Introduction
Candida albicans is an opportunistic yeast pathogen that has been established as a persistent and growing threat for critical ill patients over the last three decades. Candida species are the most common fungal species to cause invasive infections and at least one half of candidemia cases in non-neutropenic patients occur in an ICU or surgical ward1. Invasive candidiasis is associated with poor prognosis, with mortality rates of up to 30%2,3. Above all, C. albicans ranks as the fourth most common cause of bloodstream infection, carrying one of the worst prognoses3–5. The increase of Candida infections in the ICU comes at the same time as medical progress with the development of broad spectrum anti-bacterial therapy and immunosuppressive therapy1. There is also a close connection between the increased use of intravascular medical devices and the advent of bloodstream infections among the most frequent and potentially lethal nosocomial infections5–7. Catheter implantation frequently provides a gateway for the systemic entry of Candida and other pathogens from diverse sources. Additionally, hydrophobic catheter surfaces are a favorable habitat for pathogens that are able to form biofilms. They provide pathogens with a reservoir that is difficult to eradicate even by high dose antimycotic therapy, as resistance to antifungals is increased 10 to 1000 times compared to planktonic cells8,9. Known mechanisms of antifungal resistance under biofilm conditions are the upregulation of drug efflux pumps, sequestration of antifungals by matrix glucans and the development of a persister cell subpopulation10–12. Therefore, hydrophobic attachment to tissues and artificial surfaces can be regarded as a key virulence determinant of Candida spp. Adhesion of Candida and other fungi to polymeric materials correlates with cell surface hydrophobicity (CSH) phenotype13, though tissue and plastic binding are also mediated by adhesins14. The CSH1 gene product has been shown to be one of the mediators of CSH phenotype, localizing on the yeast cell surface and enhancing cell hydrophobicity as well as fibronectin binding15–17. It is thought to be induced by germ tube formation of single attaching yeast cells, subsequently evolving into a dense network of hyphae and pseudohyphae18. Quorum sensing of the densely packed cells regulates transcription of glucans that form a polymeric intercellular matrix19,20. We and others have recently shown that substances of diverse structure and origin may induce Candida pathogenicity factors. 17-β-estradiol increased growth and germ tube formation mediated increased temperature resistance by induction of the Hsp90 chaperon and elevated expression of the multidrug transporters CDR1 and CDR2. As coumarin and phenol also upregulated Hsp90 and CDR1, the authors of this study concluded that the response to estrogen might be rather unspecific21,22. Cigarette smoke induced the expression of histolytic enzymes and increased candidal adhesion in vitro23. Rifampicin, a common antibiotic, induces MDR-1 expression by Candida albicans that in turn can lead to modestly elevated minimal inhibitory concentrations (MIC) for fluconazole by some isolates24,25. Rifampicin-impregnated central-venous catheters have been increasingly used in clinical trials and one of these showed significantly increased Candida colonization26. To investigate whether rifampicin used in antibacterial catheter coatings to control bacterial catheter-related bloodstream infections may boost Candida virulence on these devices, we examined the possible induction of C. albicans SC5314 virulence factors involved in surface adhesion and biofilm formation by this drug.
Methods
C. albicans strains and growth conditions
C. albicans wild-type strain SC531427 was kindly provided by J. Morschhäuser, Institute for Molecular Infection Biology, Würzburg, Germany. The strain was kept as a frozen stock in glycerol at -80°C. For experiments, frozen cells were streaked on yeast-dextrose agar plates (5 g of yeast extract, 10 g of peptone, 20 g of dextrose, 15 g of agar, 40 mg gentamicin per litre), incubated at 30°C overnight and sub-cultured in YNB liquid medium (0.67% Yeast Nitrogen Base, 0.5% dextrose) at 30°C. Cultures were diluted in 25 mL YNB medium to an optical density at 600 nm (OD600) of 1 and were incubated at 30°C for 4 h with gentle shaking. For induction of the rifampicin response, 40 µg/mL rifampicin was used if not indicated otherwise.
Reagents
Unless stated otherwise, all reagents have been purchased at Sigma-Aldrich (Taufkirchen, Germany). Compounds were dissolved in acetone (menadione), DMSO (rifampicin) or water (all others).
Metabolic activity assay
Biofilm formation of SC5314 was quantified by the metabolic activity retained on a 96-well polystyrene plate (Falcon; BD Labware, Franklin Lakes, NJ) as described by Rammage and co-workers28. In brief, 105 cells /well were allowed to adhere to the plate for 48 h. After three times washing thoroughly with phosphate-buffered saline (PBS) (Gibco; Invitrogen, Carlsbad, CA), 2 h incubation with 2,3-bis(2-methoxy-4-nitro-5-sulfo-phenyl)-2H-tetrazolium-5-carboxanilide (XTT) substrate (0.5 mg/mL + 1 µM menadione in Ringer’s solution) was performed in the dark. Absorbance of reduced XTT was measured in a microtiter plate reader (Tecan, Maennedorf, Switzerland) at 490 nm.
Microsphere adherence
To assay Candida cell hydrophobicity, the number of adherent micro particles (Serva, Heidelberg, Germany) per cell was quantified as described by Hazen & Hazen29. 100 µL of a 2×106 cells/mL SC5314 suspension in ice cold PBS were mixed with 100 µL microsphere solution (~ 8,4×108 particles/mL) in acid-washed glass vessels. Two minutes of incubation at room temperature was followed by 30 s of rigorous mixing. Samples of this mixture were subjected to phase contrast microscopy (Axiolab / Axiovert 200 + Axiocam HRc microscope; Carl Zeiss Microimaging, Esslingen, Germany). Relative hydrophobicity was determined as the fraction size of cells with three or more adhering particles.
Hydrocarbon extraction
Extraction of Candida cells from an aqueous suspension with xylene was performed by a protocol adapted from Rosenberg and co-workers30. In brief, 4 mL of SC5314 suspension were vortexed with 1 mL of xylene for 2 min in glass tubes. After 15 min incubation at 37°C, tubes were cooled to room temperature and the OD600 of the aqueous phase was determined (Biophotometer, Eppendorf, Hamburg, Germany). Samples without xylene treatment served as controls. Relative hydrophobicity was calculated as the ratio of control samples to xylene treated samples.
Fibronectin binding
For the detection of fibronectin binding, Candida cells were sub-cultured in medium supplemented with 0.001% or 0.0001% human fibronectin (Sigma-Aldrich) for 1 h. Subsequently, cells were examined microscopically or washed 3x with PBS and subjected to immunoblot analysis.
Germ tube induction
To induce germ tube formation, 1×106 C. albicans yeasts/mL were transferred into the cell culture medium RPMI 1640 (Biochrom, Berlin, Germany) supplemented with 10% (v/v) (Sigma-Aldrich) FCS, 2 mM L-glutamine, penicillin (100 U/mL) and streptomycin (100 µg/mL) (Biochrom KG) and seeded into a 24-well plate. After 1 h, pictures were taken by phase contrast microscopy and germ tube length was determined with the Photoshop 6 measure tool (Adobe, San Jose, CA).
RNA preparation and reverse transcription-PCR (RT-PCR)
Total RNAs were extracted from C. albicans cells by use of the MasterPure™ Yeast RNA Purification Kit (EPICENTRE, Madison, Wisconsin). Contaminating DNA was removed by the TURBO DNA-freeTM Kit (Ambion Inc, Austin, Texas). Briefly, RNA was incubated at 37°C for 30 min with 2 U TURBO DNase per 10 µg of RNA. DNase was inactivated by adding DNase inactivation reagent for 2 min at room temperature. Total RNA concentrations were spectrophotometrically quantified. RNA samples were stored at -80°C or were used immediately. cDNA was synthesized with oligo(dT)-primers (New England Biolabs, Frankfurt, Germany) and Superscript™ III reverse transcriptase (Invitrogen, Karlsruhe, Germany), using 1 µg of total RNA. Controls examined C. albicans elongation factor 1 (EFB1) housekeeping gene, which contains an intron of 365 bp. Absence of genomic DNA was verified by a single intronless PCR product of EFB1. PCR were performed according standard protocols with 0.5 U Taq DNA polymerase (Roche, Mannheim, Germany). To amplify CSH1 or EFB1 the primers AGT AGA AAG CAT ATC TTA GCC G (fwd) and GCT TGT TGT CTA AGA ACT GC (rev) or AGT CAT TGA ACG AAT TCT TGG C (fwd) and ATC AAC TTC ATC ATC AGA ACC G (rev) were used. Cycling conditions were 94°C for 30 s, 63°C for 30 s and 72°C for 60 s for CSH1 and 94°C for 30 s, 60°C for 30 s and 72°C for 60 s for EFB1. Only samples from the exponential phase of PCR amplification were examined. Equivalent volumes of PCR product were analysed on 1.8% agarose gels stained with ethidium bromide.
Analysis of protein expression
Proteins were isolated by the method of Hiller et al31. Equal protein amounts as determined by Bradford assay were separated on a 10% SDS gel and transferred to nitrocellulose membranes by wet-blot. Further membrane protein binding was blocked by incubation in 5% skimmed milk powder containing wash buffer. CSH1p or fibronectin were detected by subsequent incubation with mouse anti-CSH1 (1:10,000; kindly provided by D. Singleton) or rabbit anti-human fibronectin (1:4000; Sigma-Aldrich) primary antibodies and horseradish peroxidase (HRP) labeled rabbit anti-mouse or swine anti-rabbit IgG secondary antibodies (both 1:1000; DAKO, Glostrup, Denmark), respectively. Chemiluminescence of Amersham ECL reagent (GE Healthcare, Waukesha, WI), was detected on CL-XPosure Film (Thermo, Rockford, IL).
Statistical analysis
Unless indicated otherwise, data shown are representative of at least two independent experiments. Differences between mean values were analyzed using two tailed Student’s t test. P < 0.05 was considered statistically significant.
Results
Rifampicin induces SC5314 cell surface hydrophobicity
The cell surface hydrophobicity (CSH) phenotype was identified as a major factor conferring virulence to Candida species by many means, first of all by enhancing attachment to tissues and foreign materials, thus being a prerequisite for efficient biofilm formation13,32. To examine whether rifampicin might increase C. albicans CSH phenotype expression, we quantified the adhesion of microspheres to SC5314 with or without rifampicin treatment. Microsphere adherence to SC5314 cells robustly doubled in response to rifampicin treatment, showing increased hydrophobicity and suggesting CSH phenotype induction (Figure 1A and 1B). Another method established for the measurement of microbial surface hydrophobicity is the measurement of extraction from an aqueous suspension with an organic solvent30. After rifampicin treatment, extraction of SC5314 was significantly increased, providing additional support for increased hydrophobicity (Figure 1C).
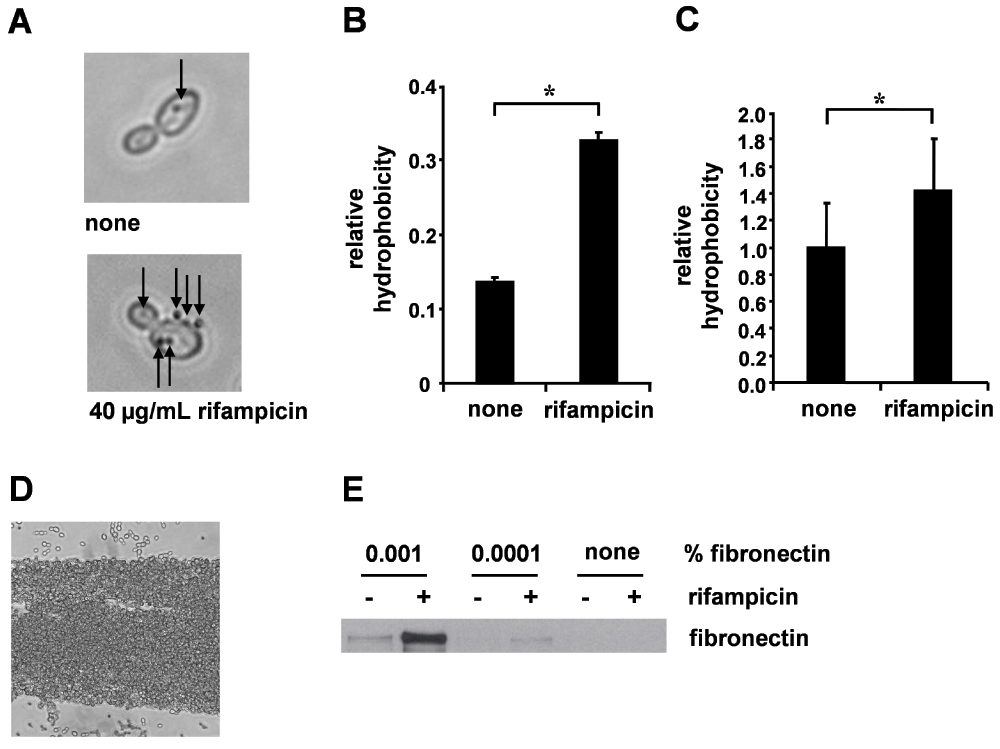
Figure 1. Rifampicin enhanced SC5314 hydrophobicity and fibronectin binding.
(A) Microsphere adherence to SC5314 after treatment with 40 µg/mL rifampicin. Representative phase-contrast picture, arrows indicate adherent microspheres. (B) Relative hydrophobicity; means + SD of three independent microsphere adherence experiments. (C) Rifampicin enhances extraction by the organic solvent xylene. Mean relative hydrophobicity normalized on untreated controls calculated from four independent extraction experiments + SEM. *Significant difference (p < 0.05). (D) Detection of fibronectin retention. Representative phase-contrast picture showing the formation of fibronectin fibers thickly coated by clustered Candida cells (condition with fibronectin and rifampicin). (E) Western blot of SC5314 with or without rifampicin and fibronectin (0.001%, 0.0001%) pre-treatment for 1 h.
Enhanced fibronectin binding after rifampicin treatment
The affinity of Candida for fibronectin has been known for a long time and has been regarded as a virulence property enabling tissue adhesion by extracellular matrix binding, thus promoting initiation as well as dissemination of candidiasis15. Strikingly, microscopic observation revealed dense clustering to long, bottle brush like aggregates (Figure 1D). Western blot analysis of SC5314 cultured in medium supplemented with human plasma fibronectin in the presence or absence of rifampicin proved strongly enhanced fibronectin retention of rifampicin treated cells after thoroughly washing (Figure 1E).
Rifampicin promotes SC5314 biofilm formation
Biofilm formation by Candida spp. on tissues or implanted materials is a process with high clinical impact, as it mediates increased resistance to antifungal agents and protection from host defenses. Additionally, biofilms act as pathogen reservoirs, boosting persistent infection9. To get a quantitative idea of how rifampicin affects Candida retention on plastic materials, we assayed XTT reduction of adhering C. albicans SC5314 after culture in polystyrene wells for 48 h, untreated or treated with 40 µg/mL rifampicin. We observed an 1.6-fold significant increase in metabolic activity retained after thoroughly washing on the culture plate induced by rifampicin treatment, indicating that rifampicin enhances biofilm formation by C. albicans
(Figure 2A).
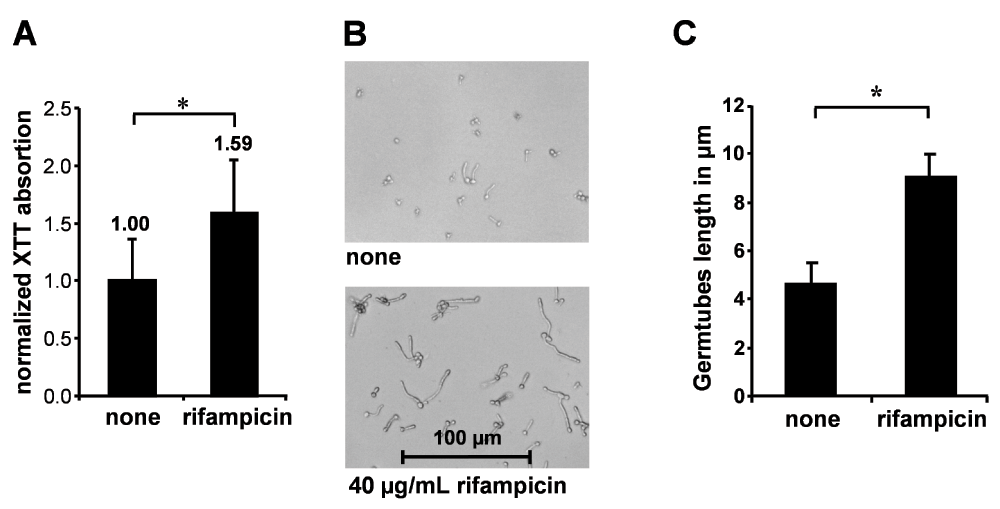
Figure 2. Rifampicin increased biofilm formation on polystyrene and induced germ tube formation.
(A) Biofilm formation of SC5314 after treatment with 40 µg/mL rifampicin. Mean and SD of XTT absorption normalized on untreated controls of three independent experiments; *difference is statistically significant (p < 0.05). (B) Rifampicin-induced germ tube formation, representative phase-contrast microscopy. (C) Germ tube length in µm; mean + SD of three independent experiments. *Significant difference (p < 0.005).
Rifampicin promotes germ tube formation
The morphological differentiation ability of C. albicans plays an important role in biofilm maturation that follows hydrophobic attachment. Mutants deficient in the yeast phenotype show reduced adhesion to tissues and polymers and are more easily removed under high-salt assay conditions, indicating improved anchoring of the biofilm by yeast phenotype cells. However, the dimorphic phenotype enables the formation of thicker biofilms with higher cell numbers than yeast-only strains that may display superior resistance to chemical and mechanical stress, respectively33. In our experiments, rifampicin treatment turned out to profoundly induce germ tube formation, accelerating germ tube growth leading to increased hyphae to yeast cells ratio. Average germ tube length was doubled 4 h after induction (Figure 2B and 2C). Interestingly, our data are paralleled by the observations of other authors who report enhanced filamentous growth induced by tetracycline and 17-β-estradiol21,34.
CSH phenotype is mediated by CSH1p expression
CSH1 gene expression may be a mediator of the CSH phenotype15–17. Upon rifampicin treatment, an immediate increase in CSH1 transcription could be detected in SC5314, as well as sustained high level expression of CSH1 mRNA until 4 h after induction (Figure 3A). Induction of CSH1 transcription is matched by elevated CSH1p protein levels in SC5314 lysates (Figure 3B).
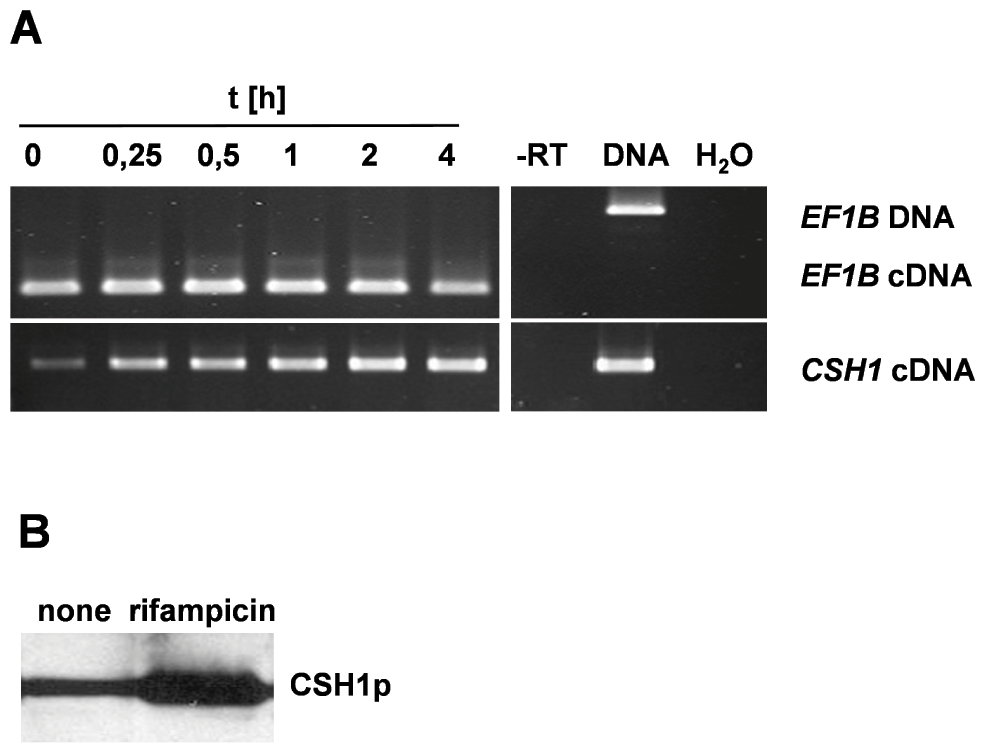
Figure 3.
CSH1 RNA and protein expression.
(A) Kinetic of rifampicin (20 µg/mL) induced CSH1 mRNA expression in SC5314. Samples were collected at indicated time points during rifampicin exposition. (B) Csh1p expression in SC5314. Western blot of equal protein amounts after rifampicin-induction (40 µg/mL, 4 h).
Discussion
In this work we addressed the question of whether rifampicin might promote Candida albicans biofilm formation, thus potentially aggravating the threat of catheter-related and other infections by this pathogen. We could show increased SC5314 cell hydrophobicity, indicating a shift to a CSH phenotype. It has to be taken into account that a recent report failed to correlate the hydrophobicity of 50 clinical C. albicans isolates with their adhesiveness to polystyrene35. Other reports however, have shown hydrophobicity not only to correlate with Candida adhesiveness but to be part of concerted pathogenicity factor expression23,36. Fibronectin binding was also strongly increased after rifampicin treatment, showing the induction of an additional trait contributing to increased adherence. In line with these findings we could demonstrate that rifampicin treatment induces enhanced Candida retention on plastic by metabolic activity assay. Additionally, rifampicin enhanced germ tube formation, known to promote an exploratory and invasive lifestyle. Reports showing germ tube formation to be induced a few hours after CSH increase or surface attachment strongly argue it to be part of the biofilm program29. Germ tubes also enhance biofilm compression strength and mediate phagocyte escape, suggesting a key role in biofilm pathogenesis18,37. Csh1p is an aldo-keto reductase and is homologous to aryl-alcohol dehydrogenases in Saccharomyces cerevisae17. CSH1 transcription is activated by Zap1, a negative regulator of the matrix component soluble β-1,3 glucan and thought to mediate the Zap1 effect by intercellular signalling20. To answer the question of whether CSH1 expression might be part of the CSH phenotype induced by rifampicin, we analysed CSH1 mRNA and protein levels in C. albicans SC5314. We found CSH1 mRNA upregulation and an increase in CSH1p protein levels.
Interestingly, CSH1p deficiency has been shown to drastically reduce the fibronectin binding properties of C. albicans, thus opening the possibility that increased fibronectin binding results from CSH1p upregulation, though host fibronectin binding seems to be mediated by multiple Candida surface proteins15,16. Previously we demonstrated that rifampicin upregulated C. albicans MDR1 expression24. Co-induction of CSH1 and MDR1 has also been shown for a fluconazole-resistant C. albicans patient isolate38. Moreover, Mdr1 has been shown to be upregulated immediately after adhesion39, and both CSH1 (orf19.4477) and MDR1 (orf19.5604) transcription is mediated by the multidrug resistance regulator Mrr1p40. This raises the possibility that Mrr1p may be a target of rifampicin in C. albicans. Taken together, the results of this study show a significant upregulation of Candida virulence determinants that promote pathogenic biofilm behaviour by the antibiotic rifampicin. Thus, antibacterial rifampicin coatings of intravascular medical devices could potentially oppose efforts to diminish their microbial colonization. This effect could also be suspected to interfere with the promising effects of some recent antimycotics in inhibiting biofilm growth41. Furthermore, the data shown here contribute to the growing evidence showing that miscellaneous structurally unrelated substances that are xenobiotic to yeasts are capable of inducing mediators of Candida virulence and drug resistance. Eventually, enhanced induction by cooperation of several of these substances cannot be excluded (e.g. tetracycline + rifampicin coated catheter in combination with estrogen)21,26,34.



Comments on this article Comments (0)