Introduction
Lack of control over stressors may be a predisposing factor for anxiety disorders, such as post-traumatic stress disorder (PTSD), depression, and drug dependence. However, not all individuals who experience stress develop these disorders suggesting a mechanism for resilience1. To explore this relationship at a neurobiological level, we utilized the stressor controllability paradigm2,3. Behavioral control over tail shock in rats prevents the physiological and behavioral consequences of equivalent uncontrollable shock as measured by anxiety tests (social interaction, shuttle box escape times, etc)4. Moreover, a week after escapable shock (ES) treatment, if the rat is subjected to an uncontrollable stress situation, such as social defeat or inescapable shock (IS) treatment, the rat will still behave as a naïve home caged (HC) rat on anxiety tests5, a process termed “behavioral immunization”6. The ventral medial prefrontal cortex (vmPFC) has been implicated in the protective effects of ES through a series of pharmacology experiments controlling cortical excitability. Inactivation of the vmPFC by GABA agonists during ES treatment results in a loss of the typical protective effects; conversely, pharmacological activation of the vmPFC with GABA antagonists during the IS treatment results in a gain of the protective effects7,8. We have previously shown that after two hours post-treatment, the intrinsic excitability of the deep layer vmPFC pyramidal neurons is increased in rats that received ES but not IS treatment compared to naïve HC rats9. However, the balance of excitation/inhibition is what ultimately determines the overall excitability, we therefore set out to gather data on the overall state of the inhibitory circuit by recording spontaneous inhibitory postsynaptic currents (sIPSCs) from the layer 5/6 pyramidal neurons. This in turn will allow us (and other groups) to build detailed computer simulations to better understand the cortical circuitry, and how inhibitory plastic changes occur shortly after stressful situations.
Materials and methods
Rats
Twenty seven adult (60–70 days old and weighing 275–350 g at the time of testing) male Sprague-Dawley derived rats were bred and reared at the University of Colorado, Boulder, USA. Rats were housed with free access to food and water in groups of two or three in a vivarium with a 12-hour light/dark cycle. Stress exposure occurred in the first four hours of the light phase. All behavioral procedures were approved by the University of Colorado Institutional Animal Care and Use Committee. Eleven rats received ES treatment, 7 received IS treatment and 9 rats remained in their home-caged environment.
Stress induction procedures
Rats received ES, IS or remained naive. One hundred electric tail-shocks were administered through copper electrodes augmented with electrode paste by a Precision Regulated Animal Shocker (Coulbourn Instruments, Allentown, PA, USA) to rats restrained in 14 × 11 × 17 cm (length × width × height) acrylic boxes with a wheel 7 cm wide and 9.5 cm in diameter located on the wall opposite the tail. Each box was enclosed in a sound-attenuating chamber. Tail-shocks were presented on a variable interval-60 second schedule (VI-60). For rats that received ES, turning the wheel at the front of the chamber terminated each tail-shock after ¼ turn of the wheel. If the response was performed within 5 seconds of shock onset, the response requirement doubled for the next trial and proceeded until a maximum of 4 wheel-turns was reached. If a response was made after 20 seconds the requirement was reduced by half; if no response was made by 30 seconds the shock was terminated and the requirement was reset to ¼ turn. In order to maintain escape behavior the shock intensity was 1.0 mA for the first 33 trials, 1.3 mA for the following 33 trials and 1.6 mA for the remaining 34 trials. Since only one rat per day could be used in electrophysiology studies, we chose to deliver IS on a schedule generated from archival ES escape latency data8. On a trial-by-trial basis, the mean escapable latency, the standard deviation, and the maximum latency and minimum latency were computed from a random sample of 10 archival ES subjects. Each rat in the IS group received a unique series of 100 shocks. Shock durations were computed by taking a random interval from between the average minimum and the trial mean ± standard deviation. If the maximum shock length was less than the mean + standard deviation, then the maximum shock length served as the range maximum. The algorithm produced a series of shocks akin to those generated by the ES rats included in the patch clamp studies. Animals in the HC control group remained in their cages.
Solutions and drugs
Standard artificial cerebrospinal fluid (aCSF) and recording solutions were used; aCSF composition was (in mM) NaCl 124, KCl 2.5, NaHCO3 26, MgCl2 2, CaCl2 2 and glucose 10; pH = 7.4; 310 mOsm/l and the recording solution (in mM) CsCl 115, HEPES 10, KCl 20, MgCl2 2, Na2ATP 3, Na2GTP 0.3, biocytin 0.1%, pH = 7.3, 280 mOsm. 0.3% Biocytin (Sigma) was added to the recording solution to visualize the recorded cell type and location.
Prefrontal cortex slices
Two hours after shocking, ES and IS rats were anesthetized with isofluorane (MWI, Boise, ID, USA), perfused with aCSF (4°C) and their brains were extracted. Coronal slices (300 µm) were taken from the prefrontal cortex using a vibratome (VT-1000P, Leica Microsystems, Nussloch, Germany). Slices were placed in oxygenated aCSF (95% O2) at 35°C for 30 minutes and then kept at room temperature until slices were removed for electrophysiological recordings.
Electrophysiology
Whole-cell voltage-clamp recordings were obtained at 33 ± 2°C. Patch-clamp electrodes were pulled (Flaming/Brown P-97, Sutter Instruments, CA, USA) from 1.5 mm outer diameter borosilicate glass (Sutter Instruments, CA, USA) and filled with a CsCl-based intracellular solution. Electrode resistance was 3–5 MΩ in the bath and series resistance was less than 30 MΩ during the recordings. Slices were visualized using a 40× water immersion objective (Zeiss, Germany) mounted on an Infinity-tube FM-100 (Infinity Photo-Optical Co, Boulder, CO, USA). Voltage-clamp recordings were obtained with a BVC-700 amplifier (Dagan, Minneapolis, MN, USA), with no compensation. Data acquisition and analysis were performed using custom software written for Igor Pro (Wavemetrics Inc., Lake Oswego, OR, USA). Inhibitory postsynaptic current (IPSC) analysis was performed using a custom analysis program developed in the Cooper laboratory as described below.
Histology
After recording, the slice was removed from the recording chamber and placed in 4% paraformaldehyde. Recorded neurons were visualized by incubation with the Vectastain PK-6100 ABC reagent (Vector labs, Burlingame, CA, USA) and di-amino benzidine (DAB, Sigma-Aldrich) solution (1 tablet/ 5 ml). Slices were floated onto glass slides and cover-slipped with DPX mounting solution (Sigma-Aldrich). Only neurons with a pyramidal morphology and soma in prelimbic (PL) layers 5/6 were included for analysis.
Results
Customized detection and analysis of spontaneous inhibitory post-synaptic currents using Igor Pro
In order to analyze the data we developed a new script for the scientific/engineering program Igor Pro. The advantages of such a script are as follows: 1) It allows for multiple events analysis. This is particularly important for analyzing sIPSCs, since a large number of interneurons in the cortex are fast spiking or bursting10 and this results in a large number of “multiple” events. 2) It incorporates seamlessly with Igor Pro, a program used by many groups to collect and analyze electrophysiological data. This allows the program to analyze the collected traces within the experiment file and allows us to take advantage of the included mathematical and graphical functions in Igor Pro. 3) It is fully modifiable, any user knowledgeable in Igor Pro programming can add or remove any features that are needed for their particular purposes. 4) During the user-controlled portion of the program, it allows the user to manually define events that were not detected automatically, and finally, 5) The script is available at no charge.
The events are detected by analyzing the first and second derivative of the raw data trace. The raw data is smoothed using a binomial (Gaussian filter) method11 using a 500 point sliding window and the point-to-point derivatives are calculated using the functions included in Igor Pro (Euler method12). The first derivative represents the change in the trace results in peaks that are at the beginning of the event (Figure 1), while the second derivative maximums are where the local minimums are located. A simple algorithm checks if the putative peak is the lowest (highest for outward events) point in a 5 ms region and corrects the event detection accordingly. During the user portion of the program, the user can accept detected events, add events or reject events. The user is also able to correct the baseline and peak of the current analyzed event. Alternatively, the user may opt not to check the proposed analysis and accept the given solution by the program without checking. The program output consists of probability histograms for amplitude, inter-event interval (IEI) and frequency of events, their respective cumulative distribution curves and an average event trace from where the decay time constant is calculated. The user at this point is able to place constraints to the analyzed data by rejecting events less than a certain amplitude, for example, if smaller events are within the noise level. All data are then summarized and presented in a single page layout.
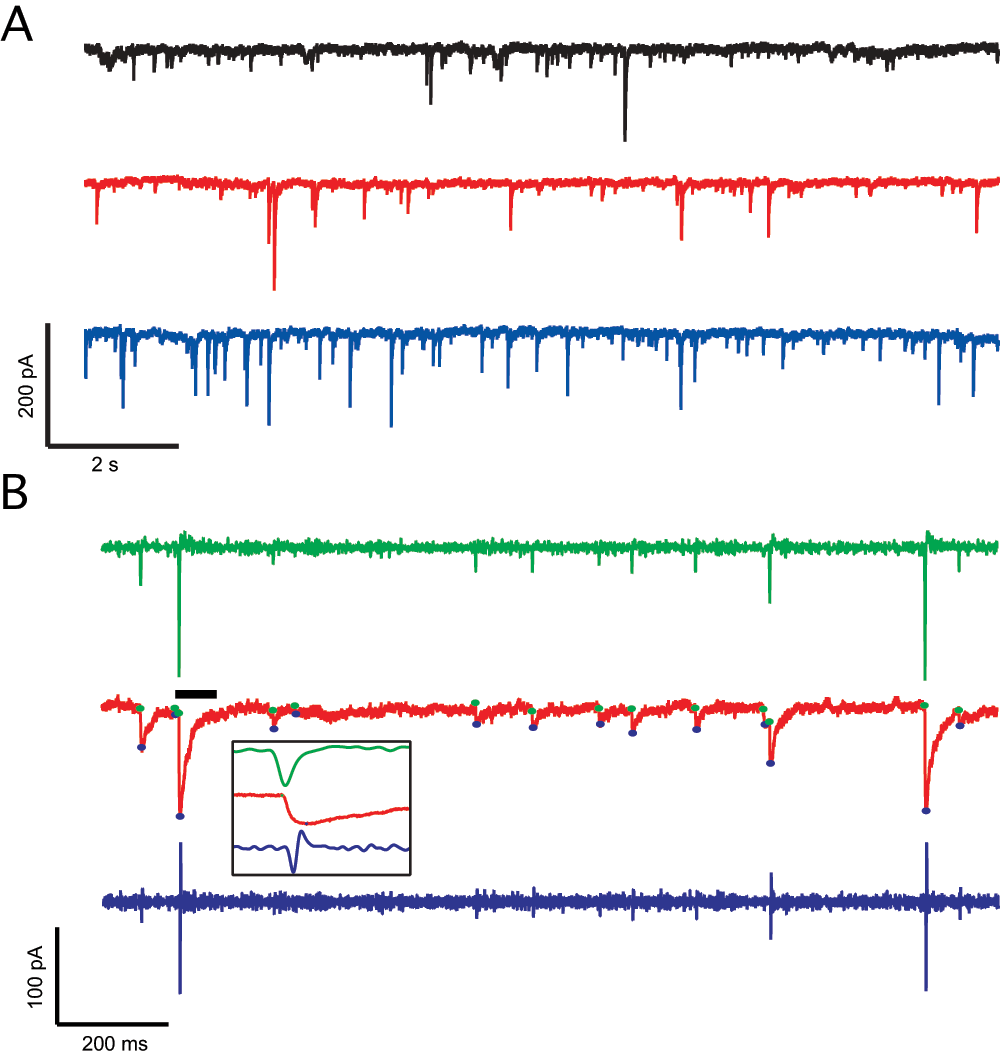
Figure 1. Detection of spontaneous inhibitory synaptic currents (sIPSCs) using the custom IGOR Pro script.
A. Sample traces from the three different groups: Home caged (black), escapable shock (red) and inescapable shock (blue). B. ISPC analysis, from the raw data (red) the first (green) and second derivative (blue) are taken. The peaks of the first derivative indicate the fast change in current in the raw data and therefore indicate the event initiation, the maximum in the second derivative indicates the local minima or the peak of the event; utilizing this information, the baseline (green circle) and peak (blue circle) are calculated. Afterwards, the program allows for the acceptance or rejection of the proposed events. Inset: Expanded event and derivatives (black bar).
Quantifying inhibitory postsynaptic currents (IPSCs) following controllable and uncontrollable stress in the prelimbic (PL) region of the prefrontal cortex (vmPFC): In order to understand the overall state of the inhibitory circuit in the PL we studied sIPSCs frequency and amplitude from pyramidal neurons in the deep layers of the cortex (Figure 2). The sIPSCs from home cage (HC, n=11), IS (n=7) and ES (n=9) groups were measured using the detection method described above (also see data set). Descriptive cumulative histograms of the amplitude and frequency of sIPSC events show an increase in the sIPSC frequency, but not amplitude under IS conditions. The increased sIPSC event frequency appears to be reduced in the ES group.
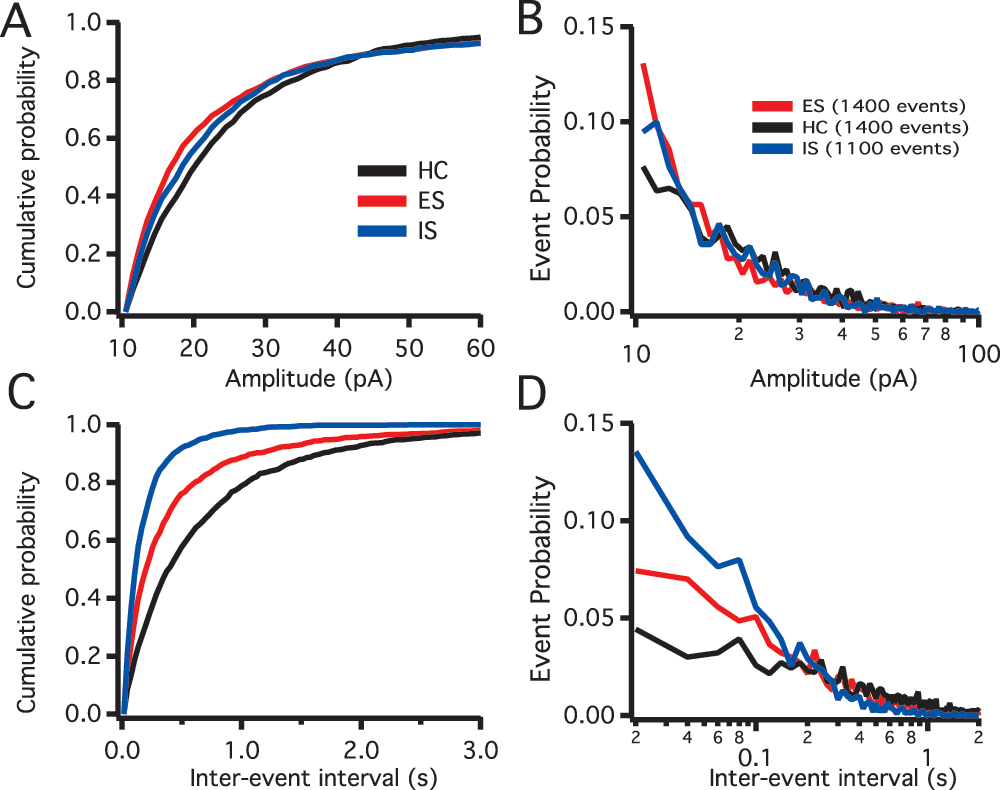
Figure 2. Spontaneous inhibitory synaptic current (sIPSC) distributions.
A. sIPSCs amplitude in pico amperes (pA) cumulative distributions for the different treatments, home caged (HC, black), escapable shock (ES, red) and inescapable shock (IS, blue). B. sIPSCs semi-log amplitude histograms, HC (11 rats, 14 recorded neurons), ES (9 rats, 14 recorded neurons) and IS (7 rats, 11 recorded neurons). C. sIPSC inter-event interval (IEI) cumulative distributions showing the three treatments overlaid. D. sIPSCs semi-log IEI histograms showing the three treatment groups overlaid.
Summary
Here we present whole-cell patch clamp recording data sets from IS, ES and HC rat pyramidal neurons and an analysis program. Preliminary analysis suggests that control over stress appears to blunt the enhanced inhibition of the vmPFC that results from lack of control. Further detailed statistical analysis of sIPSC frequency and amplitude is necessary to indicate a pre- versus post-synaptic mechanism. We have made this data available together with a software program to allow users to adjust event thresholds and incorporate the results into computational modeling simulations of prefrontal cortical networks.
Author contributions
DCC and JAV conceived the study and designed the experiments. JAV and WJ carried out the sIPSC recordings. JAV wrote the IGOR Pro script. DCC and JAV prepared the manuscript. All authors were involved in the revision of the draft manuscript and have agreed to the final content.
Competing interests
No relevant competing interests were disclosed.
Grant information
This work was supported by National Institute on Drug Abuse grant R01-DA24040 (to DCC).
Acknowledgments
The authors would like to thank Steve Maier and John Christianson for their conversation and support, and Matthew Pomrenze for his help in caring for the animals.
References
- 1.
Mancini AD, Bonanno GA:
Predictors and parameters of resilience to loss: toward an individual differences model.
J Pers.
2009; 77(6): 1805–32.
- 2.
Weiss JM:
Effects of coping responses on stress.
J Comp Physiol Psychol.
1968; 65(2): 251–60.
- 3.
Maier SF, Seligman ME:
Learned Helplessness: Theory & Evidence.
J Exp Psychol.
1976; 105; 3–46.
- 4.
Maier SF, Watkins LR:
Role of the medial prefrontal cortex in coping and resilience.
Brain Res.
2010; 1355: 52–60.
- 5.
Amat J, Aleksejev RM, Paul E, et al:
Behavioral control over shock blocks behavioral and neurochemical effects of later social defeat.
Neuroscience.
2010; 165(4): 1031–8.
- 6.
Williams Jon L, Maier Steven F:
Transituational immunization and therapy of learned helplessness in the rat.
J Exp Psychol Anim Behav Process.
1977; 3(3): 240–252.
- 7.
Amat J, Baratta MV, Paul E, et al:
Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus.
Nat Neurosci.
2005; 8(3): 365–71.
- 8.
Amat J, Paul E, Watkins LR, et al:
Activation of the ventral medial prefrontal cortex during an uncontrollable stressor reproduces both the immediate and long-term protective effects of behavioral control.
Neuroscience.
2008; 154(4): 1178–86.
- 9.
Varela JA, Wang J, Christianson JP, et al:
Control over stress, but not stress per se increases prefrontal cortical pyramidal neuron excitability.
J Neurosci.
2012; 32(37): 12848–53.
- 10.
Markram H, Toledo-Rodriguez M, Wang Y, et al:
Interneurons of the neocortical inhibitory system.
Nat Rev Neurosci.
2004; 5(10): 793–807.
- 11.
Haralick R, Shapiro L: Computer and Robot Vision, Addison-Wesley Publishing Company.1992; 1, pp 346–351.
- 12.
Atkinson KE:
An Introduction to Numerical Analysis (2nd ed.). New York: John Wiley & Sons, (1989).


Comments on this article Comments (0)