Keywords
Chronic kidney disease; epidemiology; genetic renal diseases; renal hypodysplasia; urinary bladder, neurogenic
Chronic kidney disease; epidemiology; genetic renal diseases; renal hypodysplasia; urinary bladder, neurogenic
In chronic kidney disease (CKD) there is permanent bilateral kidney damage, structural or functional, with or without a decrease in glomerular filtration rate. Reported childhood CKD rates are far lower than that of young adults and depend on discovered cases, possibly missing asymptomatic cases.
In our population-based study, we found pediatric CKD rates 10 times higher than previous studies, 50% of them CKD stage 1. This may explain the CKD burden beyond pediatric age.
Chronic kidney disease (CKD) is defined as evidence for bilateral kidney damage for more than 3 months, which can be structural or functional, with or without a decrease in glomerular filtration rate1. The presence of CKD at any stage is a strong risk factor for renal function deterioration during lifetime, many times beyond the pediatric age2.
The prevalence of pediatric CKD (p-CKD; stage ≥2) in developed countries (such as in Italian3 and Spanish studies4) has been estimated to be as high as 75 cases/106 population at risk. However, until recently, such estimates were based on CKD retrospectively detected by tertiary care centers, which does not allow the determination of true incidence as patients who do not have advanced CKD ("overt CKD") may not be identified. In addition, these pediatric numbers are much lower than reports from American young adults (up to 40,000/106 for ages 20–40 by the National Health and Nutrition Examination Survey (NHANES studies)5. Thus, the extent of p-CKD may be underestimated.
The Negev population receives tertiary care services through a single medical institution, the Soroka University Medical Center (SUMC), which serves a population (including referrals) of 300,000 children and takes care of more than 15,000 deliveries a year. The SUMC Pediatric Nephrology service is the only caregiver for children in the area affected by kidney and urinary tract diseases. The population served by this hospital is composed of two major subpopulations, usually living in separate settlements: Jews (75%) and Arab-Bedouins (25%). The consanguinity rate among Jewish couples is nowadays low and estimated as 2.3%, including 0.8% first cousin marriages6. The Negev Bedouins' current estimated population is 200,000. The total fertility rate in the Bedouin-Muslim population in 2006 was 7.3, compared with to 3.3–4.1 in other Muslim populations in Israel and 2.62 in the Jewish population in southern Israel. In Bedouin society, cousin marriage is the preferred type of marital union and traditionally involves marriage between first-degree cousins7,8. Currently, 60% of this population is under 19-years-of-age9. More than half of the Bedouin population live in unrecognized villages and shanty towns, and suffer from a high rate of unemployment. However, all Israelis are entitled to medical care as a result of a National Health Insurance plan introduced in 1995. The reported pediatric CKD stage 5 end-stage renal disease (ESRD) incidence for 2006 in this area was 19 cases/106/year10, similar to reports from developed countries11. Previous studies from this center provide reliable population-based epidemiological data12,13. The purpose of this study was to assess the prevalence and etiology of p-CKD in Southern Israel.
The study protocol was approved by the hospital's ethics committee (approval number: 10568). We reviewed all recorded cases of p-CKD among inhabitants of the Negev area (and not referrals from other country areas) during the January 1994-December 2008 period, based on a review of medical records of children detected by the Pediatric Nephrology service to have this condition. This was double-checked by screening the hospital's Medical Records department discharge diagnoses to look for additional children (age 0–19 years) with CKD diagnosis. For the retrospective analysis, we graded based on the worst CKD stage reached by the children. We calculated CKD prevalence and stage according to the living child’s status (CKD stage 1–5) as at Dec 31, 2008. In addition, we prospectively collected all new CKD cases diagnosed in our hospital, from January 1, 2009 until December 31, 2010. Data from medical records were entered into standardized forms, including demographics, age of onset, underlying renal disease, etc. The standardized form used is provided in the data file.
Estimated glomerular filtration rate (eGFR) was calculated by the original Schwartz formula14. Hypodysplasia was defined by imaging studies as a congenital reduced kidney size and maldevelopment of the renal tissue (including renal scars), with or without associated malformations in the urinary tract (hydronephrosis, hydroureter, vesicoureteric reflux), in both urinary tract systems. Unilateral urinary tract anomalies and/or a single kidney were not included unless an additional feature of CKD (such as proteinuria or hypertension) was present. Obstructive uropathy was divided between neurogenic bladder and congenital obstruction. Genetic renal disease was diagnosed only for monogenic diseases that are known to be associated with a decreased renal function with age. Therefore, diseases such as Bartter syndrome or nephrogenic diabetes insipidus were not included. CKD stage 1 was defined as an eGFR of equal or more than 90 ml/min/1.73 m2 (a normal GFR value) but with the presence of either persistent albuminuria (as may exist for reflux or diabetic nephropathy) or bilateral structural renal anomalies (as in polycystic kidney disease, hypodysplasia, or bilateral renal scars). CKD stage 2 was defined as a eGFR between 60–90 ml/min/1.73 m2; CKD stage 3 was a eGFR between 30–60 ml/min/1.73 m2, CKD stage 4 was a eGFR between 15–30 ml/min/1.73 m2 and CKD stage 5 was defined as a GFR of < 15 ml/min/1.73 m2.
A direct age adjustment method was performed to compare the prevalence and incidence of p-CKD between the Jewish and Bedouin populations using children under the age of 19 as standard population. Population statistics were obtained from The Israeli Central Bureau of Statistics national census. Statistical analysis was performed using the SPSS software, version 18.
We identified 192 living p-CKD patients at December 31, 2008 (46.1% of Jewish origin, 53.4% males) during the retrospective study period. The population at risk at that time was 241,400, as provided by the Israeli Census Bureau (http://www1.cbs.gov.il/reader/shnaton/templ_shnaton_e.html?num_tab=st02_10x&CYear=2010), providing a point prevalence of 795.4 (95% confidence interval (CI): 690.6–916.0) per million (Table 1). The age-adjusted Jewish p-CKD prevalence rate was 628.2 (95% CI: 549.2–751.9) per million, whereas the age-adjusted Bedouin p-CKD prevalence rate was 850.2 (95% CI: 764.4–1000.8) per million. Incidence for the whole study period (1989–2009) was 45.4 (95% CI: 39.7–53.0) per million. When different pediatric age groups were analyzed separately, a higher rate was found in younger children (age 0–4 yrs) of Bedouin origin than children of Jewish origin. Rates were similar between the two groups at ages 5–14 and 15–19 years (Figure 1).
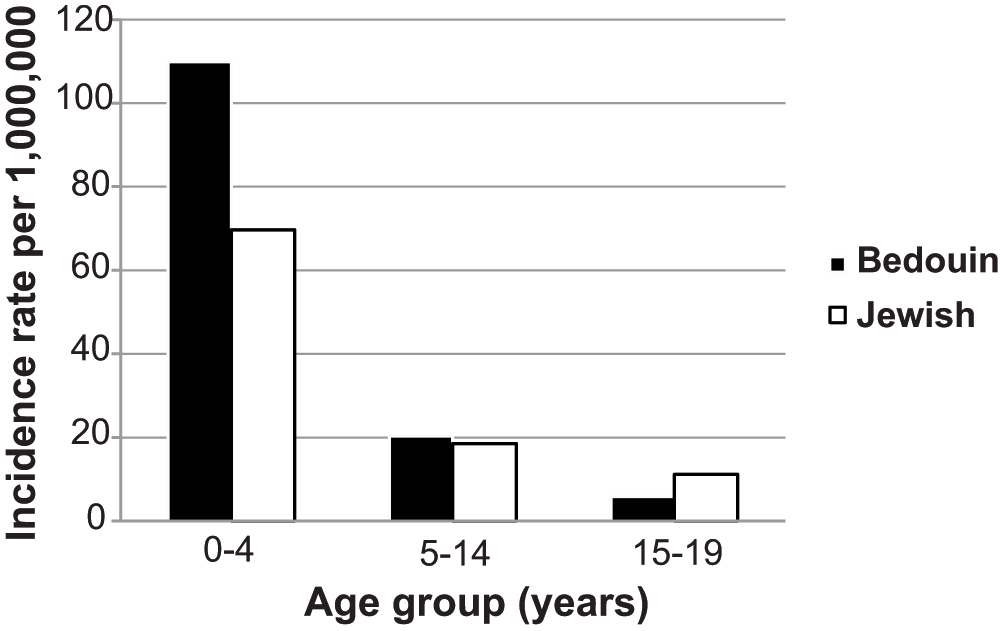
X axis: age groups (years). Black bars: children of Bedouin origin. White bars: children of Jewish origin. No p values for comparison are provided since the total population (and not a sample) is analyzed.
* Refers to living patients aged < 19 years on December 2008. Prevalence is adjusted for age. ** Mostly autosomal dominant polycystic kidney disease (ADPKD); *** Mostly atypical hemolytic uremic syndrome and infantile nephronophtisis. No p values for comparison are provided since the total population (and not a sample) is analyzed.
The mean age of diagnosis was 4.9±6 years, but the median age of diagnosis was 1 year. The main p-CKD etiologies were: hypodysplasia: 35%; obstructive uropathy: 13%; genetic renal diseases: 28% and glomerulonephritis: 15%.
The proportions of children in each CKD stage were as follows: stage 1: 50%; stages 2–4: 30%; stage 5: 20%. One hundred thirty-one children were diagnosed with CKD stage 1, which included 44 children with bilateral renal hypodysplasia (with or without renal scars), 10 children with obstructive uropathy (five of them with congenital obstruction of both kidneys and five with neurogenic bladder and bilateral renal scars), 35 children with genetic renal diseases (14 with autosomal dominant polycystic kidney disease (ADPKD) and 10 with the autosomal recessive form (ARPKD)), and 28 children with chronic glomerulonephritis (10 with IgA nephropathy, five children with Henoch Schoenlein purpura nephropathy and residual proteinuria). Miscellaneous diagnoses14 included seven children and adolescents with diabetic nephropathy.
Children of Bedouin origin had a lower median age of diagnosis (0.3 vs 4 years in Jews, p < 0.01). Twelve children died during the retrospective study period, 11 of them of Bedouin origin, six of them reached ESRD due to two genetic renal diseases: atypical hemolytic uremic syndrome and infantile nephronophtisis. The other five children had hypodysplasia, four of them as part of multiple congenital anomalies, and only one of these 5 children reached ESRD. The one child of Jewish origin that died had Lowe syndrome and died of neurologic complications at CKD stage 2. The same rate of Jewish and Bedouin children (15%) had a renal transplant.
The prevalence of CKD at stage 2 or above was 331/106 (95% CI: 266.3–412.4), a higher number than that reported from the series in Spain and Italy 71/106 and 75/106 (Table 2). The male: female ratio and age of diagnosis were similar between the series. However, the fractions of CKD children who had a genetic renal disease or obstructive uropathy were higher in our area.
* Age at registration: 6.9±5.4. ** Including congenital obstructive uropathy.
| Measure | Southern Israel | Spain | Italy |
|---|---|---|---|
| Reference | This study | Areses et al.4 | Ardissino et al.3 |
| Date of evaluation | Dec 31, 2008 | Dec 31, 2008 | Jan 1, 2001 |
| Age for inclusion (yr) | < 19 | < 18 | < 20 |
| Population at risk (106) | 0.24 | 11.3 | 16.8 |
| Prevalent cases | 80 | 605 | 1197 |
| Prevalence (cases/106) | 331.4 | 71 | 75 |
| % male | 72 | 66 | 66 |
| Age of diagnosis (yr) | 2.7±4.8 | 3.9±5 | NA* |
| CKD etiology (%) | |||
| Hypodysplasia | 52.8 | 59 | 58** |
| Genetic renal disease | 28 | 14 | 15.6 |
| Obstructive uropathy | 10.1 | NA | 3.7 |
| Glomerulonephritis | 4.5 | 3 | 5.8 |
| CKD stage (%) | |||
| 2 | 50 | 42 | 39 |
| 3 | 21.2 | 40 | 35 |
| 4 | 15 | 15 | 26 |
| 5 | 13.8 | 4 | Not included |
During a subsequent 2 year period (January 2009 – December 2010), we identified 26 new children with CKD, providing a yearly incidence of 53.8 (95% CI: 31.5–92.1) new cases/106/yr (half of them at CKD stage 2 or above). The mean age of diagnosis for CKD (stage 2 or above) in the prospective cohort was 2.7±4.8 years (interquartile range: 0–6 yrs, median age: 0.5 yrs).
The p-CKD prevalence reported in this study is higher than that reported for other recent series from developed countries, such as Italy and Spain (Table 2). We chose to compare our data with these two European countries because of the similarities in accessibility to medical services. A Universal Health Care system by law has been implemented in Israel since 1995 and provides comprehensive medical care to all Israelis. Infant mortality has dropped in Israel in the past decades and is now comparable to most developed countries15. In addition, access to prenatal diagnosis by different screening tests including frequent fetal ultrasound studies has become the standard of care for the great majority of the Israeli population: 90% of Israeli pregnant women have at least one fetal ultrasound performed (Drs Shochat and Romano-Zelicha, Israeli Ministry of Health survey, personal communication). This may allow the detection of many congenital anomalies of the kidneys and urinary tract as well as some of the genetic renal diseases that cause CKD (such as polycystic kidney disease). It may also be the reason for the low median age of detection of p-CKD in our cohort (1 year for the general cohort and as low as three months for the Bedouin population) (Table 1). The lower mean age of diagnosis for the Bedouin population may reflect the higher rate of genetic renal diseases in our area (28% in Southern Israel vs. 14% and 15.6% in Spain and Italy respectively)11. In addition, we found a higher rate of obstructive uropathy due to neurogenic bladder, most of these cases in the Bedouin population, similar to reports from Turkey16, again raising the possibility of the contribution of consanguinity or poverty (such as lower access to folic acid supplementation). However, a similar male predominance and high prevalence of renal hypodysplasia as the main etiology for CKD is seen in these three series.
It must be remembered that this apparent higher p-CKD rate is still an underestimation of the probable true p-CKD prevalence that may be detected if population screening studies are performed. Such studies have been performed in recent years in Australia, where persistent albuminuria or hypertension were found in 1–2% of prospectively screened school-age children17. Similar reports come from Japan, Taiwan and Korea18. Furthermore, the striking difference in CKD rates between pediatric and young adult populations needs to be addressed. The overall prevalence of CKD stages 1 to 4 in adults increased significantly in the 1999 to 2004 period compared with 1988 to 1994 (13.1 versus 10.0 percent respectively)19. Data from the NHANES 2003–2006 survey, with GFR estimated by the CKD-Epidemiology Collaboration study (CKD-EPI) equation, showed that the overall prevalence of CKD stages 1 through 5 among adults is 14.2%20. In other countries, CKD prevalence ranges between 1 to 30%21. This is not only true for the aged population, since a CKD (stage ≥2) prevalence rate as high as 4% (or 40,000/106 has been reported for the 20–40 year old population22.
There are fewer reports on the incidence of CKD among adults. In the Framingham cohort (n = 2580), after a mean follow-up of 18.5 years, 244 participants (9.4%) had developed CKD stage 3 and above23, which is a yearly incidence of about 500 new patients/million/year. If one assumes an overall CKD prevalence of 10% (or 100,000 per million) this provides a prevalence-to-incidence ratio of 200 in adults, which is expected given the chronic nature of CKD.
A major cause of mortality in adult CKD is increased cardiovascular comorbidity, in addition to ESRD progression24. When compared to the pediatric population (which includes the first two decades of life vs. the subsequent average of six decades in adults), where no clinical cardiovascular morbidity has yet developed and mortality is lower than adults, one would expect a higher than reported prevalence-to-incidence ratio, which has been only around 6:1 in various previous studies. In our study, we report a ratio of around 15:1. This suggests an underdiagnosis of CKD in the pediatric population. In addition, all studies performed to-date exclude CKD stage 1, which may be incorrect given the more long-term follow-up required for pediatric patients. The potential for gradual clinical progression, even from CKD stage 1, has been demonstrated for renal hypodysplasia in Italian25 and Dutch26 surveys, and is a well recognized requirement for diabetic nephropathy, in both type I27 and, more importantly, type II diabetes mellitus28. Supporting all these observations is a recent series of publications based on a true population frequency in Turkey, which demonstrate that the prevalence of childhood CKD approaches 1%, a number in the same order of magnitude as that of the younger adult cohort in the United States29.
In summary, we report on a higher prevalence and incidence for p-CKD in Southern Israel and propose that, if proper population-based screening studies are performed, then higher p-CKD values should be found in other developed countries. This may expand the contribution of pediatric renal disease to CKD even beyond the pediatric age.
DL conceived the study design, participated in data collection and elaboration, as well as writing the final manuscript version. RS participated in data collection and reviewed the manuscript draft. AK participated in data collection and analysis. AV an epidemiologist, reviewed the data quality, helped in the validation of data and its adjustment for local population data. HS participated in data collection, conceived and reviewed the manuscript draft. All authors reviewed and agreed on the final version of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 13 Sep 13 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)