release, which combines with other cell components to produce inflammation, oxidative stress, DNA damage, and altered gene expression.9,10
Piperine is a bioactive alkaloid responsible for the pungency of black pepper (Piper nigrum) and long pepper (Piper longum).11,12 Chronic administration of piperine has demonstrated antioxidant properties and cognitive benefits.13 Accumulating evidence supports the therapeutic potential of piperine and its metabolites in treating cancer, cardiovascular and hepatic diseases, and neurological diseases. In the case of piperine, to understand the tradeoffs in its use, particularly in the Central Nervous System, it is vital to evaluate the pharmacological profile alongside the associated toxicity. In a model of PD (Parkinson’s Disease), it was shown that the administration of piperine prevented the death of neuronal cells14 and in a model of AD (Alzheimer’s Disease), enhanced cognition,15 while also reducing depression, as seen in the works of16 and associated with Huntington’s Disease.17
According to the World Health Organization,450 million individuals worldwide suffer from mental or cognitive disorders. Like other plants of the Piperaceae family, the fruits of black pepper (Piper nigrum), long pepper (Piper longum), and some other piper plants contain an alkaloid piperine (1-piperoylpiperidine) that has a nitrogenous and pungent characteristic. Current studies in pharmacology suggest that piperine exerts anti-inflammatory and analgesic properties,18 anticonvulsant,19 and antiulcer20 activities, as well as depression,21 and cytoprotection and antioxidant effects.22 This study aims to evaluate the harmful effects of titanium dioxide nanoparticles (TiO2NPs) and assess the potential protective roles of piperine alone or in combination with TiO2NPs (PIP-TiO2NPs) to alleviate TiO2NP-induced physiological neurological disorders.
According to the instructions provided by Ref. 23, titanium tetrachloride (TiCl4) and 99.9% ethanol are mixed at room temperature for 30 minutes while stirring at 600 rpm under a fume hood. The clear solution that resulted was used for manufacturing nanoparticles.
Two and a half grams of black pepper extract (piperine) was dissolved in 25 mL of deionized desalinated water according to Ref. 24, stirred at 300 rpm for 10 to 20 minutes with a magnetic hotplate stirrer at room temperature, and then filtered through Whatman® filter paper. The resulting clear solution was used to produce nanoparticles.
Green titanium dioxide (TiO2) nanoparticles were synthesized by adding 14 mL of titanium tetrachloride (ACROS Organics, France) TiCl4 99.99% to 140 mL of absolute ethanol CH3CH2OH 99.99% (Ferak, Germany) with an ethanol ratio. The reaction was carried out at room temperature with stirring (600 rpm) and continued for 30 minutes under a hood due to the large amount of Cl2 and HCl produced. The solution was allowed to rest and cool to room temperature, and then the solution’s pH was measured in the range 1–2.23 Two and a half grams of piperine powder was dispersed in 25 ml of DD water at a ratio of 1:1024 and filtered through Whatman® filter paper. The resulting clear solution was used for the synthesis of nanoparticles. Then, the piperine solution was mixed with titanium in a ratio of 2:1 under stirring (700 rpm) for 3 hours at room temperature, where a clear yellow solution was formed. After that, the mixture was stirred for 30 minutes while 10–15 mL of ammonia (BDH, England) was added dropwise to maintain pH 6–7.25 After centrifuging and rinsing three times with deionized water to get rid of contaminants, the mixture was dried for three hours at 100 °C and calcined for two hours at 500 °C.26
UV-visible (ultraviolet-visible) spectroscopic
The stability and optical properties of nanoparticles were analyzed using UV-visible spectroscopy (Shimadzu, Japan). After diluting a small amount of the sample in distilled water, the UV-visible spectrum of the reducing agent was measured. To detect the bioreduction of piperine-TiO2 nanoparticles, a UV-visible spectrophotometer was used to measure surface plasmon resonance (SPR) in the wavelength range 200–800 nm. Electromagnetic waves were analyzed spectroscopically in the wavelength range of 190–1100 nm. Surface plasmon resonance is represented as peaks in the UV-visible absorption spectra of nanoscale particles.27
Diffraction of X-rays (XRD)
To characterize the formation, size and crystallinity of piperine-TiO2 nanoparticles, an XRD pattern (Philips, Holland) was obtained after measuring the Bragg angle at 2θ. After centrifuging the nanoparticles for 30 minutes at 10,000 rpm, they were cleaned repeatedly using 20 milliliters of deionized water. A simultaneous theta-2 theta scan with a scan rate of 0.2000°/min was employed to acquire XRD patterns in the 10,000–90,000° range. The Debye–Scherrer equation was used to determine the crystallite size28:
Fourier transform infrared spectroscopy (FTIR)
The prepared piperine-TiO2 nanoparticles in dry pellet form were subjected to FTIR spectroscopy (ABB/Spectro Lab, England). The number of scans was approximately 24, and the laser phase, F and D amplitudes were 35. Conventional KBr pellet technique was used for FTIR studies. The ratio between infrared light intensity and wave number was measured in the range 400–4000 cm−1. This analysis is important for elucidating functional groups in organic solutions involved in nanoparticle synthesis. The calculated spectra show optically dependent properties of nanoparticles, such as extinction cross section, scattering-to-absorption ratio, nanoparticle size and resonance wavelength.30 The work was carried out in the laboratories of the Ministry of Science and Technology, the Institute for Materials Research and the Medical Materials and Equipment Research Centre.
Scanning Electron Microscopy (SEM)
Scanning electron microscopy (TESCAN MIRA3, Czech Republic) was used to detect the size and morphology of titanium dioxide nanoparticles. Dry piperine-TiO2 nanoparticle thin films were prepared by dropping a small amount of the solution onto a cover glass grid and allowing it to dry at ambient temperature before observation. SEM was performed under the following parameters: Mag = 2305×, signal A = SE2, HV = 30.00 kV and WD = 12.6 mm.31
Transmission Electron Microscopy (TEM)
A transmission electron microscope (Philips EM208S, Holland) was used to examine the size and shape of piperine-TiO2 nanoparticles. The procedure of dropping dried materials onto TEM grids and letting them dry frequently results in nanoparticle accumulation.32
EDX (energy-dispersive X-ray) spectroscopy
The elemental composition of the particles under SEM analysis was evaluated using EDX spectroscopy.33 This work was completed at Tehran University Central Lab.
Forty adult male rats weighing 185 ± 15 g were obtained from the College of veterinary medicine, University of Baghdad. The animals were housed in cages, each cage housed five rats and was cleaned daily, in the animal facility of the College of Medicine, University of Fallujah. They were maintained under standard laboratory conditions (temperature 22 ± 2°C, relative humidity 55 ± 10%, and a 12 h light/dark cycle) and had free access to tap water and a standard commercial pellet diet throughout the experiment. The rats were randomly divided into four equal groups (n = 10 per group) and treated for 35 days, as shown: Control group: Rats were injected intraperitoneally (i/p) with sterile distilled water, G1 group: Rats were administered piperine orally 40 mg/kg. B.w.34 (Piperine was obtained as Black Pepper Extract from Charge Products/United Kingdom), G2 group: Rats were administered TiO2 NPs (i/p) of 50 mg/kg. B.w.,35,36 G3 group: Rats received a combined 75 mg/kg treatment. B.w. i/p of Piperine- TiO2NPs,37 TiO2 nanoparticles and the combined piperine–TiO2 formulation were prepared in the laboratory according to the referenced methods cited for each compound. Randomization was performed using simple random allocation, and all doses were calculated relative to body weight (mg/kg).
Blood samples were collected via the Retro-orbital sinus technique, from anesthetized rats by intramuscular injection of 16 mg Xylazine + 60 mg ketamine i.m./kg B.W.38 (Rambon/Germany), sample were placed in non-heparinized gel tubes and allowed to stand for 30 minutes before being centrifuged (for 15 minutes at 3000 rpm) and stored in firmly sealed tubes for further analysis at -20°C until analysis for the following parameters: Noradrenalin, glutamate, and gamma-aminobutyric acid (GABA) (Cloud-Clone Corp/USA). Serum total antioxidant capacity (TAO-C, U/mL) was measured calorimetrically using a Kit (YL Biotech/ China). Serum reactive oxygen species concentration was determined calorimetrically using an ELISA kit (SUNLONG/China) following the manufacturer’s instructions. Serum malondialdehyde (MDA) levels were determined colorimetrically.39
Cyclooxygenase (COX) Gene expression in brain tissues was determined using quantitative real-time PCR (qRT-PCR). Relative transcript levels were calculated using the comparative Ct methodology (ΔΔCt), normalized to 12S-rRNA, and expressed relative to the control group.40 The COX gene was amplified using the following primers ( Table 1), as previously described by reference.41
At the end of the experiment, euthanasia was performed using an overdose of anesthetic, which is an AVMA-approved method. After euthanasia, the rats remained under deep anesthesia and their brains were carefully removed from the skull for histological examination. The cerebral cortex and hippocampus were dissected, blotted, and fixed in 10% neutral buffered formalin for histological processing. Paraffin-embedded brain sections were stained with Hematoxylin-Eosin (H and E) stains according to Bancroft’s methods.42
Data were analyzed using SPSS statistical software (version 31).43 One-way ANOVA followed by the Least Significant Difference (LSD) test assessed differences among groups at a significance level of P < 0.05.
The mean serum total antioxidant capacity (TAO-C) concentration in all treated groups is shown in Figure 1. Significant (P < 0.05) increases in serum TAO-C were observed following treatment with piperine (G1) alone (A17.01±0.70a) or PIP-TiO2NPs (G3) (B11.09±0.27c) compared to TiO2NPs (G2) (B 8.77±0.15d) at the 5th weeks of experiment However, when compared with the control group (A17.01±0.70a), all treated groups exhibited a significant (P < 0.05) decrease in TAO-C levels.
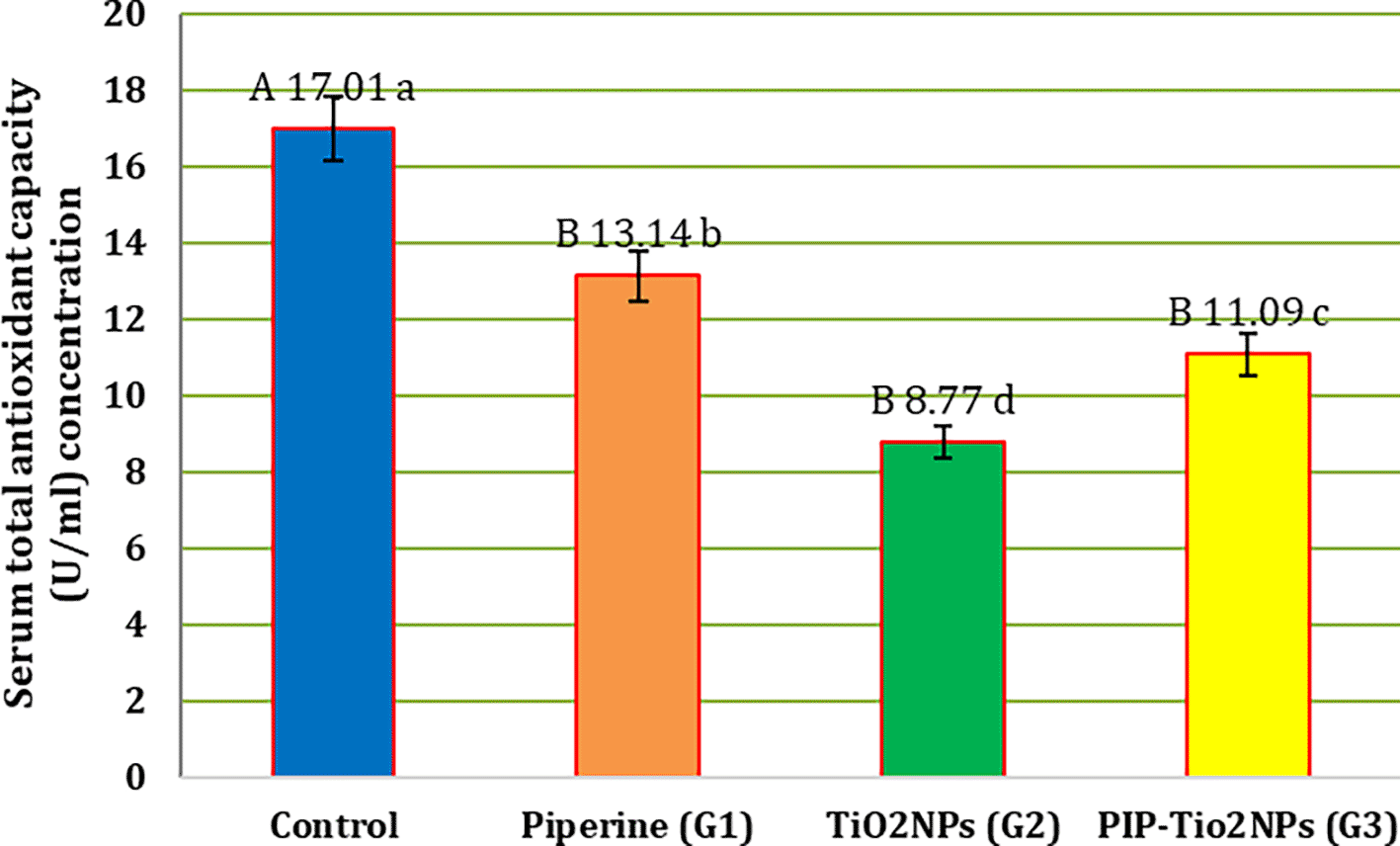
Values are expressed as mean ± SE (n = 10). C: control; G1: piperine 40 mg/kg B.W.; G2: TiO2NPs 50 mg/kg i.p.; G3: PIP–TiO2NPs 75 mg/kg i.p. for 5 weeks.
Figure 2 shows all experimental groups’ serum reactive oxygen species (ROS)levels. The G2 treatment group (intraperitoneal titanium dioxide nanoparticles at a dose of 50 mg/kg/day) showed a significant (P < 0.05) decrease in serum ROS levels (A 530.66 ± 2.19 a) compared to other treatment groups. The result also showed that treatment with G1 (orally piperine 40 mg/kg B.W) (A 434.61 ± 6.42 c) or its incorporation with G3 (i.p PIP-TiO2NPs 75 mg/kg. B.W), a significant (P < 0.05) reduction in this parameter (A 481.71 ± 10.03 b) as contrasted with the G2 treated group. In contrast, the results indicated that these groups (G1 and G3) had a significant (P < 0.05) decline as contrasted with the control group (A 396.45 ± 9.43 d), and a significant (P < 0.05) difference was also observed between G1 and G3.
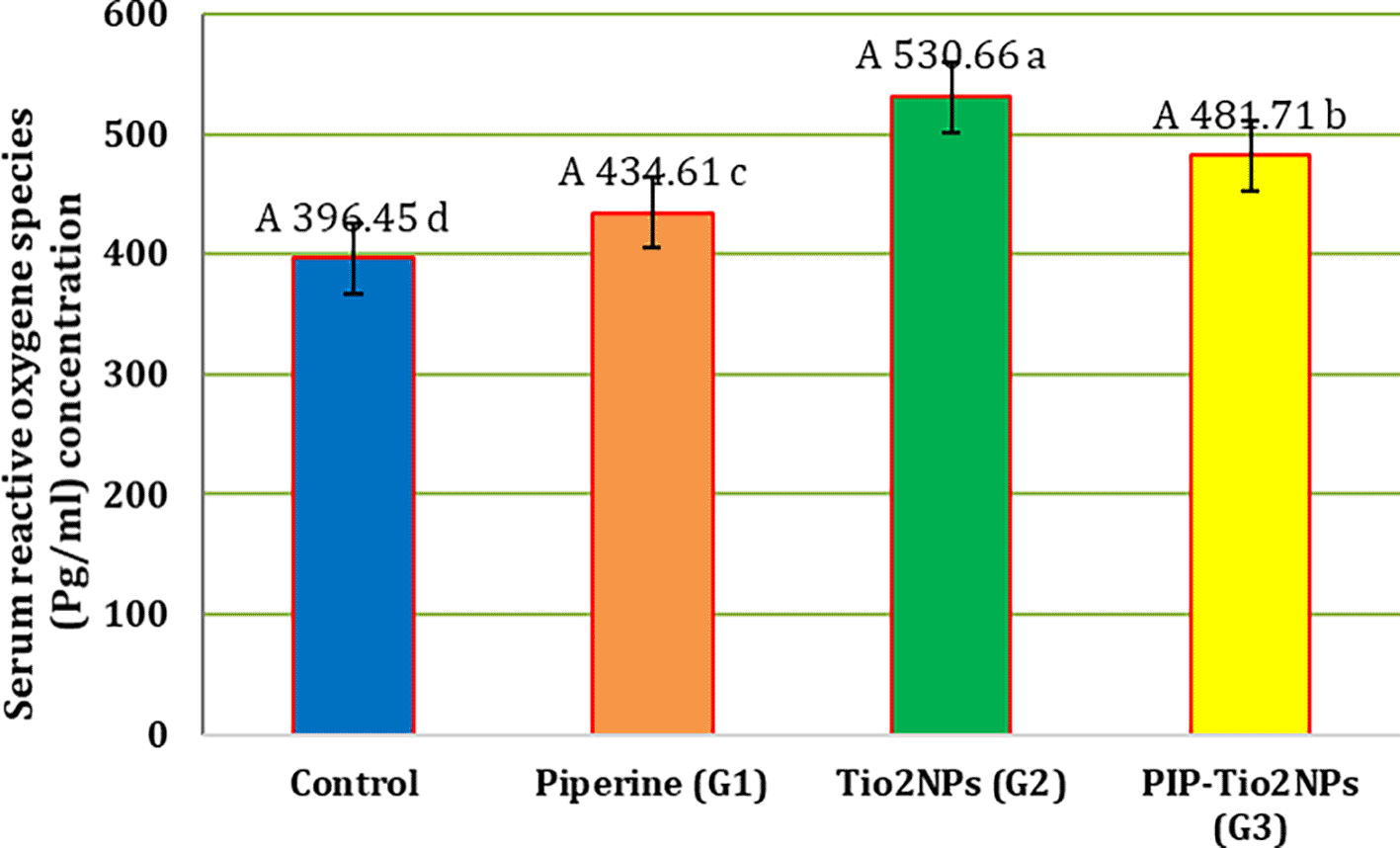
Values are expressed as mean ± SE (n = 10). C: control; G1: piperine 40 mg/kg B.W.; G2: TiO2NPs 50 mg/kg i.p.; G3: PIP–TiO2NPs 75 mg/kg i.p. for 5 weeks.
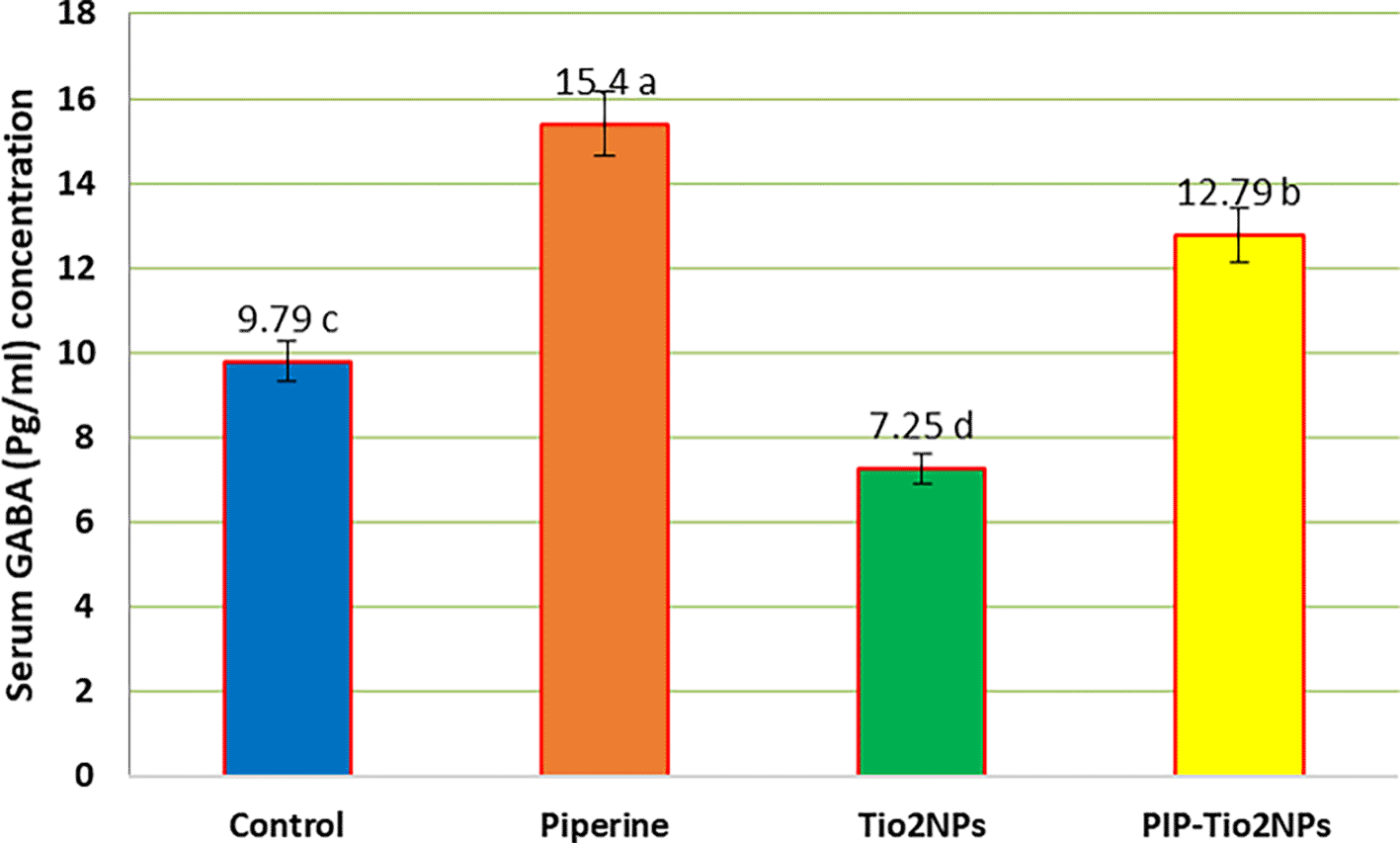
Figures 3, 4, and 5 illustrate the mean serum concentration of neurotransmitters (glutamate, noradrenaline and gamma-aminobutyric acid (GABA)) concentrations of the control and other groups, the result exhibits a significant (P < 0.05) decrease in serum neurotransmitter concentrations in rats treated with TiO2NPs (G2) compared with control and other treated animal groups (Piperine and pip-TiO2NPs). On the contrary, rats treated with piperine (G1) exhibit the highest mean value, indicating a pronounced effect compared to those treated in the G2 and G3 groups after five weeks of the experiment. Also, the rats treated with PIP-Tio2NPs (G3) show an increase in the mean value compared to Tio2NPs alone (G2).
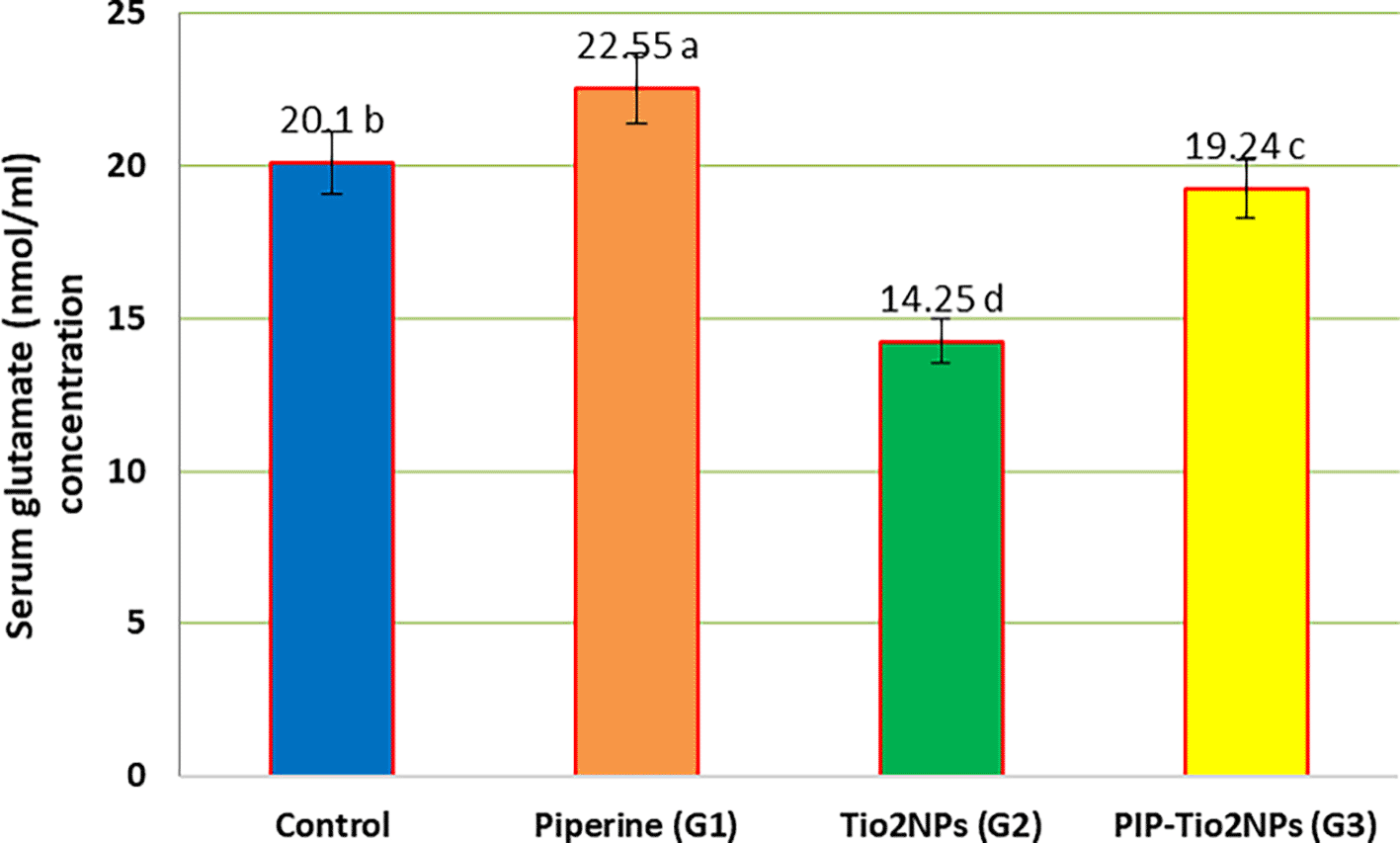
Values are expressed as mean ± SE (n = 10). C: control; G1: piperine 40 mg/kg B.W.; G2: TiO2NPs 50 mg/kg i.p.; G3: PIP–TiO2NPs 75 mg/kg i.p. for 5 weeks.
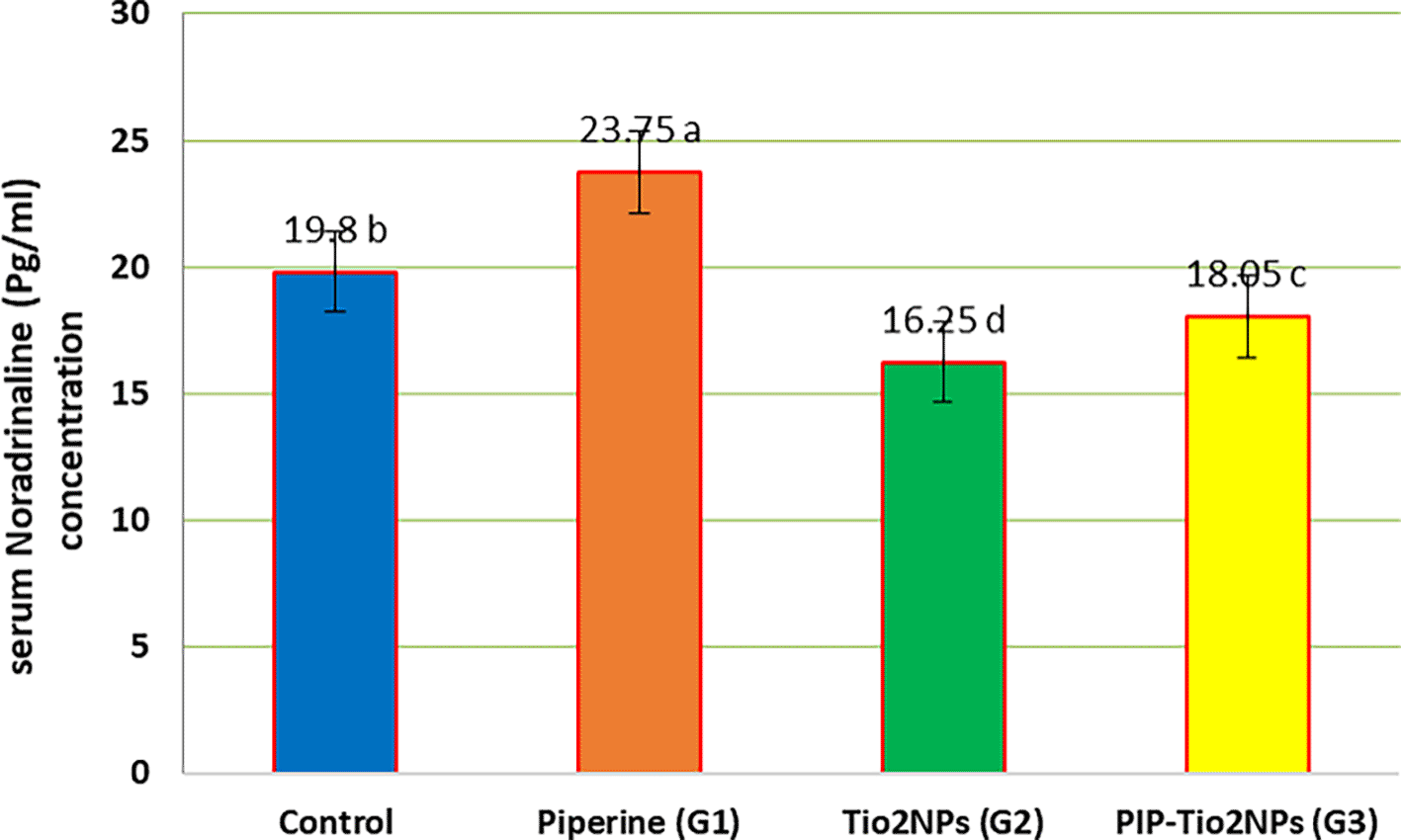
Values are expressed as mean ± SE (n = 10). C: control; G1: piperine 40 mg/kg B.W.; G2: TiO2NPs 50 mg/kg i.p.; G3: PIP–TiO2NPs 75 mg/kg i.p. for 5 weeks.
Values are expressed as mean ± SE (n = 10). C: control; G1: piperine 40 mg/kg B.W.; G2: TiO2NPs 50 mg/kg i.p.; G3: PIP–TiO2NPs 75 mg/kg i.p. for 5 weeks.
Gene expression of the rats’ brain tissue of Cyclooxygenase (COX) in all experiment groups was clarified in Figure 6 (Using the internal reference gene (12S-rRNA) as a sample normalizer). Figure 7 (This indicates a successful RNA extraction and cDNA synthesis), while Figure 9 shows the fold change comparison between the groups expressing the COX gene in the brain. A significant upregulation of COX expression was observed in the TiO2NPs group (G2) compared with the control, and a significant increase in expression of Piperine-TiOeNPs (G3) and piperine (G1) groups. However, Piperine alone produced a moderate yet significant difference in COX relative to the G2 and Control groups regarding gene expression ( Figure 8).
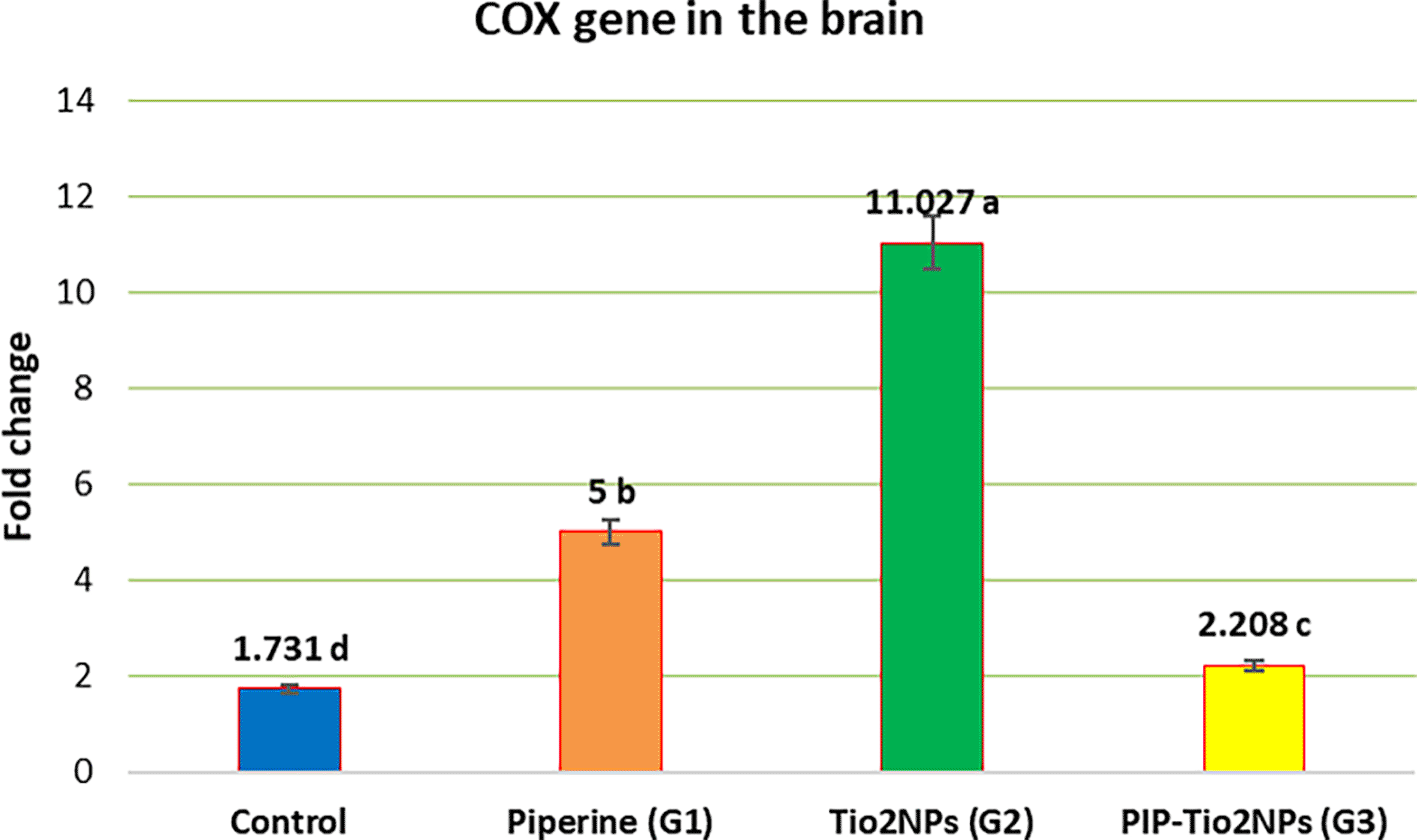
Values are expressed as mean ± SE (n = 10). C: control; G1: piperine 40 mg/kg B.W.; G2: TiO2NPs 50 mg/kg i.p.; G3: PIP–TiO2NPs 75 mg/kg i.p. for 5 weeks.
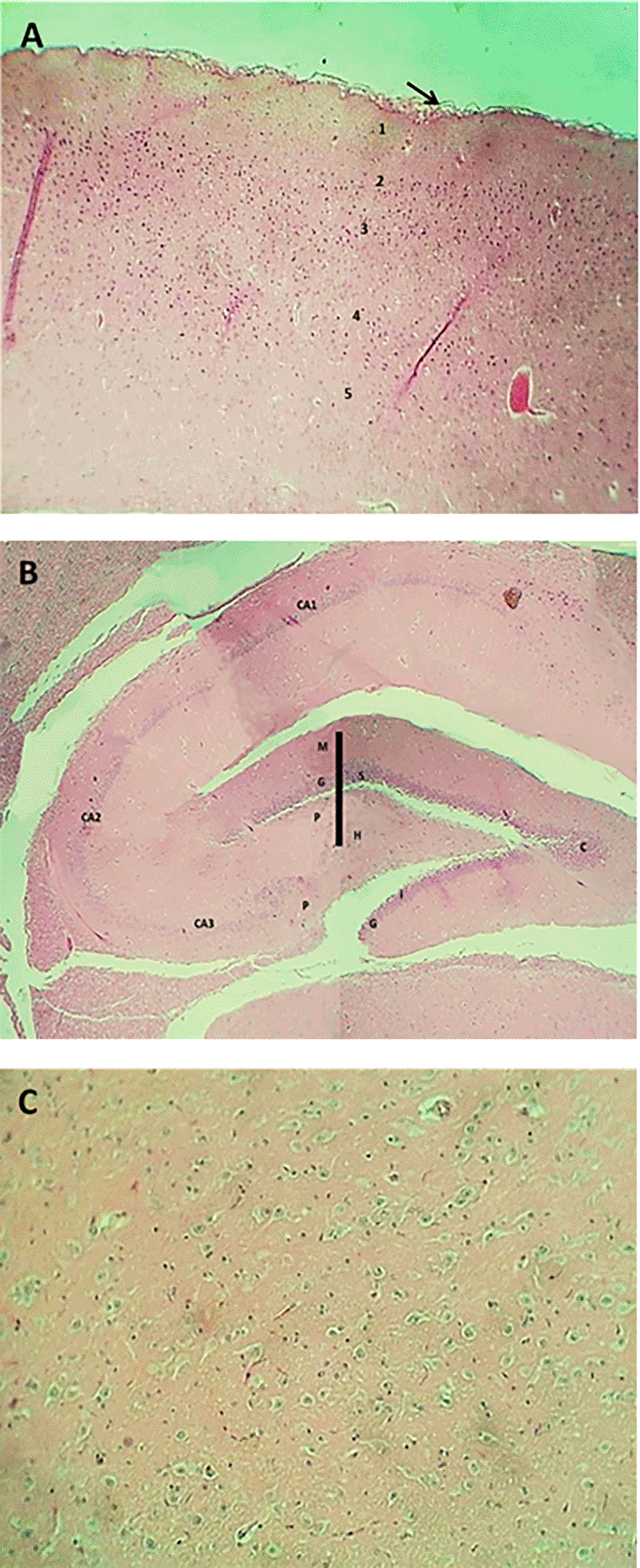
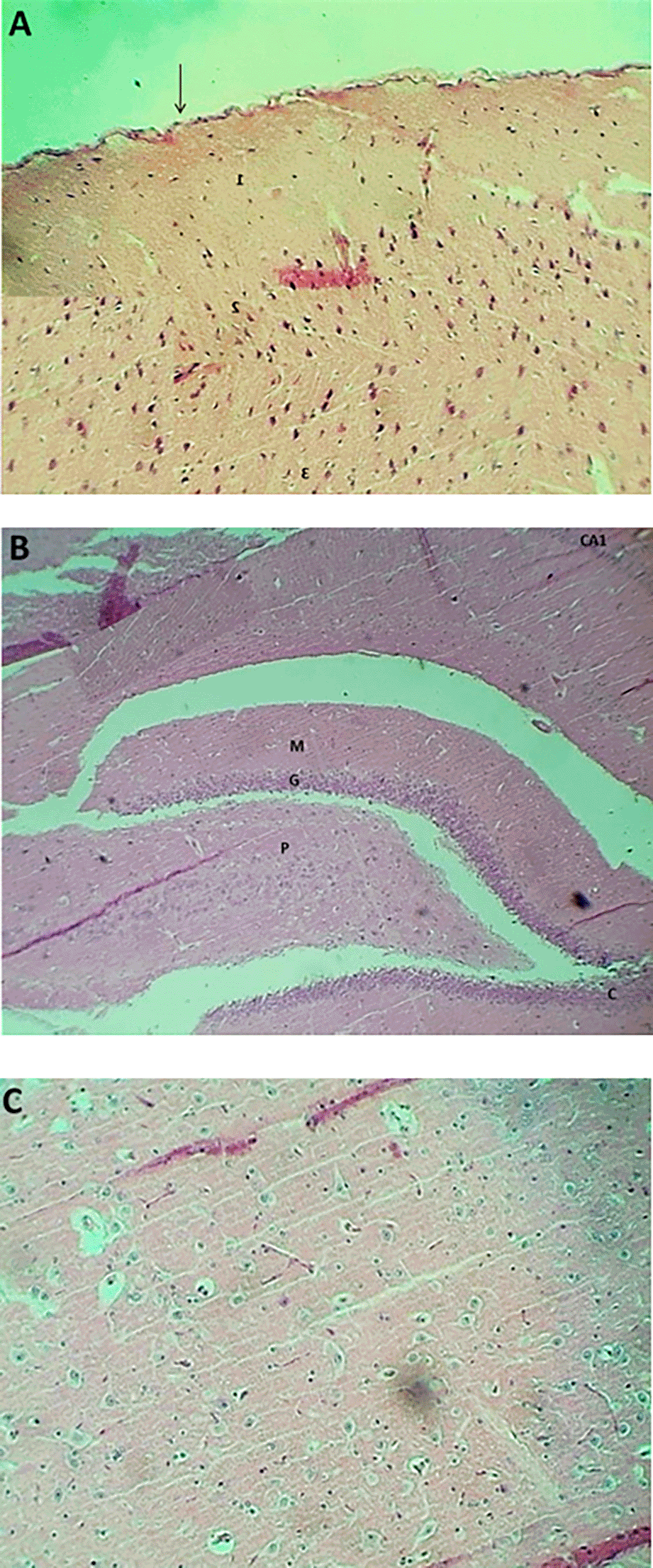
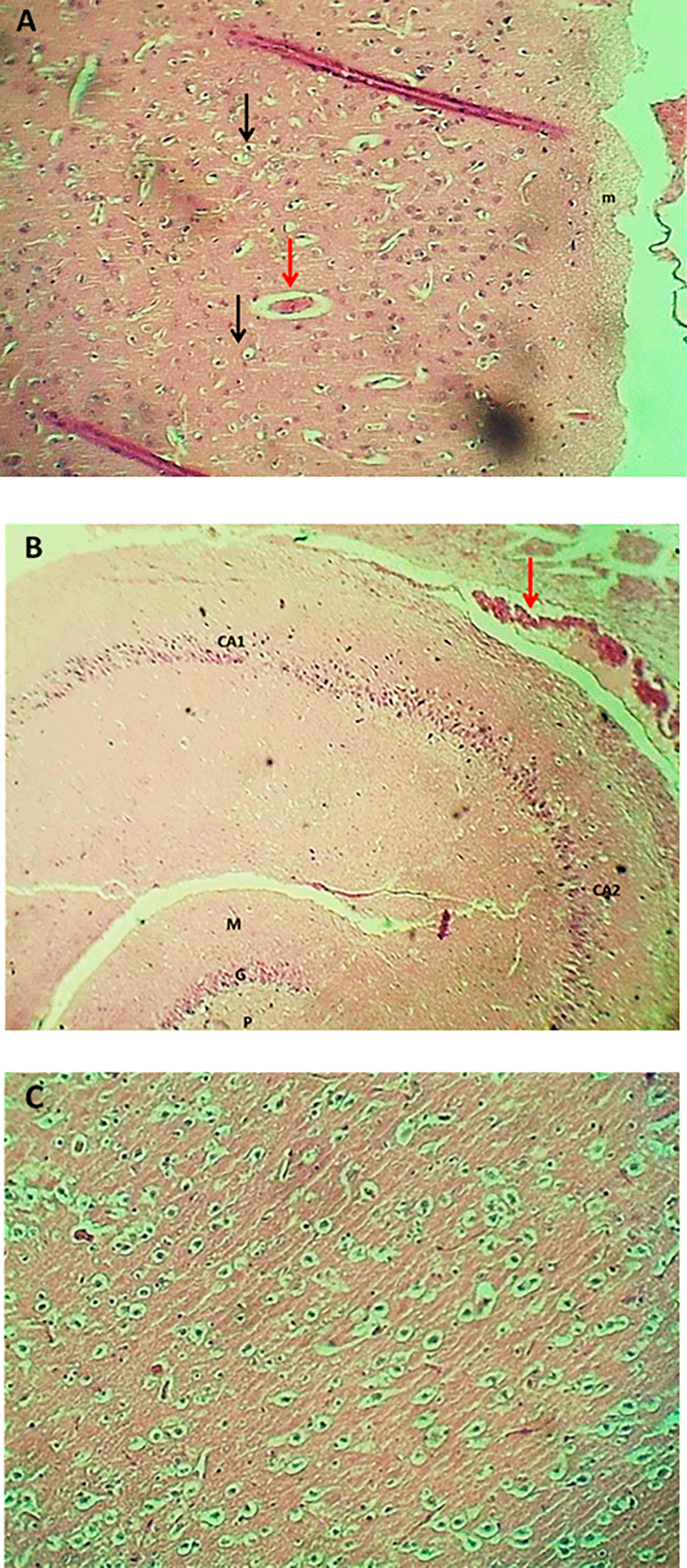
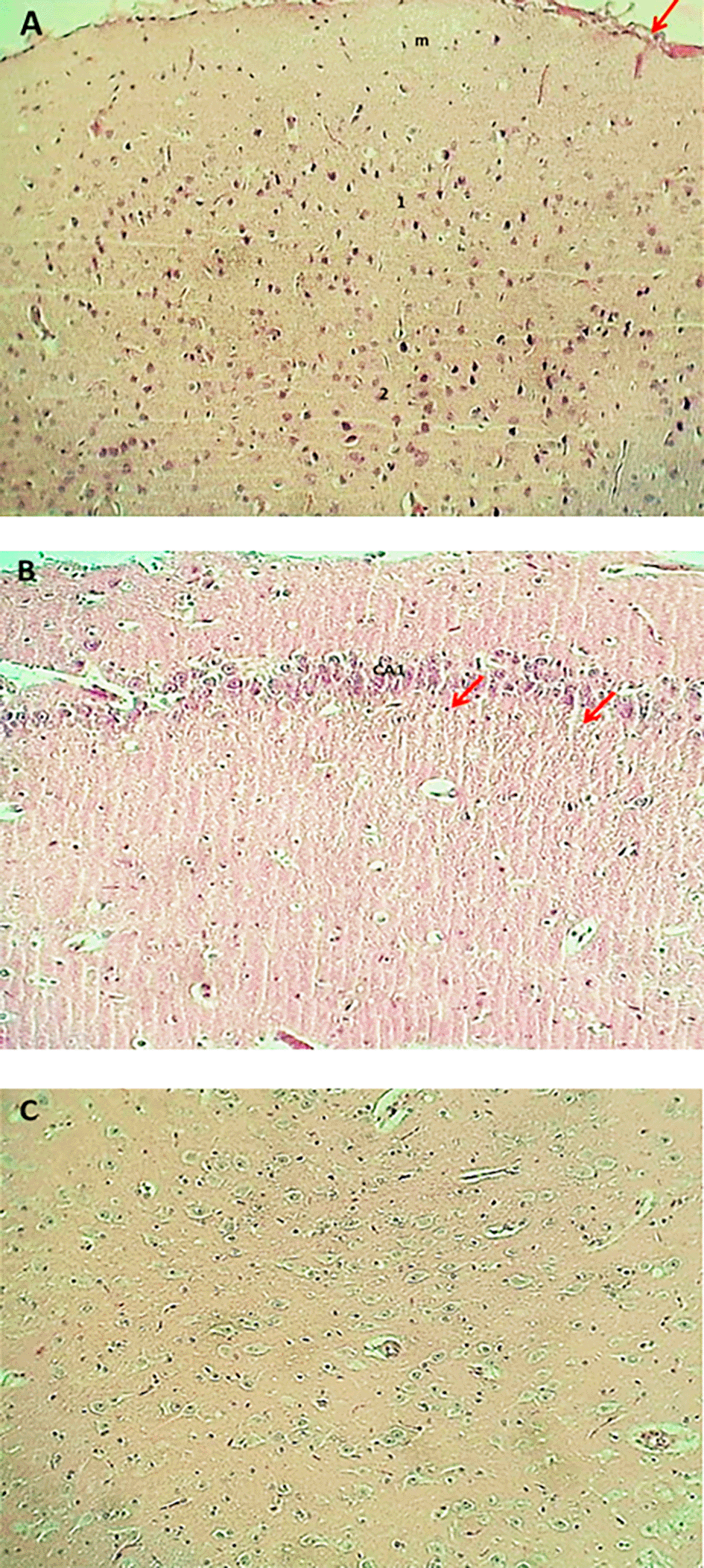
The result of the histopathological examination of the cerebral layers of the control and G1 groups (Figures 9-A and 10-A) demonstrated the typical appearance of the ganglionic, multiform, pyramidal, granular layers as well as the molecular layer, and normal glial cells with axon tracts of subcortical white matter. The hippocampus revealed a normal, distinct C-shape that reveals its layered structure, including the encompassing the Dentate Gyrus (DG) and the Cornu Ammonis (CA) areas (CA1, CA2, CA3, CA4), these areas are consistent with densely packed pyramidal neurons in the stratum pyramidale (Figures 9-B and 10-B). The thalamus also showed commonly appearing nuclei (Figures 9-C and 10-C). The histopathological results in the G2 group figures showed the cerebral layers, mild vascular congestion with little hyperplasia of glial cells within the external pyramidal cells layer, and mild demyelination of the molecular cells layer ( Figure 11-A). The figures of hippocampus showed normal neurons of CA1, two areas, normal molecular layer, granular layer, and polymorphous layer (P) of dentate gyrus, and congestion of choroid plexuses ( Figure 11-B). The thalamus also showed normal-appearing nuclei ( Figure 11-C). The G3 group histopathological figures of the cerebral layers showed normal appearance of meningeal pia mater and other cortical cerebral cell layers ( Figure 12-A). The figures of the hippocampus showed normal neurons of CA1, 2, and 3, and the figures showed moderate vaculation with glial depletion of the molecular strata of the CA1 proper hippocampus ( Figure 12-B). The thalamus also showed normal-appearing nuclei ( Figure 12-C).
A significant decrease in TAO-C and an elevation in ROS levels in the TiO2NPs (G2) treated group in the current study indicated TiO2 toxicity and a case of oxidative stress documented by Refs. 44 and 45. The following can be used to explain how TiO2NPs cause toxicity in organisms: LPO, the generation of ROS, and the electrostatic attachment of TiO2 nanoparticles to the cell membrane.46 Due to the surface area and other physical and chemical properties, TiO2 has a high capacity to generate reactive oxygen species (ROS).47,48 In addition, the toxicity of the TiO2NPPS may result from different doses, particle size, cultural medium, or test techniques.49,50 Due to their small size, large surface area, and hydrodynamic diameter, a small nanoparticle was more harmful than a large one.51 TiO2NPS is added to oxidative damage, protein oxidation, antioxidant function deficiency, and elevated brain oxidative stress.52,53 Apoptosis,54 nuclear envelope shrinkage,55 changes in the structure of macromolecules and microelements, such as copper (Cu), potassium (K), and zinc (Zn).56
The outcomes in Figures 1 and 2 confirmed a high TAO-C level and low ROS levels in the organization handled with PIP, indicating its antioxidant impact. These findings were consistent with a few studies.57–59 Besides, the capability to lessen oxidative stress is more for piperine amino acid derivatives than PIP itself.60 Numerous investigations have revealed that PIP can defend against high ranges of oxidative markers.61–63 who pronounced the co-administration of piperine with curcumin, showed reduced lipid peroxidation (LPO) and growing endogenous antioxidant enzyme levels. The capability of PIP to decrease in LPO of the mitochondrial membrane, similarly to an increase in antioxidant enzymes, PIP became utilized to deal with issues related to mitochondrial oxidative stress.64 It needs to be stated that the antioxidant property of PIP will be attributed to its antiradical and loose radical scavenging capability.65
Serum neurotransmitters concentrations analysis (Glutamate, Noradrenaline and GABA) in this study confirmed a significant differences in the values of these parameters in group of rats dealt with PIP (G1) by myself or as mixture with Tio2NPs (G3), these findings imply the function of piperine or their aggregate with TiO2NPs (PIP-TiO2NPs) in attenuated the damaging effects of TiO2NPs on a few physiological mind features, these result agreed with Ref. 55 who suggested using 2.5 mg/kg/day of PIP for 4 weeks, enhanced rats’ persistent potentiation and memory test performance; chronic PIP treatment also produced more effective synaptic plasticity compared to the achieved with donepezil.66 In the meanwhile, PIP therapy for 23 days also improved neurotransmission and locomotor activity by lowering oxidative-nitrosative stress in the hippocampus and cerebrospinal fluid and improving cholinergic function in a rat model of Alzheimer’s disease (AD).14,66 Ref. 14 who documented how piperine affected the redox state of CSF and hippocampus neurones, may undoubtedly be a factor in the medication’s ability to improve cognition. Additionally, by lowering acetylcholinesterase function, a plaque, and tangles, PIP solid lipid nano-formulation enhanced blood–brain barrier permeability and increased mobility in AD mice,67 these finding agreed with results in this experiment of G3 treated rats with piperine or piperine-Tio2NPs combination (i.p PIP-TiO2NPs 75 mg/Kg. B.W) and this may be result in enhance its potentiality in some brain dysfunctions treatment as mentioned in many studies as68 indicates that PIP administration lowered lipid peroxidation and raised antioxidants in the striatum of 6-hydroxydopamine (6-OHDA)-lesioned rats69; also found that PIP usage diminished neuronal damage in the substantia nigra in rotenone-injected PD mice and generated atuophagolysosome via Akt/mTOR pathway activation; a PIP-mediated increase in protein phosphatase 2A (PP2A) improved mitochondrial injury, mitochondrial membrane permeability transition pore dysfunction, and oxidative stress.70,71 According to Ref. 72, this alteration also increased the effectiveness of PIP derivatives in activating the Nrf2/Keap1 pathway, which provided neuroprotection in the Parkinson’s disease model and subsequently regulated brain activities. In Huntington’s disease rats, PIP therapy improved motor characteristics, striatal degeneration inside the basal ganglia, and ATP production.73 PIP therapy improved serotonin stages and adjusted the glutamate, noradrenaline, monoamine oxidase, and gamma-aminobutyric acid (GABA-ergic) pathways.74 Additionally, within the brain and serum of a pilocarpine-precipitated epileptic mouse model, PIP remedy decreased nitrite and multiplied GABA, glycine, and taurine.75
The current experiment saw a significant reduction in serum neurotransmitter concentrations in mice administered TiO2NPs (G2) compared to control and other treated groups treated groups. These results have been linked to neurodegenerative disorders, and exposure has been shown to interfere with presynaptic production and reduce the release of neurotransmitters. TiO2NPs poisoning must mainly be seen as targeting the brain, according to the growing research part.76,77 According to several studies,78,79 the TiO2NPs can form in the brain after risk. Similar to this, TiO2 has been demonstrated to easily build up in the brain after oral exposure because of its capacity to penetrate the blood-brain barrier. Consequently, it may trigger oxidative stress, apoptosis, neuronal degeneration, and neuroinflammation; these findings agree with results that showed in this study, which indicate the significant decreases in serum concentrations of neurotransmitters (G2) as a result of this exposure.80 Besides,81 showed that while oral TiO2NPs have been proven to cause oxidative stress, neuroinflammation, and altered neurotransmitter metabolism, they also are known to promote apoptosis in hippocampus, cortical, and cerebellar neurones. It has been demonstrated that TiO2NPs affect the metabolism of neurotransmitters, particularly dopamine and glutamate/glutamine.82 When mice were exposed to TiO2NPs, glutamine levels and glutamine synthetase activity decreased, but hippocampus glutamate release and phosphate-activated glutaminase activity significantly increased. Furthermore, it has been demonstrated that different types of TiO2NPs, in conjunction with oxidative stress and mitochondrial dysfunction, cause primary astrocytes to have reduced glutamate uptake.83 When combined, these results suggest that rats given Tio2NPs (G2) throughout the experiment had reduced glutaminergic neurotransmission.
Likewise, it has been demonstrated that exposure to TiO2NPs alters the metabolism of various neurotransmitters, including catecholamines in the brain. In particular, TiO2 exposure raised the levels of monoamine neurotransmitter metabolites, which are a sign of increased neurotransmitter catabolism, while significantly lowering noradrenaline and serotonin levels in the hippocampus, cerebral cortex, cerebellum, and striatum as well as dopamine content in these regions.84,85 Despite this Ref. 86, showed that oral gavage of TiO2 NPs resulted in a substantial change in the adrenergic, cholinergic, dopaminergic, and serotonergic neurotransmitter systems by lowering brain levels of noradrenaline, serotonin, and dopamine.
As demonstrated in this study, titanium promotes the generation of free radicals, which is expected to induce tissue inflammation. The purpose of this study is to investigate the physiological effects of titanium on the brain, particularly its impact on neural tissue activity and cellular function, by analyzing neurotransmitter concentrations in addition to the study assesses tissue-level damage in the brain as evidenced by COX-2 (cyclooxygenase) expression and its levels in brain tissue. Numerous intrinsic and external stimuli can cause the inflammatory response, which is catalysed by the primary enzyme cyclooxygenase-2 (COX-2).87,88 It acts as an important chemical bridge between the development of chronic inflammation and the reactive oxygen species (ROS) (ROS).89 In the treated group (G2), mice showed a significant elevation of COX-2 gene expression in their brain tissue, which supports it. This result corresponds to previous research and shows that an increase in COX-2 expression promotes prostonoid production.90,91 According to Ref. 92 are powerful regulators for brain inflammation and vascular activity. Although neurons are constitutively COX-2, other brain cells, including astrocytes, microglia and vascular endothelial cells, usually have low basic levels of COX-2, but increase strongly under inflammatory conditions.93 In addition, increased COX-2 expression is promoted in the brain by pathological conditions such as cerebral ischemia and hypoxia.94
Piperine or its combination (PIP-TiO2NPS) treated mice in groups (G1 and G3), so a significant reduction in the level of COX-2. These findings correspond to these studies, which have shown that Piperine prevents large inflammatory enzymes such as 5 -lipoxinas and cycloxicinez -1, suggesting its potential role in modifying inflammation.22,95 In addition, Piperine has cytoprotective, antioxidant and neuroprotective activities, as shown in TAO-C and ROS results.96 Despite these promising medical effects, data on Piperine painkillers, anti-inflammatory and antipyretic properties are limited.97 Also Ref. 98, demonstrated that piperine may act as a potent COX-2 inhibitor, contributing to its anti-inflammatory effects. According to Ref. 99, piperine decreased the expression of the COX-2 gene and protein in tumor cells. The formation of prostaglandin E2 (PGE2) is catalyzed by the enzyme COX-2, which binds to its corresponding G protein-coupled membrane receptors. All cell tumors showed decreased gene expression in these receptors, suggesting that COX-2’s effect on these receptors is diminished. These findings showed why the COX-2 gene expression decreases in the animals treated with piperine or their combination (PIP-TiO2NPs), followed by enhanced physiological functions of brain cells, antioxidants and neuroprotective effects.
The findings of the histopathological study in the PIP-treated group (G1) showed normal architecture of brain tissues (cerebral cortex, Hippocampus, and Thalamus) and enhancement of cellular activity as compared with rats treated with PIP-TiO2NPs or with TiO2NPs; this is consistent with the above tests for neurotransmitters and antioxidant parameters, this finding align with100 reported that the Piperine dramatically boosts quercetin’s antioxidant, anti-inflammatory, and neuroprotective benefits in rats given iron supplements and rotenone to cause Parkinson’s disease.101 The presented histopathological results from the G2 group, treated with titanium dioxide-nanoparticles (TiO2-NPs), reveal a nuanced picture of early-stage neurotoxicity characterized by selective damage and a clear glial response. The conclusions are fine because of their mild and specific nature, an exposure model with underwear or low-khurak suggests. There is increasing evidence that titanium-based products can be neurotoxic. For example, memory loss in the mouse model after intravascular delivery of titanium implantation or titanium nanoparticles (TiO2NPs) was reported by Ref. 102. Blood-brain barrier (BBB) is compromised. This is a theory that is excluded to explain this effect. This is confirmed by research by Ref. 103, where it was found that TINAP improved paracellular permeability in the in vitro human BBB model. This discovery is compatible with the lower level of the significant BBB band in the hippocampus of mice treated with TiO2NP. Many ways can cause tiotoxicity. In addition, new research by Ref. 104 indicates that other procedures are likely to include, such as the regulation of the intestinal brain axis through epigenetic modification and intestinal microbiota changes. Some studies, including Ref. 105 no average amount of tension in brain tissue, which indicates that the basic perception of brain translation is not widely accepted.
Histopathological consequences in a combined mouse combined by PIP-TiO2NPS (G3 group) showed the protective effect of Piperine to reduce the toxic effects of TiO2NPs on brain tissue, which was not the same by reducing tissue damage compared to TiO2NPs alone, because it did not show that it did not show that it showed on the tissue of the tissues. Piperine-titanium dioxide nanoparticle (TiO2NP) shows neuroprotective properties against induced brain damage, mainly by reducing oxidative stress. This discovery matches,106 which showed that TiO2NPS causes brain injury, triggers neuroinflammation, and leads to neuronal apoptosis, while Piperine can reverse these effects, improve cognitive function, and restore redox balance.107 suggest that in different types of brain cells, there were noticeable variations in sensitivity to the harmful effects of TiO2NPS. Especially compared to the study, it was shown that both acute and long-lasting low dosage risk for TiO2NP made neuronal cells more resistant than the human glial cell line.108 In the animal model for AD, both control checks control both lipid peroxidation of piperine enzymes and non-enzymatic antioxidant levels as well as hippocampus pyramidal cells.109 In addition, it was noted that Piperine increased the amount of brain-derived neurotrophic factor (BDNF) and the growth of the hippocampus precursor cells. This means that it puts tissue and neuron cells in one or a combination due to damage caused by TiO2NPS.97 The findings indicate that piperine, either alone or in combination with TiO2NPs (PIP-TiO2NPs), is protective and reduces the damaging effects of TiO2NPs on some physiological brain functions and oxidative stress.
This study was conducted in the Animal House of the College of Veterinary Medicine and was approved by the Ethical Committee of the College of Medicine, University of Fallujah (ethical approval number: 11, dated 5/01/2025) before commencing the research work. All animals were treated according to the standards of the Ethical Committee. All information was anonymized and used for research purposes only.
Each the scientific information generated and analyzed throughout the current study are included in the study’s article. The article includes a detailed description of every testing technique, methodology, dose, and laboratory environment. Replicating the study doesn’t require any more unreported data. As a consequence, the reader may easily access all pertinent information in the book. Researchers can reach the corresponding author at dr.physiologist_med@uofallujah.edu.iq and with any additional questions.
The ARRIVE (Animal Research: Reporting of in vivo Experiments) principles were followed in the planning and documentation of this inquiry in order to guarantee reproducibility and transparency in animal research. For this publication, Pathophysiological Effects of Titanium Dioxide Nanoparticles and the Protective Role of Piperine and a Piperine-TiO2NP Combination in Rats, the completed ARRIVE checklist has been posted to an online archive. ARRIVE 2.0 checklist for Pathophysiological Effects of Titanium Dioxide Nanoparticles and the Protective Role of Piperine and a Piperine-TiO2NP Combination in Rats. [DOI 10.5281/zenodo.18220968]. Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
The authors wish to express their deepest gratitude to the College of Veterinary Medicine and the College of Medicine, Fallujah University for their valuable support in providing the necessary animal housing facilities and scientific laboratories to conduct this study.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)