Introduction
Normal kidney development and function requires coordinated cell signalling through complex molecular pathways1. The Eph receptor tyrosine kinases and their ephrin ligands, best known for their role in the development of the nervous and vascular systems, have recently been implicated in mammalian kidney development and maintenance2–4. Ephs and ephrins are divided into A and B subclasses, with ephrin-As typically binding to EphAs and ephrin-Bs to EphBs. Exceptions to this rule are ephrin-B2 and B3, which can bind to EphA4, and ephrin-A5, which can bind to EphB25,6. Within the kidney, EphBs and ephrin-Bs have been implicated in the development and maintenance of the slit diaphragm2, a component of the filtration barrier in the glomerulus, and also in renal tubule development4. However, the expression and function of the other family of ligands, the ephrin-As, has not been investigated.
A possible role for ephrin-As in kidney development and/or function was suggested by sporadic unexpected deaths in a breeding colony of mice lacking ephrin-A2 and/or ephrin-A5 (6.25% over a period of 4 years). Autopsy revealed that these mice had a single remaining kidney that had partially or fully degenerated to a fluid-filled sac. Subsequent dissections of mice used for other research purposes confirmed that mice lacking ephrin-A5 (both ephrin-A5-/- single knockouts and ephrin-A2A5-/- double knockouts, but not ephrin-A2-/- single knockout mice) often had only one kidney with the other either absent, abnormally small, or degenerating. To further investigate a possible role for ephrin-A5, we performed immunohistochemistry for ephrin-A2 and ephrin-A5 in kidneys of normal adult WT mice and carried out quantitative morphological analysis of renal corpuscles and tubules in haematoxylin and eosin stained sections of WT, ephrin-A2-/-, ephrin-A5-/- and ephrin-A2A5-/- (knockout) mice.
Materials and methods
Animals
Ephrin-A2-/-, ephrin-A5-/- and ephrin-A2A5-/- knockout mice were a generous gift from Prof David Feldheim7. These mice were backcrossed to C57Bl/6j strain for more than 13 generations and were maintained in a breeding colony at the University of Western Australia. Wild-type (WT) mice from the same genetic background (C57Bl/6j) were used as controls. Mice were housed in standard cages with clear plastic walls (17 cm × 19 cm base, 16 cm high) in a controlled environment (12/12 light/dark cycle; temperature 22°C±2°C) with food and water ad libitum. Kidneys were collected from terminally euthanased mice (160 mg/kg pentobarbitone, i.p.; Lethabarb, Virbac Australia) after transcardial perfusion with 4% paraformaldehyde (Sigma Aldrich, St Louis, Montana USA) and preserved in 4% paraformaldehyde for up to 24 months following sacrifice. The histology of renal corpuscles and tubules was examined within one kidney from six ephrin-A5 knockout mice, five ephrin-A2A5 knockout mice, three ephrin-A2 knockout mice, and seven wild type mice. Mice were aged 50–189 days at the time of sacrifice and age was included as a factor in our analysis. All procedures in this study were conducted in accordance with US NIH guidelines and approved by The University of Western Australia Animal Ethics Committee.
Tissue preparation
For immunohistochemistry, kidneys were dissected through the mid-sagittal line and were cryopreserved in 30% sucrose (VWR international BVBA, Belgium, 27480.360) in PBS overnight before freezing and cryosectioning on a Leica Cryostat CM1900 at -19°C. Sections were cut at 30 µm free-floating into PBS (NaCl, VWR international BVBA, Belgium, 27810.364, KCl, Chemsupply, Australia, Na2HPO4, VWR international BVBA, Belgium, 28026.36, H2PO4, BDH chemicals, Australia, 10203.4B) with 0.02% Sodium Azide (Sigma, USA, S-2002). Endogenous peroxidises were quenched with 10% MeOH-3% H2O2 in PBS for 20 minutes at RT, followed by 2×10 minute washes with PBS. Cells were permeabilised with Triton-X100 (BDH chemicals, Australia, prod 30632) (0.3% in PBS) for 15 min, and incubated in blocking solution (10% Normal Donkey Serum (Millipore, USA, S30–100 ml) and 5% Bovine Serum Albumin (Sigma, St Louis, USA, A-7888) in PBS) for 3 hours at RT. Sections were then incubated in primary antibodies (anti-Ephrin-A2 rabbit polyclonal, Santa Cruz Biotechnologies (California, USA) SC-912, diluted 1:200 in blocking solution; anti-Ephrin-A5 rabbit polyclonal, Santa Cruz Biotechnologies SC-20722 diluted 1:75 in blocking solution) at 4ºC overnight with agitation. Sections were washed for 3×10 minutes in blocking solution at RT and detection was carried out using the Vectastain ABC kit (Vector, USA, PK6101) following the manufacturer’s instructions. Briefly, anti–rabbit-biotinylated secondary antibody (Vector BA-1100) was diluted 1:300 in blocking solution and applied to sections for 3 hours at RT. Sections were washed 10 minutes in blocking solution, 3×10 minutes in PBS and the ABC solution was applied for 1 hour at RT. Sections were washed 3×10 minutes in PBS and DAB (Thermo Scientific, USA, 34065) applied for 3 minutes. Sections were washed in PBS for 10 minutes, dehydrated in increasing concentrations of ethanol, defatted in xylene and mounted in Entellan (Merck, Germany).
For haematoxylin and eosin (H&E) staining, kidneys dissected through the mid-sagittal line were processed through dehydration and wax infiltration procedures and then sectioned at 6 µm using a microtome. Wax sections were floated onto slides in a hot distilled water bath, de-waxed using xylene and decreasing concentrations of ethanol, stained with haematoxylin (2%; Sigma Aldrich) and eosin (1%; Sigma Aldrich), dehydrated in increasing concentrations of ethanol and mounted with Entellan.
Histological analyses
H&E stained sections photographed using an Olympus DP70 digital camera. Structural aspects of renal corpuscles and tubules within the cortex of each kidney were measured using ImageJ software. Three images were captured under light microscopy at evenly spaced positions across the cortex of each kidney section. Images were captured at 100× magnification for examining renal corpuscles and at 400× magnification for tubules.
Renal corpuscles: In order to determine cell density within glomeruli, nuclei were counted using the cell counter add-on in ImageJ. The number of nuclei within each glomerulus was then divided by the cross-sectional area of each glomerulus. In order to determine relative glomerular size, the cross-sectional area of each glomerulus was divided by the cross-sectional area of each associated renal corpuscle and was converted to a percentage. An average value for glomerular cell density and relative glomerular size were calculated for each mouse.
Renal tubules: To quantify cell density within tubule tissue, nuclei were counted using the cell counter add-on in ImageJ. The number of nuclei within each tubule was then divided by the cross-sectional area of tubule tissue. In order to determine the cross-sectional area of tubule tissue, the difference between the cross-sectional area of each tubule and the cross-sectional area of each associated tubule lumen was converted to a percentage. Only latitudinally sectioned tubules were analysed. Average values for nuclei density within tubule tissue and the cross-sectional area of tubule tissue were calculated for each mouse.
Statistical analyses
The averaged values for each renal corpuscle and tubule measurement were compared between strains using a multivariate permutational ANOVA (MANOVA) in PERMANOVA+ for PRIMER8. PERMANOVA+ is a highly robust non-parametric testing program and therefore does not require agreement with the usual ANOVA assumptions of parametric tests. The MANOVA was computed with age at sacrifice as a covariate to account for the effects of aging on kidney morphology. Each variable was then analysed univariately in PERMANOVA+ with age at sacrifice as a covariate. The four variables were also analysed between mice with and without the ephrin-A5 gene using a MANOVA in PERMANOVA+ such that ephrin-A5/ephrin-A2A5 knockout mice and ephrin-A2 knockout/wild type mice were combined to form two groups. The measured variables were then analysed between the two groups univariately in PERMANOVA+ with age at sacrifice as a covariate. These groups were formed to increase sample sizes and were considered valid because mice homozygous for the gene for ephrin-A2 were unaffected by renal failure. Data were normalised prior to conducting each MANOVA. All analyses were computed using 9999 permutations from a resemblance matrix based on Euclidian distance.
Results
Immunohistochemistry for ephrin-A2 and ephrin-A5
Ephrin-A2 and ephrin-A5 expression patterns were examined in the adult mouse kidney using immunohistochemistry (Figure 1). For both proteins, expression was strongest in the medulla in the Loops of Henle with weaker staining in the pyramids (Figure 1A,D). In the cortex, cells within the proximal and distal tubules were strongly labelled and this was most prominently visualised when tubules were cut in cross section (Figure 1B,E). Within the glomeruli, expression was detected in podocytes and in the squamous cells lining the Bowman’s capsule (Figure 1C,F). The major difference in expression pattern was that ephrin-A5, but not ephrin-A2 expression, was detected in cells along the length of the tubules.
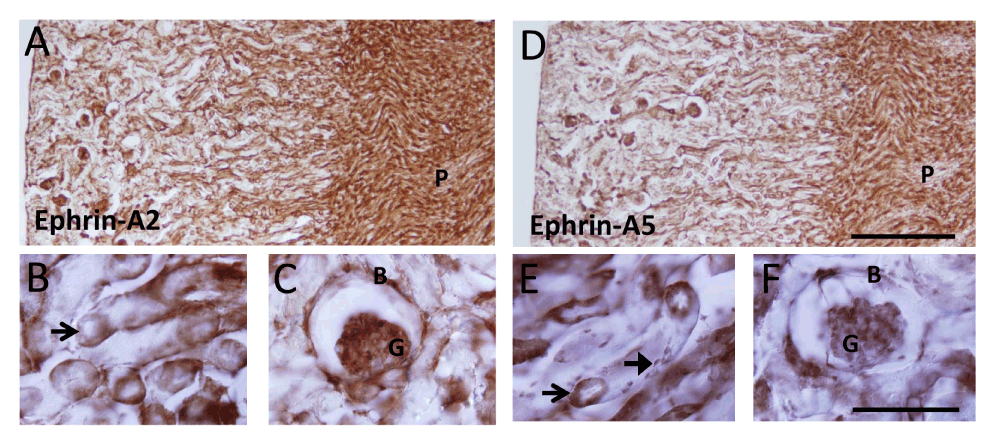
Figure 1. Photomicrographs showing immunohistochemistry for ephrin-A2 (A–C) and ephrin-A5 (D–F).
A,D: low power images showing strong expression in the medulla. P: Pyramids. B,E: High power image showing expression in cells within the proximal and distal tubules, most prominently visualised when tubules were cut in cross section (open arrows). Note expression of ephrin-A5, but not ephrin-A2 in cells along the length of the tubules (closed arrow, E). C,F: Expression was also detect in glomeruli, in podocytes and in the squamous cells lining Bowman’s capsule. Scale bars: A,D: 250 µm; B,C,E,F: 50 µm. B: Bowman’s capsule; G: glomerulus; P: pyramid.
Renal corpuscle and tubule structure between the four strains
Cell density within glomeruli and tubule tissue, glomerular size, and the cross-sectional area of tubule tissue, did not differ significantly between the four strains (multivariate analysis: p = 0.2684; pairwise comparisons are shown in Table 1). The relationship between age at sacrifice and each dependent variable was not significant (Table 1).
Table 1. Summary of univariate statistical results for the effects of strain and age at sacrifice, on renal corpuscle and tubule structure.
An alpha level of 0.05 was used to determine significance. All p-values are not significant.
| Measure | Strain | Age |
|---|
| % glomerulus | 0.146 | 0.396 |
| Glomerular cell density | 0.681 | 0.900 |
| % tubule tissue | 0.631 | 0.291 |
| Tubule cell density | 0.149 | 0.499 |
No differences in cell density within glomeruli or tubules, or in cross-sectional area of tubule tissue, were observed across the four strains (Figure 2A). Although not significant, there was a trend for glomerular size to be lower in ephrin-A2A5-/- and ephrin-A5-/- mice compared with mice from the other two strains, however this was a subtle difference that was not obvious in histological sections (Figure 2B–D). When mice were pooled based on the presence or absence of the ephrin-A5 gene to form two groups (WT and ephrin-A2-/- mice vs ephrin-A5-/- and ephrin-A2A5-/- mice), the trend for reduced glomerular size in mice lacking ephrin-A5 reached significance (p = 0.033) but no other significant differences in the other measurements were detected between the two groups.
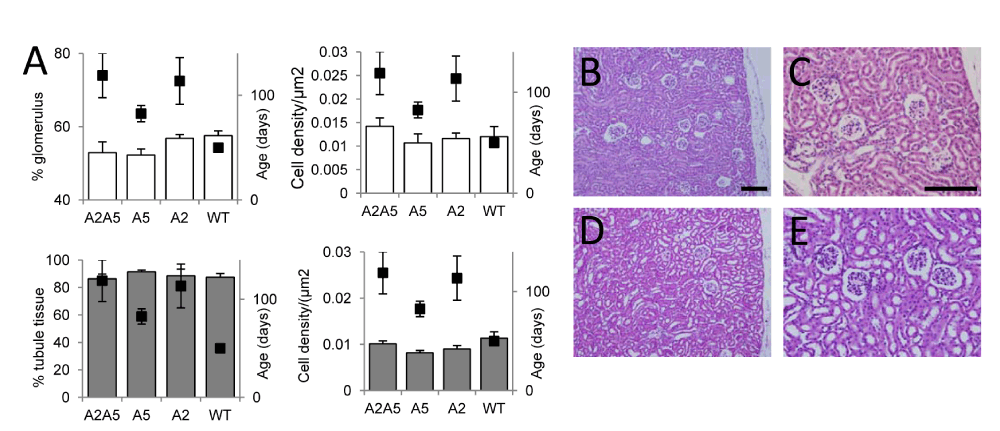
Figure 2. Analysis of kidney morphology in WT and ephrin-A2A5-/- mice.
A: Histograms showing morphological parameters measured in kidney sections (left hand axis) with average age at sacrifice (black points; right hand axis), for each strain. A2A5 = ephrin-A2A5-/- knockout mice, A5 = ephrin-A5-/- knockout mice, A2 = ephrin-A2-/- knockout mice, and WT = wild type mice. Error bars are standard error. B–D: Haematoxylin and eosin stained sections of WT (B,C) and ephrin-A2A5-/- (D,E) knockout mice. Scale bars: 100 µm.
Discussion
In summary, we show strong expression of ephrin-A2 and ephrin-A5 in the tubules and glomeruli of the adult mouse kidney. Despite the significant overlap in expression between the two proteins, lack of ephrin-A5 appears to have a stronger effect on kidney morphology with glomerular size being mildly reduced in ephrin-A5-/- mice. However, the magnitude of this change was very small and could only be detected when animals were pooled across genotypes lacking ephrin-A5.
Possible mechanisms for reduced glomerular size
Interestingly, the small reduction in glomerular size in mice lacking ephrin-A5 was not related to age and is therefore likely to be a consequence of abnormal development. Glomeruli consist of a capillary tuft surrounded by highly specialised epithelial cells called podocytes and are formed during development through coordinated interactions between vascular and epithelial tissues9. Members of the EphB and ephrin-B families have been shown to be implicated in this process2–4,10. Although Eph-ephrin interactions are most often restricted within the A or B classes, there is evidence that ephrin-A5, but not ephrin-A2, can bind to EphB26, providing a possible explanation for the ephrin-A5-/- phenotype we report. Because ephrin-A5 and EphB2 have been detected within glomeruli (ephrin-A5: present study; EphB22), and both proteins have been implicated in vascular development in other systems11,12, it is possible that EphB2-ephrin-A5 interactions may contribute to the vascular-epithelial signalling events occurring during kidney development. Our data suggesting reduced glomerular size may be secondary to a vascular phenotype that was not detected using our histological methods.
Functional consequences of reduced glomerular size
Although we cannot rule out the possibility of other cellular phenotypes in ephrin-A5-/- mice, reduced glomerular size alone may contribute to the increased incidence of kidney failure observed in these mice. The fine scale structure of a glomerulus is important for kidney function as it allows for high molecular weight proteins and red blood cells to be retained, while small molecules such as water, sugars, and electrolytes, are able to pass through to the nephron9. Reduced glomerular size would likely result in an abnormally low glomerular filtration rate in mice lacking the gene for ephrin-A5. A recent study found that age-related declines in nephron number led to a proportional increase in glomerular size relative to Bowman’s capsule, probably to compensate for the associated loss in glomerular filtration rate13. It is possible that mice lacking ephrin-A5 may be unable to compensate for the age-related reduction in nephron number. This might lead to a further reduction in glomerular filtration rate, increasing waste concentrations in the blood, and/or fluid retention which can result in hypertension and eventually renal failure14. Future studies investigating kidney function in ephrin-A5-/- mice are needed to better understand the impact of ephrin-A5 on kidney development and function.
Conclusion
The minimal phenotype, together with the relatively infrequent incidence of kidney failure, suggest that ephrin-A2 and ephrin-A5 play only minor roles in kidney development and function. This is surprising given the relatively strong and widespread expression patterns of these proteins detected immunohistochemically. A previous study showed upregulation of ephrin-A5 within a model system of the ureteric bud and hypothesised a role of this protein in the branching of the collecting duct system and/or segmentation of the nephron15. However, the normal morphology described in our study suggests that any role played by ephrin-A5 in the growth and branching of the UB is probably very minor. It is also likely that other members of the ephrin-A family are expressed in the mouse kidney and redundancy within this large family of “promiscuous” signalling molecules may compensate for the loss of individual members16,17.
Author contributions
JR conceived the study, JR, JEO and TS designed the experiments, AB, RD, CG, SL, GL, AW, MS and TS carried out the research, AB, RD, CG, SL, GL and AW analysed results, JR, AB, RD, CG, SL, GL and AW wrote the manuscript, and all authors were involved in the revision of the manuscript and have agreed to the final content.
Competing interests
No relevant competing interests were disclosed.
Grant information
Experiments were funded by project funds to AB, RD, CG, SL, GL and AW from the University of Western Australia (School of Animal Biology). Animal tissue was obtained from studies funded by a project grant from the NHMRC (no 634386) and the Neurotrauma Program of Western Australia. JR is a NHMRC Senior Research Fellow (APP1002258).
Acknowledgements
We are grateful to Leah Attwood and Helen Moulder (Animal Care Services, UWA) for expert animal care and to Marilyn Davies (Animal Care Services, UWA) for autopsy expertise.
Faculty Opinions recommendedReferences
- 1.
Uhlenhaut NH, Treier M:
Transcriptional regulators in kidney disease: gatekeepers of renal homeostasis.
Trends Genet.
2008; 24(7): 361–71. PubMed Abstract
| Publisher Full Text
- 2.
Hashimoto T, Karasawa T, Saito A, et al.:
Ephrin-B1 localizes at the slit diaphragm of the glomerular podocyte.
Kidney Int.
2007; 72(8): 954–64. PubMed Abstract
| Publisher Full Text
- 3.
Ogawa K, Wada H, Okada N, et al.:
EphB2 and ephrin-B1 expressed in the adult kidney regulate the cytoarchitecture of medullary tubule cells through Rho family GTPases.
J Cell Sci.
2006; 119(Pt 3): 559–70. PubMed Abstract
| Publisher Full Text
- 4.
Takahashi T, Takahashi K, Gerety S, et al.:
Temporally compartmentalized expression of ephrin-B2 during renal glomerular development.
J Am Soc Nephrol.
2001; 12(12): 2673–82. PubMed Abstract
- 5.
Miao H, Wang B:
EphA receptor signaling--Complexity and emerging themes.
Semin Cell Dev Biol.
Elsevier. 2012; 23(1): 16–25. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 6.
Himanen JP, Chumley MJ, Lackmann M, et al.:
Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling.
Nat Neurosci.
2004; 7(5): 501–9. PubMed Abstract
| Publisher Full Text
- 7.
Feldheim DA, Kim YI, Bergemann AD, et al.:
Genetic analysis of ephrin-A2 and ephrin-A5 shows their requirement in multiple aspects of retinocollicular mapping.
Neuron.
2000; 25(3): 563–74. PubMed Abstract
| Publisher Full Text
- 8.
Anderson M, Gorley R, Clarke K:
PERMANOVA+ for PRIMER: Guide to software and statistical methods. Plymouth, UK. 2008. Reference Source
- 9.
Little MH, McMahon AP:
Mammalian kidney development: principles, progress, and projections.
Cold Spring Harb Perspect Biol.
2012; 4(5); a008300. PubMed Abstract
| Publisher Full Text
- 10.
Kawachi H, Suzuki K, Miyauchi N, et al.:
Slit diaphragm dysfunction in proteinuric states: identification of novel therapeutic targets for nephrotic syndrome.
Clin Exp Nephrol.
2009; 13(4): 275–80. PubMed Abstract
| Publisher Full Text
- 11.
Hara Y, Nomura T, Yoshizaki K, et al.:
Impaired Hippocampal Neurogenesis and Vascular Formation in Ephrin-A5-Deficient Mice.
Stem Cells.
2010; 28(5): 974–83. PubMed Abstract
| Publisher Full Text
- 12.
Nakamoto M, Bergemann AD:
Diverse roles for the Eph family of receptor tyrosine kinases in carcinogenesis.
Microsc Res Tech.
2002; 59(1): 58–67. PubMed Abstract
| Publisher Full Text
- 13.
Murawski IJ, Maina RW, Gupta IR:
The relationship between nephron number, kidney size and body weight in two inbred mouse strains.
Organogenesis.
2010; 6(3): 189–94. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 14.
Beck LH Jr, Salant DJ:
Glomerular and tubulointerstitial diseases.
Prim Care: Clinics in Office Practice.
2008; 35(2): 265–96. PubMed Abstract
| Publisher Full Text
- 15.
Pavlova A, Stuart RO, Pohl M, et al.:
Evolution of gene expression patterns in a model of branching morphogenesis.
Am J Physiol.
1999; 277(4 Pt 2): F650–F63. PubMed Abstract
- 16.
Klein R:
Eph/ephrin signalling during development.
Development.
2012; 139(22): 4105–9. PubMed Abstract
| Publisher Full Text
- 17.
Himanen JP, Nikolov DB:
Eph signaling: a structural view.
Trends Neurosci.
2003; 26(1): 46–51. PubMed Abstract
| Publisher Full Text


Comments on this article Comments (0)