Keywords
clear cell hidradenoma; renal carcinoma; immunochemistry; cutaneous metastasis.
clear cell hidradenoma; renal carcinoma; immunochemistry; cutaneous metastasis.
We read with interest a review of 15 cases of nodular hidradenoma published recently in the American Journal of Dermat opathology1, in which Nandeesh and Rajalakshmi did not demonstrate a preponderance of clear cell populations in any of the cases. Additionally, they stated that clear cells constituted no more than 30% of the total cells in all cases1. Interestingly, here we report a rare case of nodular hidradenoma with a vast preponderance of large clear cells. To date, none of the small number of similar publications2 have described the striking similarities to metastatic cutaneous renal cell carcinoma presented in our patient.
A 60-year-old female patient was referred to the Department of Dermatology, University Hospital of Salamanca, Spain, after a growing lesion appeared on her face over the preceding 6 months. Physical examination revealed a well-demarcated reddish nodule of 0.7cm in size located on the glabellar prominence. No ulceration was noted. On palpation, a firm, dermal round lesion was noticed. Total surgical excision was performed. The specimen was fixed with paraffin. Histopathological examination (hematoxylin and eosin, viewed at ×40) demonstrated a well-circumscribed but non-encapsulated, expansive-growing nodule within the deep and mid-dermis (Figure 1a). This tumor was mainly composed of clear cells separated by fine conjunctive-vascular septae. These cells showed a large empty cytoplasm, medium-small eccentric nuclei and coarse chromatin (Figure 1b). Atypia, necrosis and mitotic figures were absent. The periphery of the lesion presented papillae and tubular formations accompanied by two alternating types of cells: those that were smaller and basophilic and those with abundant clear cytoplasm (Figure 1c). Tubular ducts were covered by cubical to cylindrical cells. Cystic areas filled by eosinophilic fluid were also present. Immunohistochemistry was positive for CK7, AE1/AE3, and slightly for p63. Conversely, negativity for CK20, CD10, Napsin-A, Vimentin and Actin was demonstrated. On account of these findings, our patient was diagnosed with a nodular variant of clear cell hidradenoma (CCH). In addition, renal ultrasonography examinations ruled out any neoplasm.
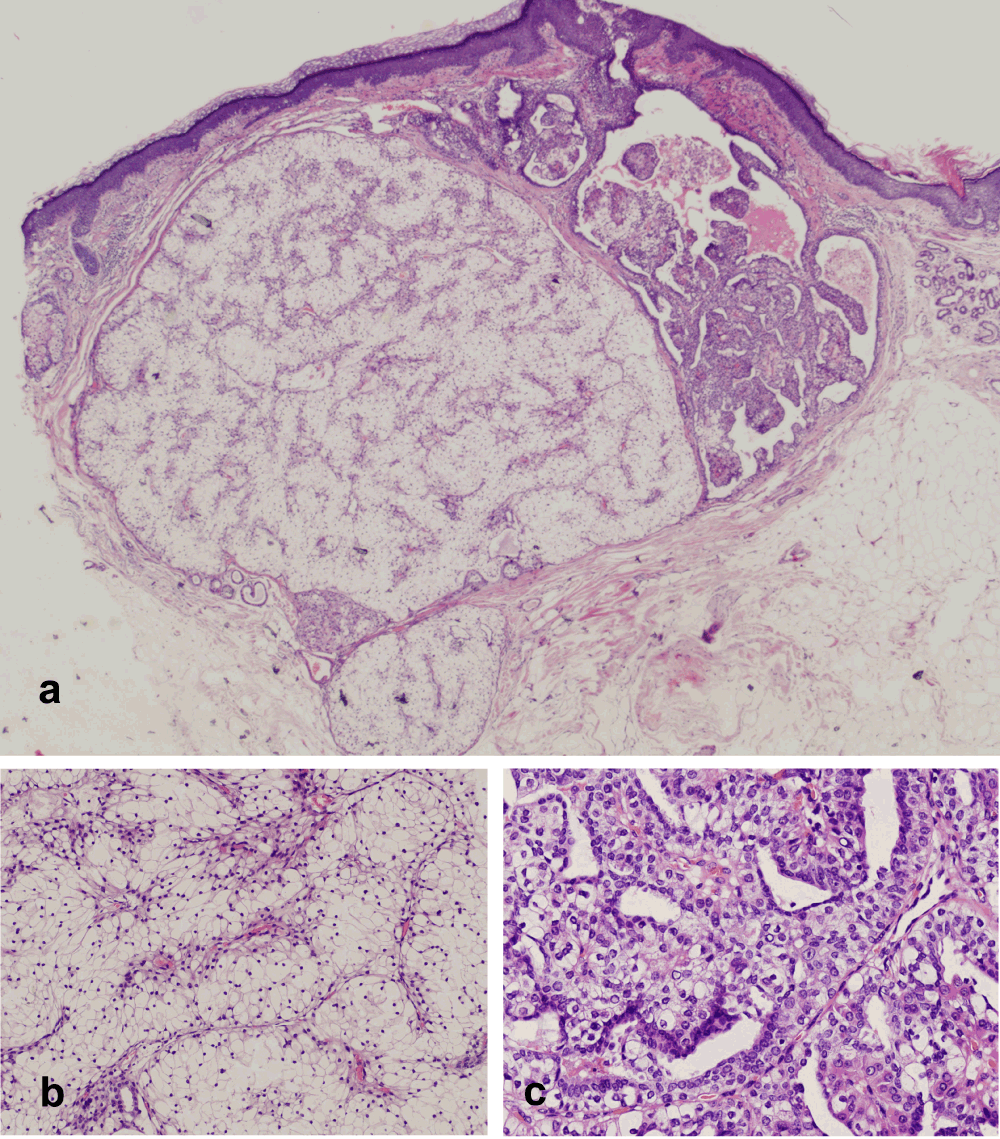
a) Microphotograph showing a well-circumscribed but non-encapsulated, expansive-growing nodule located in deep and mid-dermis (hematoxylin and eosin, ×40). b) Microphotograph showing clear cells with a large empty cytoplasm, medium-small eccentric nuclei and coarse chromatin (hematoxylin and eosin, ×400). c) Microphotograph showing the periphery of lesion, which presented papillae and tubular formations accompanied by two alternating types of cells: those smaller and basophilic and those with abundant clear cytoplasm (hematoxylin and eosin, ×400).
Hidradenoma is presented as a benign small, firm and solitary nodule. On microscopic examination, spared epidermis may be separated with a dermal well-circumscribed tumor by a Grenz zone. It may be encapsulated and involvement of subcutaneous fat tissue is uncommon3. Cytological study reveals a lesion mainly composed by two types of cells2,4. Firstly, hidradenoma may show a greater amount of a polyhedral and slightly basophilic cell type. On the other hand, a predominance of larger, rounded-shaped pale to clear cell type is characteristic of CCH, as in our case. In the latter form, nuclei are frequently located eccentrically1. CCH shows small ductular lumens accompanied by changes from apocrine glands or from epidermoid cells. Glandular structures compounded by columnar cells are frequently appreciable. Clear cells contain a variable amount of glycogen within their cytoplasm, unaccompanied by lipid material, which is responsible of their clear component. This material is periodic acid-Schiff–positive and diastase-labile1. Solid portions may alternate with cystic areas, which might be materialized as a result of tumor degeneration.
Differential diagnosis between metastatic clear renal cell carcinoma (MCRCC) and CCH may be confusing as both display clear cell preponderance, and it is essential to exclude metastatic disease because of its poor prognosis. Because of two recently reported cases of renal medullary carcinoma metastatic to cranial skin5,6, we were also obliged to rule out this possibility. Thus, immunohistochemistry becomes absolutely necessary in order to rule out its mimics2. Dorairajan et al.7 stated that skin metastasis may vary from 2.8% to 6.3% in renal cell carcinoma patients. A remarkable case of a patient with both an axillary CCH and a renal cell carcinoma was reported by Volmar et al.2. These authors emphasized the importance of immunohistochemistry in distinguishing between these entities. MCRCC has positive staining for CD10, Napsin-A and Vimentin, all of which were negative in our patient. In contrast, CCH expresses CK7+, AE1/AE3+ and CK20-2. These results were consistent with those observed in our patient. Some authors have reported a variable range of staining for CK6/18, CK8/18, CK10, CK198, 34bE12, carcinoembryonic antigen and CAM 5.29.
In summary, we report a rare case of nodular clear cell hidradenoma on glabellar prominence. This case is of interest since none of the 15 cases of nodular hidradenoma recently reviewed showed a preponderance of clear cells1. Additionally, it is also remarkable that no related publication2,5–7 have exhibited the striking similarities to renal cell carcinoma presented in our patient.
In order to avoid serious pitfalls, since the prognosis of these two conditions differs dramatically, a differential diagnosis based on immunohistochemistry is highlighted.
Written informed consent for publication of clinical details and clinical images was obtained from the patient.
José M. Mir-Bonafé: drafting the initial article Ángel Santos-Briz: clinical images and histologic description Marta González: patient's doctor and revising the manuscript Emilia Fernández-López: revising the whole manuscript and corrected errors.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Peer review at F1000Research is author-driven. Currently no reviewers are being invited.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 11 Oct 13 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)