Introduction
Global climate warming and increases in atmospheric CO2 concentration are currently key topics for scientists, politicians and the general public alike1. Such changes have been observed in the past 150 years and supported by modeling results for the longer term, e.g., warming ocean water, shrinking mountain glaciers, retreating snow cover, and CO2 concentration dynamics in Arctic/Antarctic ice cores2–6. It is projected that the global temperatures will increase by an average of 3°C with a range of 2 to 4.5°C under the scenario of doubling atmospheric CO2 concentration by the end of the century2. Global climate warming will likely have profound and diverse impacts on biological systems2,4–13.
Increases in CO2 and temperature to a certain extent should have positive impact on photosynthesis and growth, as the current atmospheric CO2 concentration is below the saturation point for RuBisCO (Ribulose-1,5-bisphosphate carboxylase oxygenase)14. Furthermore, higher CO2 concentrations suppress photorespiration and increase the partitioning of photosynthetic electron transport to carboxylation14. However, the situation will become complicated if the temperature goes beyond plants’ ability to acclimate, or when the rate of temperature increase exceeds the pace of acclimation. In such cases, temperature and CO2 will have opposite effects on photosynthesis, i.e., the higher temperature induced increase in photorespiration may exceed the beneficial effect of CO2 elevation, resulting in a decline in net photosynthesis. Consequently, the direction and magnitude of change in net photosynthesis will be determined by the relative magnitudes of the two opposite effects15. Kirschbaum16 has conducted a theoretical analysis on the dependence of photosynthesis on temperature and CO2 concentration for C3 plants and found that at 35°C, photosynthesis at the ambient CO2 concentration reaches only 50% of the rate at saturating CO2 concentration, whereas the corresponding value at 5°C is 77%. Therefore, there is greater potential photosynthetic enhancement by CO2 elevations at higher temperatures. This theory has been supported by the results of a number of studies15,17–19. Long20 has suggested that the increase in atmospheric CO2 will not only increase photosynthetic rate, but also alter the photosynthetic response to temperature. Mooney et al.21 indicate that the photosynthetic acclimation to elevated temperature and CO2 mainly involves changes in the heat stability of the thylakoids and RuBisCO activity. Hence, high temperature and CO2 elevations may have synergistic effect on photosynthesis and CO2 elevations may lead to improved acclimation to high temperatures. However, such interactions may vary with plant species22,23 and other environmental conditions. Variations in acclimation ability can change the interactions within and between species and the composition and functioning of plant communities under future climatic conditions. Furthermore, in most past studies, high temperature treatments are achieved in one step, which is in contrast with the gradual, progressive increases in temperature occurring in global climate changes.
The boreal forest is an important terrestrial ecosystem with a high carbon sequestration potential24. As the global climate change accelerates, the boreal forest has been experiencing progressive increases in temperatures and CO2. The response of the boreal forests could have great impact on the global carbon balance25. White birch is one of the most widely distributed tree species in the boreal forest. The growth conditions of white birch in northwest Ontario are characterized by a long cold winter and short summer. For example, the annual mean temperature in Thunder Bay region is 2.4°C while the January and July average temperatures are -14.9°C and 17.6°C (based on Environment Canada’s online weather records for the time period of January 1943 to December 2003). Nevertheless, based on our past experience in growing white birch seedlings in greenhouses, it appears that the species is capable of acclimating to continuous warming to more than 40°C in the early afternoon on sunny summer days (see Figure 1). In this current study, we test the hypothesis that CO2 elevation will enhance the photosynthetic performance of white birch seedlings growing in a progressively warming environment.
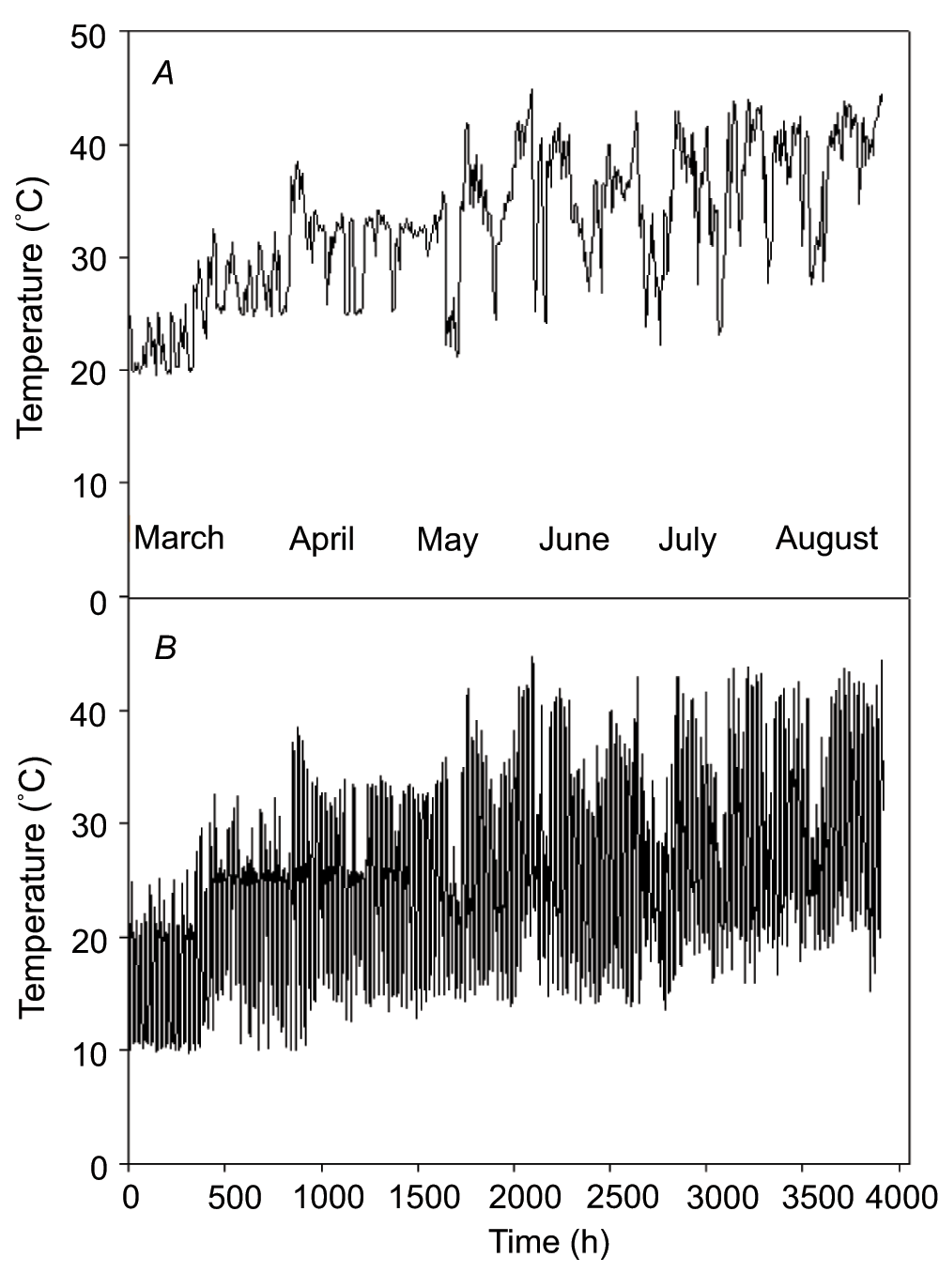
Figure 1. Time course of temperatures in the greenhouses during the experimental period (March 1 through August 15).
(A) Postmeridian (hour) pattern between 13:00 and 16:00; (B) Diel (hour) pattern between 0:00 and 24:00.
Materials and methods
Plant materials
White birch (Betula papyrifera Mash.) seedlings were grown from seeds in the greenhouses at the Thunder Bay campus of Lakehead University. The growing medium was a mixture of peat moss and vermiculite (1:1 (v/v)).
Experiment design
The seedlings were subject to a progressive warming in the greenhouses as the season progressed from March to August (Figure 1). The temperatures in all the greenhouses were monitored and recorded using a computerized environment control system (Argus, Vancouver, Canada). The highest recorded temperature in the greenhouse was 44.8°C in the later stages of the experiment (Figure 1). The seedlings were grown under two CO2 concentrations (i.e., the ambient (360 µmol mol-1) and elevated (650 µmol mol-1)). The two CO2 treatments were conducted simultaneously in separate greenhouses with identical design and dimensions. The CO2 elevation was achieved using Argus CO2 generators (Argus, Vancouver, Canada). A photoperiod of 16-hour was maintained (the natural light was supplemented by high-pressure sodium lamps on cloudy days, early mornings and late evenings).
The moisture content of the growing medium was maintained at around 50%, as measured using a HH2 Moisture Meter (DELTA-T DEVICES, Cambridge, UK). The seedlings were watered up to twice a day during the summer to maintain the soil moisture condition. The seedlings were fertilized weekly with a solution of 100 µmol mol-1 N, 35 µmol mol-1 P and 66 µmol mol-1 K.
Simultaneous measurements of in situ gas exchange and chlorophyll fluorescence
The foliage gas exchange was measured using a PP-Systems CIRAS-1 open gas exchange system (Hitchin, Hertfordshire, UK). The environmental conditions in the broad-leaf chamber were controlled automatically. The environmental conditions for measuring the Pn-Ci (Ci = intercellular CO2 concentration) curve were as follows: 26°C and 37°C air temperature, which were close to the highest temperatures in the early and late period of the experiment, 800 µmol m-2s-1 PAR (PAR = photosynthetically-active radiation) and 50% relative humidity. The in vivo maximal carboxylation rate (Vcmax), PAR-saturated electron transport rate (Jmax), triose phosphate utilization (TPU) and other relevant parameters were calculated from the Pn-Ci curves according to Farquhar et al.26, van Caemmerer and Farquhar27, Sharkey28, Harley and Sharkey29 and Harley et al.30. The Pn-Ci curves were fit using the Photosyn Assistant software (Dundee Scientific, Scotland, UK) to estimate Vcmax, Jmax and TPU. The parameters for the kinetics of RuBiscCO, i.e., Kc, Ko and τ, and their temperature dependencies were adopted from Harley et al.30 and Wullschleger31.
Three seedlings were selected randomly from each treatment combination for the measurement. The measurement was taken on the top 5th mature leaf. All the in situ measurements were made between 9:00 and 11:30 AM with the seedlings in their original positions and conditions of the treatments.
The chlorophyll fluorescence was measured using a FMS-2 portable pulse-modulated fluorometer (Hansatech Instruments Ltd. Norfolk, UK). The probe was integrated in the leaf chambers of the gas exchange system and the control software for the two systems was also integrated to allow the simultaneous measurement of gas exchange and chlorophyll fluorescence. The following variables were obtained: fluorescence intensity at any time, F; the maximal fluorescence in light, Fm’; the actual photochemical efficiency of PSII in light, (Fm’-F)/Fm’ or ΔF/Fm’, which is the efficiency under the actual degree of reaction centre closure32. Fm’ was obtained by illuminating the foliage with a pulse of strong light (around 14000 µmol photons m-2s-1) for 800 ms. The ΔF/Fm’ was measured simultaneously with each gas exchange measurement. Both gas exchange and chlorophyll fluorescence were measured after 5 months of the treatments.
The apparent rate of total electron transport (JT) and its partitioning between carboxylation (Jc) and oxygenation (Jo) were calculated based on the methods of Farquhar et al.26, Genty et al.33 and Epron et al34.
Statistical analysis
All the data were examined graphically for the normality of distribution (probability plots for residual analysis) and the homogeneity of variance (scatter plots) using the Data Desk (version 6.01, Data Description, Inc. 1996)35 before the Analysis of Variance (ANOVA) was carried out. Some of the data were log-transformed to meet the two assumptions for ANOVA. The data were analyzed using the two-way ANOVA procedure of the Data Desk. When the interaction between temperature and CO2 was significant, Scheffe’s F test for post hoc pairwise comparisons was conducted.
Results
In situ photosynthetic gas exchange
There was a significant (P<0.01) interactive effect of temperature and CO2 on Pn (Figure 2). Pn was higher (P<0.01) at 37°C than at 26°C under elevated CO2(Figure 2), but there was no significant (P>0.05) temperature effect on Pn under ambient CO2. CO2 elevation significantly increased Pn at both temperatures (P<0.05, P<0.001 at 26°C and 37°C, respectively). gs significantly (P<0.05) decreased at 37°C under both ambient and elevated CO2(Figure 2), and there was no significant (P>0.05) CO2 effect on gs. Meanwhile high temperature significantly (P<0.05) stimulated E under both ambient and elevated CO2(Figure 2). Water-use efficiency (WUE) was significantly (P<0.05) higher at 26°C than that at 37°C under both CO2 regimes. CO2 elevation greatly (P<0.001) increased WUE at both temperatures.
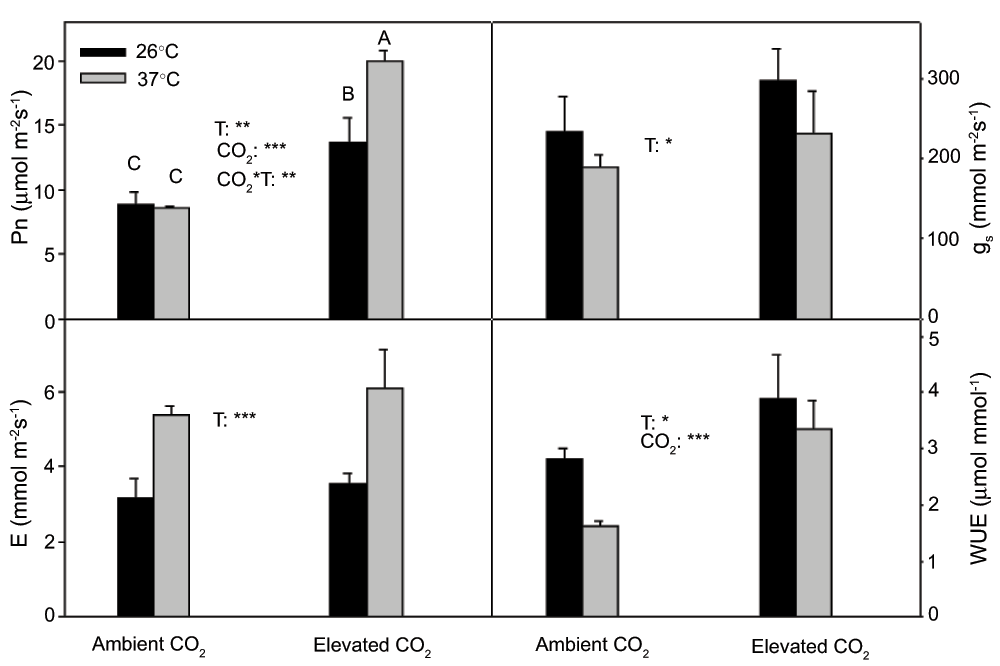
Figure 2. Pn, gs, E and WUE (mean ± SD, n=3–4) for current year white birch seedlings after they were exposed to continuous warming under ambient CO2 and elevated CO2 concentrations for 5 months.
The in situ measurements were taken at 26°C and 37°C under ambient CO2 and elevated CO2. The significance levels (*** = P<0.001, ** = P<0.01, * = P<0.05). If the interaction between measurement temperature and CO2 was significant for a given parameter, Scheffe’s F test for post hoc pairwise comparisons was conducted. Means sharing the same letter or letters are not significantly different.
High temperature significantly reduced Ci under both ambient and elevated CO2 (P<0.05, P<0.01, respectively) and, also, elevated CO2 significantly (P<0.001) increased Ci at both temperatures.
In vivo RuBisCO activity
Vcmax, Jmax and TPU at 37°C were significantly (P<0.001) higher than those at 26°C (Figure 3). The temperature dependencies of Vcmax and Jmax were changed by CO2, and those values at 37°C enhanced much more (P<0.05) under elevated CO2 than under ambient CO2.
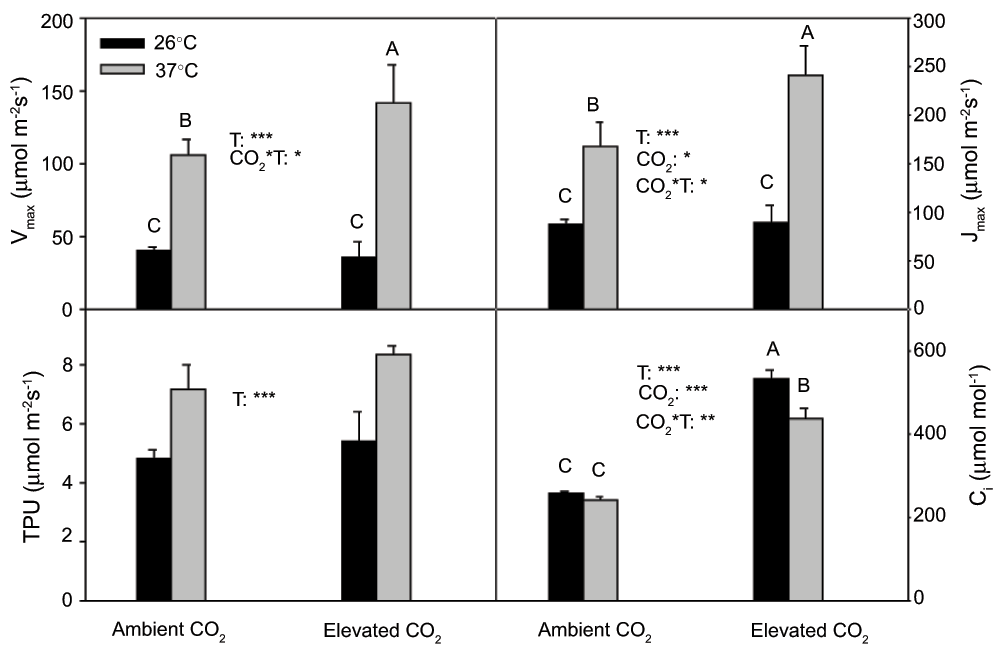
Figure 3. Vcmax, Jmax, TPU and Ci in current year white birch seedlings.
Vcmax, Jmax and TPU were derived from A-Ci curves, which were measured at 26°C and 37°C under ambient CO2 and elevated CO2. See Figure 2 for other explanations.
Photosystem II efficiency and electron transport partitioning to carboxylation and oxygenation
There was a significant (P<0.001) interactive effect of CO2 and temperature on (Fm’-F)/Fm’ and JT(Figure 4). (Fm’-F)/Fm’ and JT greatly increased at 37°C as compared to at 26°C under elevated CO2, and there was no significant temperature effect on (Fm’-F)/Fm’ and JT under ambient CO2.
The pattern of CO2 and temperature effects on Jc was almost the same as (Fm’-F)/Fm’ and JT(Figure 4), and Jc was greater (P<0.001) at 37°C than that at 26°C under elevated CO2, and there was no significant temperature effect on Jc under ambient CO2. Elevated CO2 greatly suppressed Jo/JT, and there was no significant (P > 0.05) effect of temperature on Jo/JT(Figure 4).
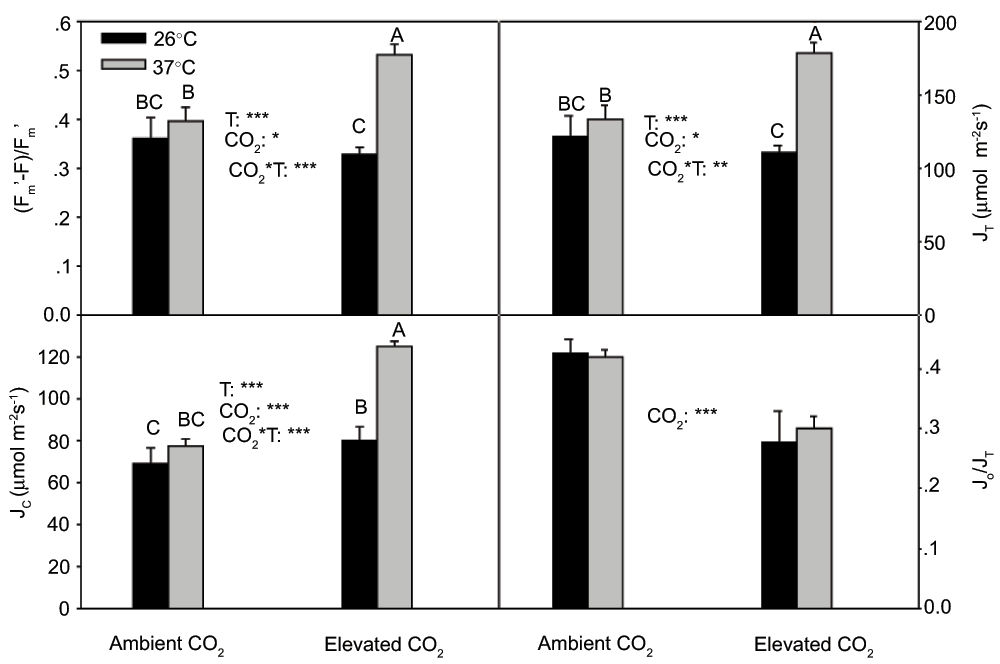
Figure 4. (Fm’-F)/Fm’, JT, Jc and Jo/JT in the current year white birch seedlings (Fm’-F)/Fm’ and JT were derived from chlorophyll fluorescence measurements, and Jc and Jo were derived from both chlorophyll fluorescence and gas exchange measurements.
See Figure 2 for other explanations.
Discussion
Our results suggest that the photosynthetic mechanisms of white birch seedlings have high capacity to acclimate to a progressively warming environment, particularly under elevated CO2. This result is in contrast to the results of most studies with a single step warming treatment. Larcher36 has suggested that plants’ optimal temperature is closely related to the climate in which they grow. The measurement temperatures of 26°C and 37°C used in this study are believed to be the normal (or optimal) and stressful temperature, respectively, for most boreal forest tree species growing at their natural environments. Zhang et al.37 have found that the Pn of mature oak even in warm-temperate zones decline greatly at temperatures over 30°C, as compared to measurements at temperatures of 20–30°C, which occurs naturally north of temperate zones or even warm-temperate zones. However, in this experiment the Pn of white birch didn’t decline at 37°C under ambient CO2, as compared to that at 26°C; furthermore, Pn increased substantially at 37°C under elevated CO2. These results indicate that the photosynthetic mechanisms of white birch acclimated to the progressive warming environment, and this high temperature acclimation was greatly strengthened by elevated CO2. Long20 argued that CO2 elevation could change the photosynthesis dependence of temperature.
The activity of RuBisCO is highly temperature-dependent. According to Jordan and Ogren38, the Rubisco’s specificity for CO2/O2 decreases as increasing temperatures over the optimal range, but the increase in RuBisCO oxygenation will exceed that of carboxylation because the solubility of CO2 declines faster than that of O2 at even higher temperatures, resulting in a decline in net photosynthetic rate. White birch’s acclimation to warming was also evidenced by Vcmax measured at the two different temperatures and two CO2 regimes. Vcmax at 37°C was much higher than at 26°C under both ambient and elevated CO2, indicating a shift in the temperature dependency of RuBisCO. Furthermore, the partitioning of total electron transport to oxygenation was not significantly different between the two temperatures under either ambient CO2 or elevated CO2, suggesting that the higher temperature did not change the RUBisCO specificity for CO2/O2 which could be a contributing factor for the enhanced acclimation of photosynthesis to the progressive warming. Overdieck et al.15 have also found that both the temperature treatment alone and the combination of elevated CO2 and temperature depressed Vcmax in Scots pine at temperatures below the optimum range, but increased Vcmax when the temperature was above the optimum. Additionally, the magnitude of the change in Vcmax increased as temperature increased.
The decrease in Ci at the high temperature could be attributable to either enhanced RuBisCO activity or declines in stomatal conductance or both. Not only Vcmax, but Jmax and TPU were also higher at 37°C than at 26°C, suggesting that the CO2 assimilation process, including carboxylation, electron transport for RuBP regeneration, ATP supply and the translocation of the primary photosynthates, all maintained at high levels in the warm environment. In this study, there was no down-regulation of RuBisCO activity in association with the CO2 elevation, to the contrary, CO2 elevation greatly increased Vcmax and Jmax at 37°C, as well as Pn at both 26°C and 37°C.
While high temperature enhanced Vcmax under both ambient and elevated CO2, the increases in actual PSII efficiency (ΔF’/Fm’) and Jc associated with the high temperature only occurred under elevated CO2, suggesting that the high temperature did not significantly affect the total electron transport, and its partitioning to carboxylation, t under the ambient CO2. Conversely, the partitioning of total electron flow to oxygenation increased more than 40% in response to the high temperature under elevated CO2. The reduced electron transport partitioning to carboxylation and low Ci might explain why Pn was relatively low at 37°C under the ambient CO2, even though the corresponding Vcmax was quite high, implying that the slow electron transport to carboxylation and CO2 supply at high temperature under ambient CO2 didn’t match the high activity of RuBisCO. This again confirms Kirschbaum’s theoretical analysis that photosynthesis has a higher potential to be stimulated by CO2 elevation at high temperatures than at low temperatures16.
Author contributions
QLD and SZ conceived and designed the experiment. SZ conducted the measurements and analyzed the data. SZ and QLD wrote and revised the manuscript.
Competing interests
No competing interests were disclosed.
Grant information
This study was supported by Lakehead University, Canada Foundation for Innovation, Ontario Innovation Trust and NSERC Discovery Grants to Q.L. Dang (Project # 203198-2008).
Acknowledgements
The authors thank Dr. J. Wang at Lakehead University for providing white birch seeds, and Dr. K. Brown for statistical advice.
Faculty Opinions recommendedReferences
- 1.
Kerr RA:
Climate change. Scientists tell policymakers we're are all warming the world.
Science.
2007; 315(5813): 754–757. PubMed Abstract
| Publisher Full Text
- 2.
IPCC:
Climate Change 2007: Impacts, Adaptation, and Vulnerability. Cambridge Univ. Press 2007; pp 976. Reference Source
- 3.
Keeling CD, Whorf TD:
Atmospheric CO2 records from sites in the SIO air sampling network. In: Trends: a compendium of data on global change. Oak Ridge National Laboratory, US Department of Energy 2002. Reference Source
- 4.
Parmesan C:
Influences of species, latitudes and methodologies on estimates of phenological response to global warming.
Glob Change Biol.
2007; (13): 1860–1872. Reference Source
- 5.
Root TL, Price JT, Hall KR, et al.:
Fingerprints of global warming on wild animals and plants.
Nature.
2003; 421(6918): 57–60. PubMed Abstract
| Publisher Full Text
- 6.
Rosenzweig C, Karoly D, Vicarelli M, et al.:
Attributing physical and biological impacts to anthropogenic climate change.
Nature.
2008; 453(7193): 353–357. PubMed Abstract
| Publisher Full Text
- 7.
Both C, van Asch M, Bijlsma R, et al.:
Climate change and unequal phenological changes across four trophic levels: constraints or adaptations?
J Anim Ecol.
2009; 78(1): 73–83. PubMed Abstract
| Publisher Full Text
- 8.
Bradshaw W, Holzapfel C:
Genetic shift in photoperiodic response correlated with global warming.
Proc Natl Acad Sci U S A.
2001; 98(25): 14509–14511. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 9.
Colwell R, Brehm G, Cardelus C, et al.:
Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics.
Science.
2008; 322(5899): 258–261. PubMed Abstract
| Publisher Full Text
- 10.
Parmesan C, Yohe G:
A globally coherent fingerprint of climate change impacts across natural systems.
Nature.
2003; 421(6918): 37–42. PubMed Abstract
| Publisher Full Text
- 11.
Sinervo B, Méndez-de-la-Cruz F, Miles DB, et al.:
Erosion of lizard diversity by climate change and altered thermal niches.
Science.
2010; 328(5980): 894–899. PubMed Abstract
| Publisher Full Text
- 12.
Umina PA, Weeks AR, Kearney MR, et al.:
A rapid shift in a classic clinal pattern in Drosophila reflecting climate change.
Science.
2005; 308(5722): 691–693. PubMed Abstract
| Publisher Full Text
- 13.
Walther GR, Post E, Convey P, et al.:
Ecological responses to recent climate change.
Nature.
2002; 416(6879): 389–395. PubMed Abstract
| Publisher Full Text
- 14.
Amthor JS:
Respiration in a future, higher-CO2 world.
Plant Cell Environ.
1991; 14(1): 13–20. Publisher Full Text
- 15.
Overdieck D, Kellomäki S, Wang KY, et al.: Do the effects of temperature and CO2 interact? In: European Forests and Global Change: the likely impacts of rising CO2 and temperature. (Eds. Jarvis PG).X Cambridge University Press, 1998; pp 236–273. Reference Source
- 16.
Kirschbaum MUF:
The sensitivity of C3 photosynthesis to increasing CO2 concentration: a theoretical analysis of its dependence on temperature and background CO2 concentration.
Plant Cell Environ.
1994; 17(6): 747–754. Publisher Full Text
- 17.
Idso KE, Idso SB:
Plant responses to atmospheric CO2 enrichment in the face of environmental constraints: a review of the past ten years’ research.
Agricultural and Forest Meteorology.
1994; 69(3–4): 153–203. Publisher Full Text
- 18.
Idso SB, Idso KE, Garcia RL, et al.:
Effects of atmospheric CO2 enrichment and foliar methanol application on net photosynthesis of sour orange tree (Citrus aurantium; Rutaceae) leaves.
Am J Bot.
1995; 82(1): 26–30. Publisher Full Text
- 19.
Tissue DT, Thomas RB, Strain BR, et al.:
Growth and photosynthesis of loblolly pine (Pinus taeda) after exposure to elevated CO2 for 19 months in the field.
Tree Physiol.
1996; 16(1–2): 49–59. Publisher Full Text
- 20.
Long SP:
Modification of the response of photosynthetic productivity to rising temperature by atmospheric CO2 concentration, has its importance been underestimated?
Plant Cell Environ.
1991; 14(8): 729–739. Publisher Full Text
- 21.
Mooney HA, Bjorkman O, Collatz GJ, et al.:
Photosynthetic acclimation to temperature in the desert shrub, Larrea divaricata I. Carbon dioxide exchange characteristics of intact leaves.
Plant Physiol.
1978; 61(3): 406–10. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 22.
Stanghellini C, Bunce JA:
Response of photosynthesis and conductance to light, CO2, temperature and humidity in tomato plants acclimated to ambient and elevated CO2.
Photosynthetica.
1993; 29: 487–97.
- 23.
Teskey RO:
Combined effects of elevated CO2 and air temperature on carbon assimilation of Pinustaeda trees.
Plant Cell Environ.
1997; 20(3): 373–380. Publisher Full Text
- 24.
Apps MJ, Kurz WA, Luxmoor RJ, et al.:
Boreal forest and tundra.
Water, Air, and Soil Pollution.
1993; 70(1–4): 39–53. Publisher Full Text
- 25.
Peng CH, Apps MJ:
Simulating carbon dynamics along the Boreal Forest Transect Case Study (BFTCS) in central Canada. 2. Sensitivity to climate change.
Global Biogeochem Cycles
1998; 12(2): 393–402. Publisher Full Text
- 26.
Farquhar GD, von Caemmerer S, Berry JA, et al.:
A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species.
Planta.
1980; 149(1): 78–90. Publisher Full Text
- 27.
von Caemmerer S, Farquhar GD:
Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves.
Planta.
1981; 153(4): 376–87. Publisher Full Text
- 28.
Sharkey TD:
Photosynthesis in intact leaves of C3 plants: physics, physiology and rate limitations.
Botanical Review.
1985; 51(1): 53–105. Publisher Full Text
- 29.
Harley PC, Sharkey TD:
An improved model of C3 photosynthesis at high CO2: Reversed O2 sensitivity explained by lack of glycerate reentry into the chloroplast.
Photosynth Res.
1991; 27(3): 169–178. Publisher Full Text
- 30.
Harley PC, Thomas RB, Reynolds JF, et al.:
Modelling photosynthesis of cotton grown in elevated CO2.
Plant Cell Environ.
1992; 15(3): 271–82. Publisher Full Text
- 31.
Wullschleger SD:
Biochemical limitations to carbon assimilation in C3 plants – a retrospective analysis of the A/Ci curves from 109 species.
J Exp Bot.
1993; 44(5): 907–920. Publisher Full Text
- 32.
Bilger W, Björkman O:
Role of the xanthophyll cycle in photoprotection elucidated by measurements of light induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis.
Photosynth Res.
1990; 25(3): 173–185. Publisher Full Text
- 33.
Genty B, Briantais JM, Baker NR, et al.:
The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence.
Biochim Biophys Acta.
1989; 990(1): 87–92. Publisher Full Text
- 34.
Epron D, Godard D, Cornic G, et al.:
Limitation of net CO2 assimilation rate by internal resistances to CO2 transfer in the leaves of two tree species (Facus sylavatica L. & Castanea sativa Mill.).
Plant Cell Environ.
1995; 18(1): 43–51. Publisher Full Text
- 35.
Data Desk. Version 6.01, Data Description, Inc., Ithaca, New York, USA 1996. Reference Source
- 36.
Larcher W:
Physiological plant ecology: ecophysiology and stress physiology of functional groups. Springer-Verlag, Berlin Heidelberg New York 2003. Reference Source
- 37.
Zhang S, Li Q, Ma K, et al.:
Temperature-dependent gas exchange and stomatal/non-stomatal limitation to CO2 assimilation of Quercus liaotungensis under midday high irradiance.
Photosynthetica.
2001; 39(3): 383–388. Publisher Full Text
- 38.
Jordan DB, Ogren WL:
The CO2/O2 specificity of ribulose 1,5-bisphosphate carboxylase/oxygenase.
Planta.
1984; 161(4): 308–313. Publisher Full Text




Comments on this article Comments (0)