Introduction
Three dimensional tissue cultures are gaining popularity because they mimic the natural cell environment1,2 and are used in many applications such as drug discovery, toxicity testing and tissue engineering. Scaffoldless 3D tissue cultures may be superior to 3D tissue cultures prepared by immobilizing cells in extracellular matrices such as alginates, agarose or chitosan3. The advantages of scaffoldless tissues include avoiding immune responses to the scaffolds, degradation of scaffolds in vivo, the effects of non-physiological mechanical properties of the scaffold and avoiding cross reactivity to scaffolds during in vitro testing. There are at least four common methods reported in the literature used to prepare these 3D spherical tissue cultures or multi-cellular tumor spheroids (MCTS) without the need for any scaffolds or matrices. They include (a) the use of centrifugal force to produce cell pellets4, (b) the hanging drop technique5,6, (c) culturing cells at high density to enable self assembly, or (d) placing cell suspensions onto hydrophilic surfaces (agarose or alginate based hydrogels) so that electrochemical forces coerce cells to assemble into spherical structures7. However, these methods are often time consuming and the results are often poorly reproducible8.
The use of synthetic polymer hydrogels may overcome the limitations associated with the previously described 3D tissue culture methods and simplify 3D tissue manufacture. For example, synthetic hydrogel polymers can be used to manufacture microarrays, 96 or 384 well plates and can be made available off-the-shelf. Preparing 3D tissue structures using commercially manufactured hydrogel plates can be as simple as pipetting a known quantity of cells into these plates, incubating the plates for a certain time period (usually 24 h) and then pipetting out the formed 3D cell aggregates for further tests. In fact, Le Gac et al (2008) showed that polydimethylsiloxane (PDMS) microwell arrays may overcome some of the limitations (e.g. reproducibility, sterility, and low production yield) associated with conventional 3D tissue manufacture9. Synthetic polymer hydrogel materials have been used for a long time in the large scale manufacture of soft contact lenses. Therefore, they may also be ideal hydrogel candidates for large scale manufacture of these microarrays, 96 or 384 well plates, etc. The first logical step is to evaluate whether the synthetic polymer hydrogels used to prepare soft contact lenses have the properties required to guide cells (e.g. chondrocytes) to form 3D tissue-like structures. This property was evaluated by simply placing bovine chondrocytes or porcine dentine pulp cells in commercially available soft contact lens and observing whether they can guide the cells to form 3D structures.
Methods
Cell culture
Bovine chondrocytes were harvested from the femoral cartilage of a 2 yr old cow obtained from a local slaughter house and the porcine dental pulp cells were harvested from the dental pulp of a euthanized 3–4 month old female Yorkshire pig. The cells were isolated from their respective tissues within 4 h of tissue harvest. It should be noted that to avoid yeast contamination, the porcine dental pulp tissue was briefly (< 30 sec) washed with 70% isopropyl alcohol. To isolate the cells, the tissues were cut into very small pieces using a scalpel blade and washed twice using regular Dulbecco’s Modified Eagle Medium DMEM, (Invitrogen, Carlsbad, CA) and 5 ml solution of TrypLE™ Express (Invitrogen, Carlsbad, CA) containing 10 mg collagenase type 2 (Worthington, Biochemical Corp., Lakewood, NJ) was added to the washed pieces in a 50 ml centrifuge tube. The centrifuge tube was placed in a shaker water bath for 2 h and maintained at 37°C. The dissociated cells along with the remaining partially dissociated tissue fragments were centrifuged to remove the collagenase and washed twice using regular DMEM media. The washed cells along with the tissue fragments were then placed in T25 cell culture flasks containing high glucose concentration DMEM media supplemented with 10% fetal calf serum (Invitrogen, Carlsbad, CA), 100 units/ml penicillin, and 100 µg/ml streptomycin (complete medium). The cell culture flasks were maintained at 37°C in a humidified atmosphere containing 5% CO2. The cells were allowed to grow in a monolayer for 15 days. Every time (3–5 day interval) the cells reached confluence, they were trypsinized (TrypLE™ Express, Invitrogen, Carlsbad, CA), passaged at 1:1 dilutions and grown in complete medium.
Induction of 3D cell self-assembly using commercially available soft contact lens or 2% agarose hydrogel surfaces
2% agarose hydrogels (control group) were prepared by mixing 2 g of agarose in 100 ml PBS and boiling the mixture in a microwave oven. The clear agarose solution was then poured into six well plates and was allowed to form a hydrogel. For the contact lens group, (chemical composition: galyfilcon A (Acuvue advance™, Johnson & Johnson Vision Care, Inc, Jacksonville, FL), enfilcon (AVAIRA™, Cooper Vision, NY) or Etafilcon A (Acuvue2™, Johnson & Johnson Vision Care, Inc, Jacksonville, FL)), the contact lenses were removed from their sterile packaging and one placed in each well of a 12 well plate. Once placed in the plates, the contact lenses maintained their concave shape and were able to hold up to 200 µl of media without any shape deformation. 100 µl of media containing 6.0 × 105 – 5 × 106 of the desired cells (counted using the automated cell counter, TC10, Bio-Rad Laboratories, Inc., Hercules, CA) were placed carefully into the contact lens or on top of the 2% agarose hydrogels using a pipette. The cells were allowed to coalesce for 3 hours and then 3 ml of complete medium was added to each well surrounding the contact lens to keep them moist (Figure 1). Within 24 hours, the cells coalesced completely and formed a single 3D tissue like structure. The micromass was removed, placed in fresh 12 well culture plates and the soft contact lens or agarose plates were discarded. The constructs were maintained for 3 weeks and the media was changed regularly every 3–4 days. The experiments were conducted in triplicates. After 3 weeks, the micromass was then frozen sectioned; hematoxylin and eosin (H&E) stained and observed under a microscope.
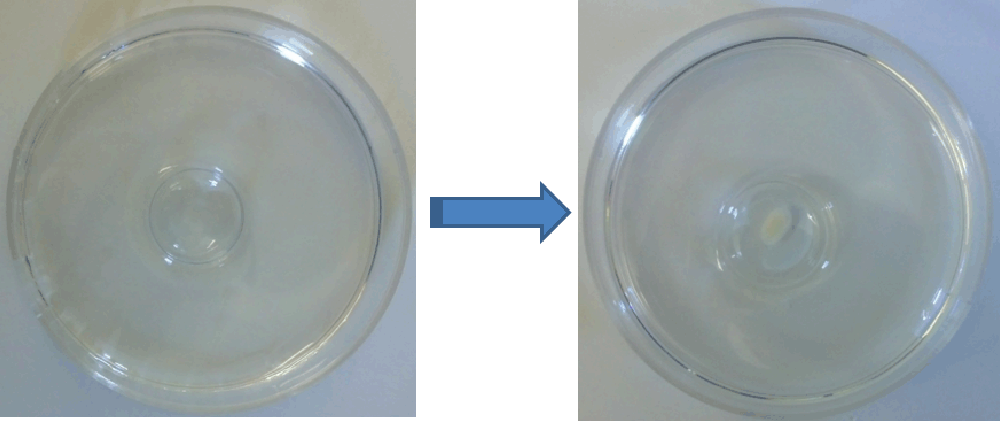
Figure 1.
Experimental set up used to evaluate the formation of 3D tissue structures using synthetic polymer hydrogels (in the form of a contact lens, pictured left) and a photograph of a 3D micromass formed using chondrocytes (5 × 106 cells) after 3 weeks (right).
Results
Both bovine femur chondrocytes and porcine dental pulp cells self-assembled when placed in contact either with the contact lens or 2% agarose hydrogel surfaces. None of the cells seemed to adhere to the soft contact lens or 2% agarose hydrogel surfaces. The micromass formed was firm after 24 h and did not crumble during handling (e.g. pipetting). This shows that the cells were already producing an extracelluar matrix to hold them together. However, these micromasses continue to shrink and by day 10 it reaches a stable size. After 3 weeks, the final diameter of the micromass depended on the initial number of cells used. The micromass (irregular in shape) was at least 1.5 mm in diameter when 6 × 105 cells were used and 2–3 mm in diameter when 1.2 × 106 cells were used. Visual examination of 3D constructs showed that the type of contact lens used did not seem to make a difference in the size of the micromass at either day 5 (Figure 2) or day 21 (Figure 3). However, further studies are needed to evaluate whether there are any differences in the matrix composition between 3D constructs formed in agarose gels versus different soft contact lens hydrogel surfaces. From a handling point of view thicker contact lenses worked better (e.g. enfilcon (AVAIRATM, Cooper Vision, NY)) and tended to maintain their shape during media placement (Figure 4). Both galyfilcon and etafilcon had similar thickness/handling properties (they both tended to fold easily) and were thinner compared to the enfilcon. It should be noted that the ease of handling is based purely on thickness of the lens, rather than the materials themselves. An example of the frozen section of micromass tissue formed with either bovine femur chondrocytes or porcine dental pulp cells is shown in Figure 5a and 5b respectively. Hematoxylin and eosin (H&E) stain was used to visualize the cells and the presence of extracellular matrix produced by the cells.

Figure 2.
Chondrocytes grown in (top to bottom) hondrocytes grown in (top to bottom) enfilcon (AVAIRA™, Cooper Vision, NY), galyfilcon A (Acuvue advance™, Johnson & Johnson Vision Care, Inc, Jacksonville, FL), and etafilcon A (Acuvue2™, Johnson & Johnson Vision Care, Inc, Jacksonville, FL)) at 5 days.
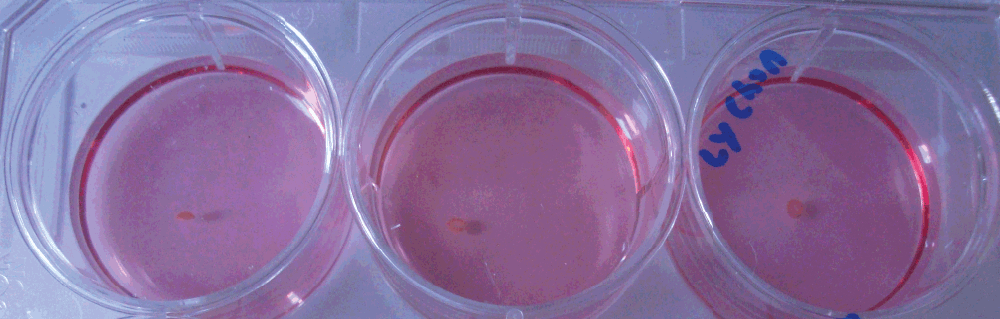
Figure 3. Micromass at day 21.
On visual inspection of the micromass it was concluded that the type of contact lens used did not seem to make a difference. The micromass (left to right) was formed in galyfilcon A (Acuvue advance™, Johnson & Johnson Vision Care, Inc, Jacksonville, FL), enfilcon (AVAIRATM, Cooper Vision, NY), and etafilcon A (Acuvue2™, Johnson & Johnson Vision Care, Inc, Jacksonville, FL) respectively.
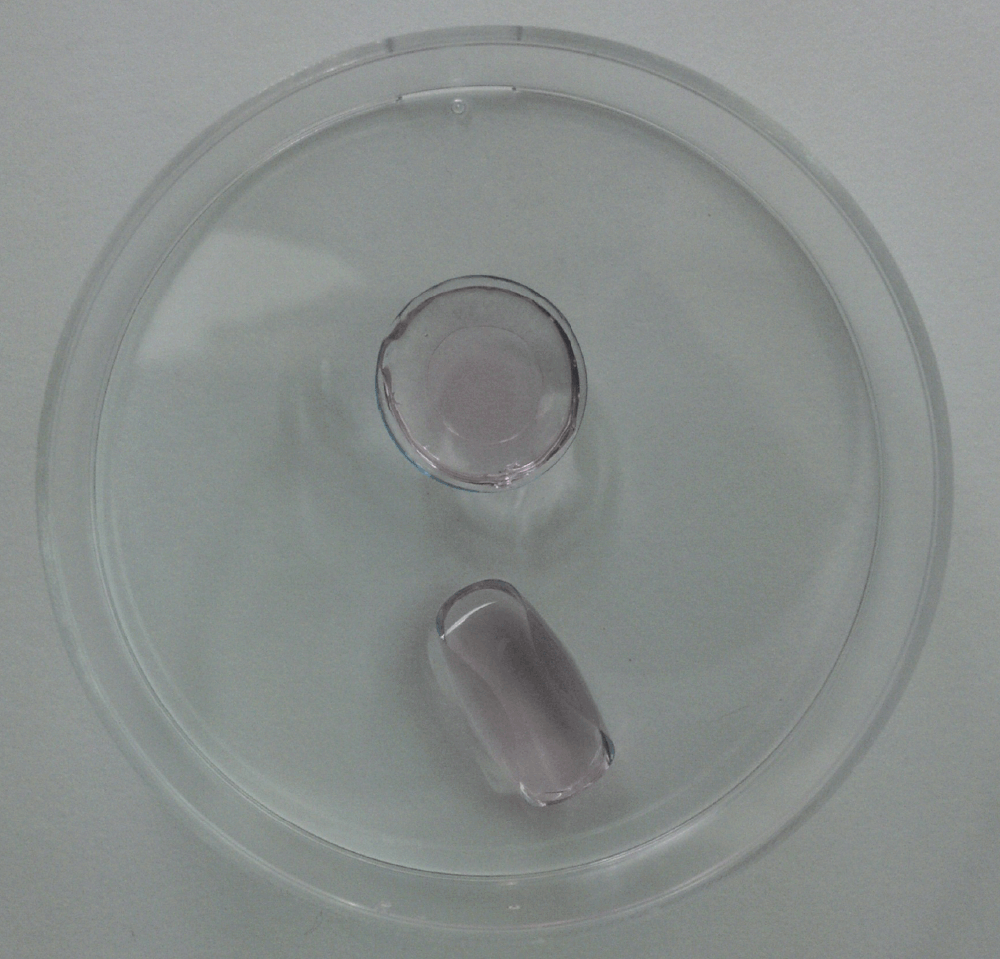
Figure 4.
Thinner contact lenses (bottom) tended to fold and release the cell contents during initial cell placement.
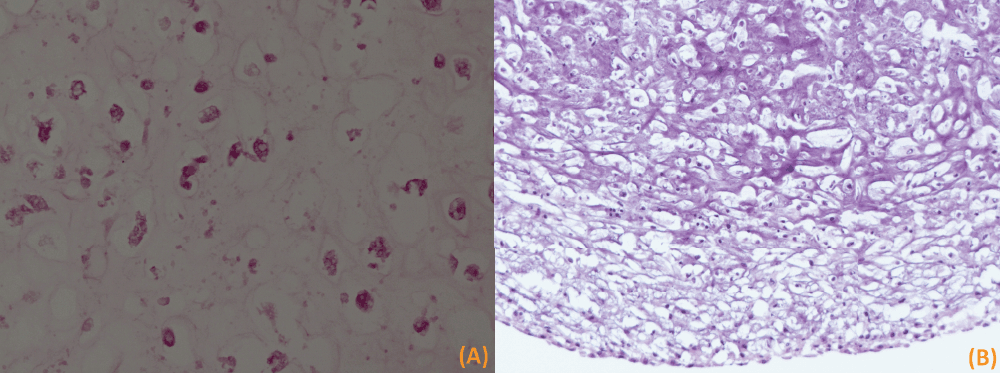
Figure 5a and 5b.
Frozen H&E section (400X) of 3 week old 3D tissue structure formed using bovine femur chondrocytes (a) and porcine dental pulp cells (b). The microscopy slide shows viable cells embedded in a hyalinized background (hydrogel material). The cells show rounded slightly eccentric nuclei with a clear space around them.
Discussion
Hydrogels such as agarose, alginate or PDMS have been used before to guide or coax cells to form 3D multicellular tissues3,9. This study evaluated the use of synthetic polymer hydrogels used to prepare soft contact lenses to do the same. The results showed that both chondrocytes and dentine pulp cells reproducibly formed 3D tissue structures when placed inside these soft contact lenses. Therefore, the study demonstrated that the synthetic polymer hydrogels (e.g. galyfilcon A, enfilcon or etafilcon A or even poly HEMA10) may provide an alternative to agarose, alginate or PDMS based hydrogel surfaces.
It is well known that soft contact lens can be manufactured in large scale without much variability within batches and stored for a long time in sterile solutions. The same technology can theoritically be used to manufacture and store 3D cell aggregation devices in the form of microarrays, 96 or 384 well plates using these synthetic polymer hydrogels. A commercially available 3D cell aggregation device manufactured using synthetic polymer hydrogels has the potential to simplify and help large scale manufacture of reproducible 3D cell cultures for various applications including in vitro chemical toxicity testing and preparation of small scaffold-less tissue engineered constructs.
Author contributions
SS conceived the study. SS and GF designed the experiments and carried out the research. EW and MS contributed to the design of experiments and the preparation of manuscript. JK helped with the histology sections. All authors were involved in the revision of the draft manuscript and have agreed to the final content.
Competing interests
No competing interests were disclosed.
Grant information
This study was supported by Albert Einstein Society, Philadelphia, PA with an internal grant provided to Solomon Samuel.
Faculty Opinions recommendedReferences
- 1.
Caron MM, Emans PJ, Coolsen MM, et al:Redifferentiation of dedifferentiated human articular chondrocytes: comparison of 2D and 3D cultures.
Osteoarthritis Cartilage.
2012; 20: 1170–1178.
- 2.
Mandl EW, Van Der Veen SW, Verhaar JA, et al:Multiplication of human chondrocytes with low seeding densities accelerates cell yield without losing redifferentiation capacity.
Tissue Eng
2004; 10: 109–118.
- 3.
Bernstein P, Dong M, Corbeil D, et al:Pellet culture elicits superior chondrogenic redifferentiation than alginate-based systems.
Biotechnol Prog
2009; 25: 1146–1152.
- 4.
Detzel CJ, Van Wie BJ: Use of a centrifugal bioreactor for cartilaginous tissue formation from isolated chondrocytes.
Biotechnol Prog
2011; 27: 451–459.
- 5.
Tung YC, Hsiao AY, Allen SG, et al:High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array.
Analyst
2011; 136: 473–478.
- 6.
Timmins NE, Nielsen LK: Generation of multicellular tumor spheroids by the hanging-drop method.
Methods Mol Med
2007; 140: 141–51.
- 7.
Hu JC, Athanasiou KA: A self-assembling process in articular cartilage tissue engineering.
Tissue Eng
2006; 12: 969–979.
- 8.
Napolitano AP, Dean DM, Man AJ, et al:Scaffold-free three-dimensional cell culture utilizing micromolded nonadhesive hydrogels.
Biotechniques
2007; 43: 494–500.
- 9.
Le Gac S, Rivron N, Wijnperle D, et al:Cellular microtissues spontaneously formed in a microfabricated device for angiongenesis. In: 12th International Conference on Miniaturized Systems for Chemistry and Life Sciences, 12–16 October 2008, San Diego, CA.
- 10.
Phung YT, Barbone D, Broaddus VC, et al:Rapid Generation of In Vitro Multicellular Spheroids for the Study of Monoclonal Antibody Therapy.
J Cancer
2011; 2: 507–514.





Comments on this article Comments (0)