Introduction
Past studies on the Na, K-ATPase have mostly relied on the conventional single topology scheme consisting of one large α-subunit (about 100 k Da) that offers a single transmembrane channel for transporting both Na and K across the plasma membrane, and a glycoprotein β-subunit (about 60 k Da) needed for functional regulations1. However, the single topology Post-Albers (PA) scheme fails to explain several fundamental issues related to the operation of the Na, K-ATPase system, such as, half of the sites reactivity2, double ATP binding sites3, simultaneous binding and transport of Na and K across the membrane4,5 and extensive binding of ouabain to the Na, K-ATPase6. The cardiac glycoside, ouabain, is well-known to bind to the Na, K-ATPase and inhibit its function by binding to parts of the ATPase molecule exposed to the cell exterior. An alternate model for P-2 ATPase function devoid of these limitations has recently been described7 based on the orientation of critical reactive sites of the functional ATPase molecule across the membrane as discussed below.
The PA scheme visualizes Na-dependent phosphorylation of the α-subunit by ATP (a kinase step) followed by K-dependent dephosphorylation (a phosphatase step) during each reaction cycle. The activity of K-dependent p-nitrophenyl phosphatase (K-pNPPase), which is always co-purified with the Na, K-ATPase system was assumed to represent the phosphatase step of the total ATPase reaction. However this assumption was subsequently proven to be wrong since K-pNPPase activity reflects the ion channel activity across the membrane rather than being a partial reaction of the ATPase8. The K-site responsible for the turn-over of the ATPase reaction has greater affinity to potassium than the K-site needed for ion-channel function (reflected by K-pNPPase activity) just mentioned8. We made an orderly investigation on the bilayer orientation of various functional sites of the gastric H, K-ATPase system using tightly sealed gastric microsomal vesicles capable of H, K-ATPase-dependent accumulation of H in exchange for K8. Based on the orientation of the ATP and pNPP hydrolytic sites together with the respective K regulatory sites across the plasma membrane, a mirror image orientation of the two α-subunits (for a functional P-2 ATPase complex) was proposed (Figure 1). It may be noted that there are differential inhibitors such as thiocyanates, isothiocyanates9 and polyamines10 that do not affect H, K-ATPase activity (proton production) but do inhibit the K-pNPPase activity (proton transport).
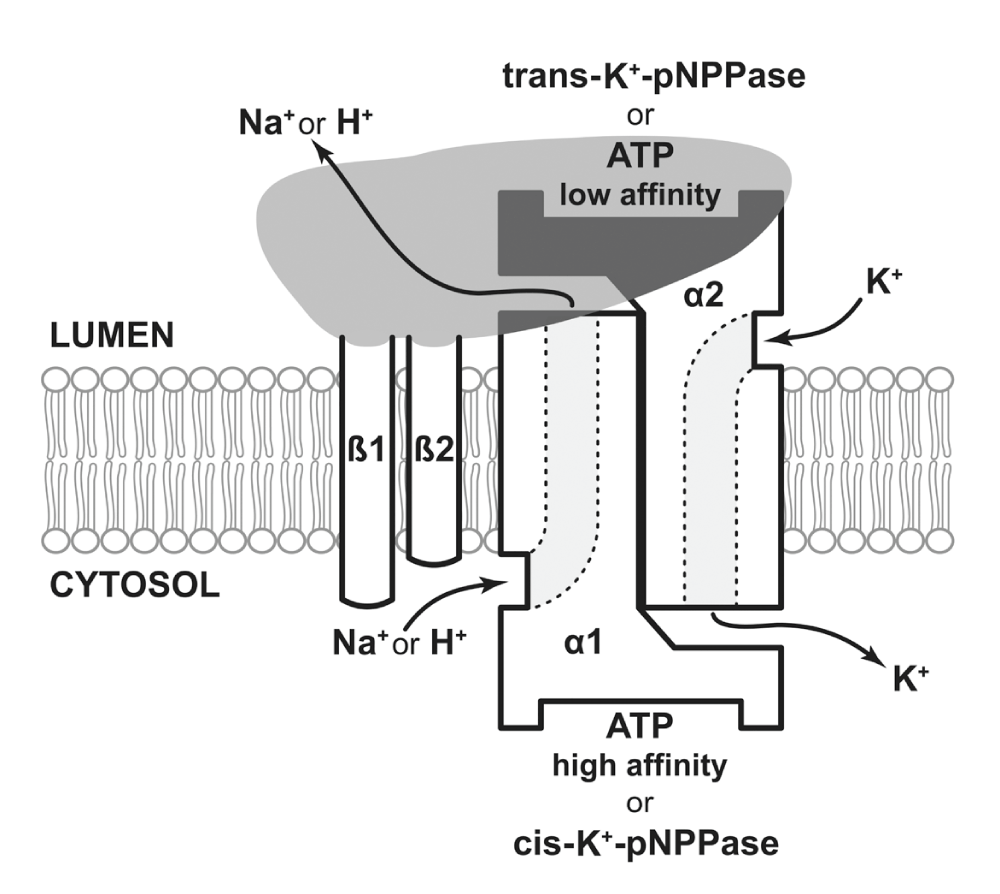
Figure 1. Dual topology of the P-2 ATPase system showing bilayer orientation of α and β subunits with related ion-channels.
Two identical subunits, α1 and α2 (not isomers) are shown in mirror images across the membrane with embedded-ion channels in contact, and are held with two closely associated β-subunits facing the lumen. Recent evidence of a direct β-β cross-linkage in the native state suggests that β1 and β2 are held closely together with the α1α2 assembly13. The ATP hydrolytic site (separate from the cis-pNPPase site) on the α1, and trans-cytosolic non-hydrolysable ATP-binding site and the corresponding trans-pNPPase site on the α2 are shown. Besides ATP-binding the intimate association among α2, β1 and β2 on the cell exterior is expected to modulate the ATPase function in various ways including the reception of extracellular signals14. The high affinity K+ site for ATPase stimulation is located across the bilayer on α2 and the corresponding high affinity H+ or Na+ site is on the cytosolic side of the α1 enabling access gating. The low affinity K binding sites responsible for releasing the transported ions are present at or near the exit end of the related ion channel on each side of the bilayer. Under appropriate conditions the pump would also transport Ca/H in altered states12.
Thus, contrary to the conventional single topology PA model, the updated dual topology model7 has two ion channels designated for Na and K respectively, and two neighboring β-subunits (one for each channel) supporting the channel function. The model7 fits well with the behavior of the gastric H, K-ATPase system regulated by its 80 k Da cytosolic activator, HAF12. In addition, studies on cytosolic regulation of the kidney Na, K-ATPase11 by the 170 k Da cytosolic activator protein, NaAF, and Ca (which shows abrupt inhibition of the Na, K-ATPase between 10–25 µM Ca) revealed a delicate regulation of the activator dependent Na, K-ATPase system. Also, NaAF purified from brain and kidney tissues are equally effective in stimulating the purified Na, K-ATPase in brain and kidney tissue and vice versa11 showing similar regulatory role of NaAF in brain function. These aspects of the structure, function, and regulation of the P2-ATPase system will be discussed in the following sections in relation to brain function.
Dual topology P2-ATPase with dual channels for the simultaneous two-way transport of two cations across the bilayer
The dual topology model (Figure 1) was constructed based on ample evidences from the gastric H, K-ATPase and Na, K-ATPase system and was extended to Ca-ATPase based on analogy and circumstantial evidence in the literature7. Later it was recognized that the gastric H, K-ATPase12 and the ubiquitous Na, K-ATPase function as a Ca-ATPase in altered states.
The functional heterodimeric P-2 ATPase pump (Figure 1) consists of two α (α1 and α2) and two β (β1 and β2) subunits embedded within the plasma membrane. The adjoined α subunits with embedded ion channels are believed to oscillate laterally within the plane of the membrane during the pump operation7. Oscillation of the ion channels is initiated by ATP-induced phosphorylation of the pumping enzyme at the catalytic α1 site with simultaneous binding of high affinity Na to a nearby site in the cytosol. This reaction initiates the spreading of domain-domain interaction between the membrane-embedded parts of the α1 and α2-subunits accompanied by the binding of high affinity K at the trans-cytosolic α2 site. This binding in turn induces the rapid dephosphorylation helping the ATPase molecule to return to the original configuration along with a harmonious shift of the adjacent ion channels back to its initial state. The entire process creates a peristaltic movement of both ion channels for the vectorial translocation of Na and K across the membrane in opposite directions. The peristaltic movement of the H, K-ATPase system is supported by proper fluidity of the surrounding annular phosphatidyl choline (PC) molecules (at 37°C) consisting of 40% saturated fatty acid and the rest mono- and di-unsaturated fatty acids in equal proportion15,16.
Amongst various cell types there are four different isoforms of α-subunits (with molecular masses of 95–110 k Da) that constitute the trans-bilayer ion channels and three isoforms of β-subunits (β1 and β2, 45 k Da each and 60 k Da β3) that support the function of the Na, K-ATPase. Three of these α-isoforms, α1, α2 and α3 and two β–isoforms, β1 and β2, are present in the nervous system19,22,23 suggesting different isoforms of the Na, K-ATPase in the brain might play important roles in different brain areas with differential modes of neurotransmission. Expression of various isoforms of the Na, K-ATPase in different expression systems revealed differences in substrate affinity and kinetic properties20,21. Different α-isoforms in various species show a high degree (76–90%) of sequence homology19 with the greatest similarities along the critical sites including the membrane-embedded ion-channels, the mid-region phosphorylation site (Asp 369) and the ATP binding site. However, contrary to the mid-region ATP-related highly conserved sites, no such strict conservation is necessary within the ion channel sites (regions exhibiting reversible domain-domain interactions) following the dual topology scheme7. In the dual topology model, energetic aspects of the access gate, selective filtering, and exit pathway of cations is appropriately explained by simultaneous binding of two different cations across the membrane and the subsequent domain-domain interactions that spontaneously follow with resultant ion transport7. The highly conserved α-regions, mentioned above, clearly point toward a common ancestral origin for the P-2 ATPase system19.
The massive amount of ATP being spent by brain tissue is used primarily by the Na, K-ATPase and Ca-ATPase pumps located along the plasma membrane to generate the carefully regulated bioelectric potential for the receiving, processing and transmission of signals. While the ligand gated Na and K channels create the action potential for signal propagation, the voltage gated Ca channels simultaneously couple the bioelectric signals to physiological events within the cells24. The influx of Ca occurring through these voltage gated Ca-channels regulates many vital cell processes such as, contraction, Na/H exchange and the release and reuptake of neurotransmitters involving fusion of vesicles to the synaptic terminal.
In the case of acid secreting parietal cells (lacking in regular Na, K-ATPase activity), the gastric H, K-ATPase has recently been suggested to function as a Na, K-ATPase at alkaline pH (in H, K-ATPase locality) in altered states12. Likewise in a high Ca environment (> 4 µM), the gastric H, K-ATPase appears to function as a temporary Ca-ATPase in altered states12. In an analogous manner, the kidney Na, K-ATPase system appears to function as an H, K-ATPase and a Ca-ATPase in altered states (see below). Such altered functions of the gastric H, K-ATPase and kidney Na, K-ATPase systems are well accommodated by the dual topology P-2 ATPase model discussed above, and suggest further that, similarly, such altered function of the Na, K-ATPase system would also exist in brain. These aspects will be discussed in detail in the following section.
Regulation of the Na, K-ATPase by the cytosolic activator protein (NaAF) and µM Ca
We previously reported11,25 the purification and characterization two unique cytosolic activator proteins of 80 k Da and 170 k Da mass for the activation of the gastric H, K-ATPase25 and kidney Na, K-ATPase11 designated as the HAF and NaAF respectively. From the degree of cross-activation of the two ATPase systems by the two activators it is clear that they share some domains essential for ATPase activation11. The ATPase activities associated with the plasma membrane fractions of low (1.06) and high (1.115) density prepared from the parietal cells and kidney cortex respectively, revealed distinctive activation by HAF and NaAF. The origin of low density membranes harvested from gastric parietal cells are derived mostly from the apical (secretary) plasma membrane (APM) and the high density membranes from the intracellular tubulovesicles (TV) which fuse with the APM following secretagogue stimulation. Such characterization has been based on differential enrichment in 5´-nucleotidase activity, Vitamin B12 binding ability, phospholipid to cholesterol molar ratio and other criteria26. The aspects on regulation of the APM associated gastric H, K-ATPase activity by the HAF, and Ca (µM) have recently been highlighted16, which will be frequently referred to in the following section due to close similarities in regulation between the HAF and the NaAF.
While both low and high density membrane fractions from kidney cortex had equal phospholipid to cholesterol molar ratios (about 0.64) characteristic of the plasma membranes, the high density membrane showed remarkable Na, K-ATPase activity (about 250 µmoles/mg protein/hour) in contrast to the low-density membrane (< 1 µmole/mg protein/hour) which suggest the high density fraction to be of basolateral origin. The low density fraction had high basal (Mg-ATPase) and negligible Na, K-stimulated ATPase activity suggesting the low density membranes to be derived from the apical plasma membrane of the cells lining the collecting duct of kidney designated as KAPM (below) by analogy with the APM of gastric cells26.
The nature of NaAF activation of the kidney Na, K-ATPase system (Figure 2) associated with the low and high density plasma membrane fractions is also closely similar to that of the HAF-activated gastric H, K-ATPase27. Thus, the high density basolateral membranes (BLM) had very low basal Mg-ATPase and very high Na, K-ATPase; in contrast to the KAPM which had high basal ATPase and negligible Na, K-ATPase activity. When assayed with NaAF (purified from brain tissue) the renal Na, K-ATPase activity associated with BLM was stimulated by about 70% while the KAPM Na, K-ATPase activity increased by over 50-fold (Figure 2). In other words, the Na, K-ATPase was dormant in KAPM due to inactivity of the luminal high affinity K-binding site (Figure 1) which becomes highly sensitive to K-stimulation following NaAF-activation, thus increasing the turnover of the Na, K-ATPase27. Such differential activation of the Na, K-ATPase by NaAF is consistent with their differential sensitivity to vanadate inhibition. NaAF stimulated basolateral Na, K-ATPase activity was inhibited by 50% within 1.5 µM vanadate while the KAPM associated Na, K-ATPase was totally insensitive up to 4 µM27. Almost identical vanadate inhibition was observed with the APM and TV associated H, K-ATPase system derived from gastric parietal cells26.
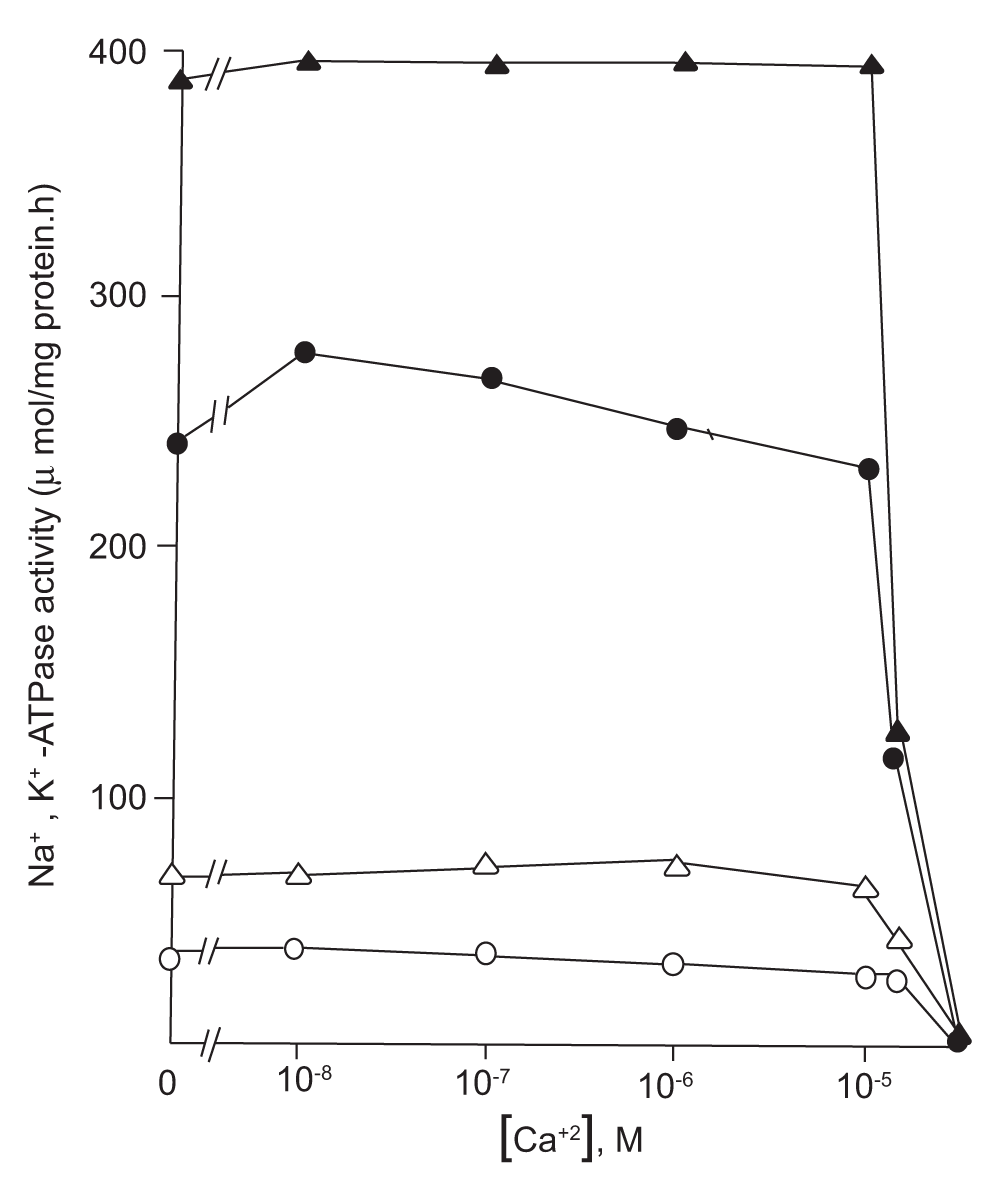
Figure 2. Effects of varying concentrations of Ca (µM) on the NaAF-activated Na, K-ATPase activity associated with the KAPM and BLM.
The concentration of free Ca was regulated by varying Ca at a fixed concentration of 0.5 mM EGTA (Figure taken and redrawn from Reference 27 by Courtesy of IJBB). The KAPM associated Mg-ATPase (basal) and Na, K-ATPase activities are represented by open circles without NaAF, and open triangles with NaAF respectively. Please note that the KAPM having very high basal activity as above had negligible (about 0.5 µmol/mg protein.h) Na, K-ATPase activity in the absence of NaAF which is not shown in Figure 2. The BLM associated Na, K-ATPase activities are represented by the closed circles without NaAF, and closed triangles with NaAF. The KAPM and BLM were isolated from dog kidney26. The NaAF was purified from dog brain using the soluble supernatant (100,000 g for 90 min) fraction overlaying the microsomal pallet using our published procedure11.
The NaAF-activated Na, K-ATPase associated with the KAPM and BLM revealed dramatic effects in response to µM Ca; activities in both membranes remained almost unaltered prior to reaching 10 µM at which point the activities suddenly dropped to less than 50%, and were abolished at 50 µM Ca27. This behavior is qualitatively similar to that of the HAF-regulated gastric H, K-ATPase which shows abrupt inhibition within a narrow range of 2–4 µM Ca although there is significant stimulation of the ATPase within a 2 µM range29,30. The lower sensitivity of the NaAF stimulated Na, K-ATPase to µM Ca probably indicates NaAF has extra Ca-binding sites (compared to HAF), which somehow makes its configuration unfit for stimulating the H, K-ATPase by restricting access of ATP to the catalytic site. It may be noted that NaAF is accustomed to a rather high Ca environment while regulating the basolateral Na, K-ATPase where the Ca-entry points (from the circulation) are in close proximity. In addition, the molecular alterations due to differences in αβ-isoforms between the kidney Na, K-ATPase and the gastric H, K-ATPase33 might also contribute to their differential sensitivity to µM Ca. The latter aspect is fully consistent with the fact that the Na, K-ATPase purified from brain and kidney tissues, which are known to be made up of different αβ-isoforms, are differentially stimulated by HAF: 50% and 100% stimulation of the brain and kidney enzymes respectively11. The kidney Na, K-ATPase is reported to be primarily of the α1β1 type while the brain enzyme has several additional αβ-isoforms19,38.
Such analogous behavior of the APM associated gastric H, K-ATPase to the KAPM associated Na, K-ATPase suggest that the latter would also act as an H, K-ATPase and Ca-ATPase in altered states based on the following analysis. For the purpose of acidification of urine in the distal convoluted tubule and collecting duct, the KAPM associated NaAF-stimulated Na, K-ATPase of the luminal cells is likely to function as a NaAF-stimulated H, K-ATPase in altered states, possibly in a low Na, low pH environment. This situation is analogous to APM associated gastric H, K-ATPase, which acts as a Na, K-ATPase in high pH (low H) high Na environment12. Also, this NaAF-stimulated Na, K-ATPase is likely to function as a provisional Ca-ATPase in altered states under conditions of high Ca (> 10 µM). This Ca-pump would switch to the Na-pumping mode of Na, K-ATPase when the local Ca concentration reaches < 10 µM. Though this remains to be tested, such behaviors would be consistent with the reported existence of H, K-ATPase in the kidney tubules47. The differential sensitivity to µM Ca between the gastric H, K-ATPase and Na, K-ATPase systems is most likely due to the tissue-specific differences in ATPase-isoforms discussed above. The NaAF regulated colonic Na (H), K-ATPase system might also play a similar role in order to maintain Na-homeostasis in the distal colon48.
One noteworthy feature of HAF is that pure HAF possess high NH2OH-insensitive protein kinase activity as revealed by the ability of HAF to phosphorylate histone, though at same time it is not auto-phosphorylated or phosphorylated by endogenous protein kinase27,31. Thus, under similar conditions of histone phosphorylation, HAF could not be phosphorylated by bovine heart kinase from Sigma used in these experiments. This rare ability to phosphorylate histone strongly suggests that the cytosolic regulators of the P2-ATPase system might be capable of self-regulating their own intracellular level of activities via gene expression. However, NaAF has not yet been tested for histone kinase activity. This is critical since the NaAF-regulated P2-ATPase system is the chief controller of ionic homeostasis in all tissues with the exception of gastric parietal cells.
The preceding aspects on cytosolic regulation of the P-2 ATPase system taken together emphasizes that each tissue or cell type possess its own Na (H), K-ATPase of distinct isoforms consisting of different combinations of the α and β isomer. This idea is consistent with the detailed studies from Sweadner and collaborators32 demonstrating the unique distribution of various combinations of αβ isoforms in the brain tissue underscoring the subtlety in P2-ATPase regulation.
Significance of these studies in brain function
The super-busy brain derives most of its energy from the oxidative metabolism of glucose in the form of ATP. As a result the supply of oxygen to our brain is highest (over10% of the total O2 consumed by an individual) making its oxygen consumption rate over 500-times higher (based on brain mass) than consumed by the rest of our body. The massive amount of ATP generated from glucose oxidation is utilized primarily for maintaining the Na/K gradient for polarization across the neuronal plasma membrane, and for the ongoing recharging of neurotransmitter vesicles before fusion, along with the up-keeping of phospholipid dynamics of the busy membrane domain of the synaptic terminal. The ATP driven Na, K-ATPase system incessantly uses this ATP to generate the bioelectric potential for the transmission of nerve impulses. The following section analyses the prospects of the NaAF-regulated Na, K-ATPase system and its altered function as a Ca-ATPase following the voltage gated channeling of Ca at the presynaptic zone during neurotransmission.
Multifunctional role of the P-2 ATPase in brain function
The brain possesses multiple isoforms of the Na, K-ATPase with a variety of αβ combinations which consist of three isoforms of the α-subunit (α1, α2, α3), and at least two (β1, β2) of the three β-subunits19,38. These ATPase-isoforms are differentially distributed in different areas of the brain serving some unique functions to these regions32,38. The nature and extent of NaAF regulation of the Na, K-ATPase isoforms acting in different altered states might also vary accordingly. The variation in the affinity of different isoforms, towards Na, K, ATP and Ca17,19,38, might be in part due to their differential susceptibility to regulation by NaAF. The resultant altered function of the Na, K-ATPase as a provisional Ca-ATPase during neurotransmission, would also be affected. Hence, the factors regulating the association of NaAF with the catalytic domain of the Na, K-ATPase, such as Ca (µM), pH and local Na concentration, need to be carefully studied. Based on the evidence discussed in this article there are critical Ca-binding sites on the NaAF molecule, which modifies its nature, and the degree of bound Ca somehow determines the access of ATP to the catalytic site. The nature of Ca-binding domains within the 170 k Da NaAF, however, would be different from the small Ca-binding proteins known to play critical roles in various tissues including neurons34.
The aspects of processing and control of Ca-signals are at the heart of brain metabolism and function, which in turn depends on appropriate altered functions of the Na, K-ATPase system in different areas of the brain. Neurotransmission mediated by the fusion of neurotransmitter vesicles is triggered by voltage gated Ca-channels that are clustered at the presynaptic zones. The depolarizing impulse for the opening of voltage gated Ca-channels is in turn mediated by the voltage gated Na-channels neighboring the Na, K-ATPase pumps (the voltage generators). The Na-channels rapidly transmit the depolarizing impulses throughout the cell and its network thereby coordinating higher processes.
The Ca-induced vesicular exocytosis, mentioned above, is carried out by a rapid (< 1 ms) increase in Ca-level (> 1000 fold) within the presynaptic button35. High Ca-microdomains are formed by the opening of Ca-channels directly teethered to docked synaptic vesicles for fusion with the presynaptic membrane36,37. The intracellular pH homeostasis involving Na/H exchange and the opening and closing of the voltage gated Ca-channels and related aspects are discussed below. The accompanying process of the reuptake of neurotransmitter vesicles and the Na/Ca exchange that remove Ca from the Ca-ATPase neighborhood fit well with the proposed scheme.
Critical role of the Na/H antiporter (NhaA) in pH homeostasis in the brain
The axonal NhaA would play a crucial role in pH homeostasis in neurons. The neurotransmitters are taken up by tiny synaptic vesicles in exchange for vesicular H, which is preloaded by the proton-pump built into the structure of neurotransmitter-vesicles. During the process of vesicle-uploading with neurotransmitters the cytosolic pH tends to go down when the NhaA starts functioning with characteristic high turnover at physiological pH by drawing extracellular Na in exchange for cytosolic H49. So, the dimeric NhaA molecule discussed below would have dual channels for the simultaneous exchange of 2H for 1Na down the respective gradients across axonal plasma membrane to maintain homeostasis49.
Cryo-electron microscopy (Cryo-EM) of 2-dimensional crystals revealed that NhaA is a dimer with 12-lipid embedded trans-membrane segments (TMS) with inverted motif in the native state49 allowing each TMS monomer to hold one built-in ion-channel. Such inverted mirror-image motif of the adjoining membrane embedded TMS ion-channels would be analogous to those demonstrated for H and K channels associated with the functional H, K-ATPase pump across gastric microsomal vesicles7, and the primary reason behind this is as follows. In mirror-image orientation the corresponding channels of NhaA for the passage of H (out) and Na (in) would have favorable polarity for effective binding affinity (loading) of H and Na at corresponding entry points (acting as sensors) with lower affinity at the exit ends for prompt unloading. The details on the transport function of the NhaA including its ion-channeling properties remains to be elucidated.
Altered function of the Na-pump as a provisional Ca-pump in the busy membrane environment
The sensitivity of the Na, K-ATPase to Ca (µM) varies considerably depending on the nature of the ATPase-isoform38; the α2β1 and α3β1 isoforms have been reported to be 10- and 100-fold less resistant to Ca-inhibition than α1β139. Hence, following depolarization of the excitable cells once the Ca-level raises to 5–10 µM the α1β1-isozyme remains fully active while the α2β1- and α3β1-isozymes will be working at about 50% of their capacity38,39.
Based on the scheme presented here the activated Ca-ATPase pump at the nerve terminal (following depolarization) would be an altered state of the existing Na, K-ATPase isoform. The provisional Ca-ATPase pump will start driving Ca out from the pre-synaptic ATPase zone at full capacity. Concurrently, the Na/Ca exchanger will remove Ca from the vicinity of the Ca-pump, replacing it with Na. Thus, with the rapid fall in Ca level and rapid rise in Na level a critical point is reached when the Ca-pump is spontaneously switched back to the Na-pumping mode; and this process of switching back and forth continues based on the local environment during nerve conduction. The mass entry of extracellular Ca through voltage gated channel of synaptic plasma membrane is likely to be influenced by the extracellular Ca-sensing receptor, CaR, discussed below.
It should be noted that the lipid dynamics of the synaptic plasma membrane skilfully accommodates all vital tasks, such as running the ATPase-pump, voltage gated Ca-channeling, fusion and reuptake of the synaptic vesicles, and maintaining CaR function in an efficient phospholipid-cholesterol-glycolipid environment. This is somewhat analogous to the busy activities at the APM during hormone stimulated fusion with the TV in parietal cells52 for inclusion of more proton pumps to meet secretary demand. It is noteworthy that using the immunohistochemical superimposition technique Caroppo et al.50 co-localized the CaR, the plasma membrane Ca-ATPase (PMCA) and H, K-ATPase on the parietal cell APM, and suggested that the usual hormone-stimulated Ca-transients in the lumen that precede gastric H-transport might act as a signal for CaR activation. Before going into more detail on CaR, however, the aspects of various lipids associated with the APM and TV are summarized below to cover the background on membrane transformation.
The total phospholipid content of the APM, 2.1 mg/mg protein, is 40% higher than the intracellular vesicles, TV (1.2 mg/mg protein), where the individual phospholipid content also varies26. Remarkably, the APM has 67 µMoles phosphatidyl choline (PC)/mg protein which is 100% more than in the TV, and the ratios of PC/phosphatidyl ethanolamine (PE) are 1.38 and 0.87 for the APM and TV respectively. It may be noted that the orientation of PE in TV is asymmetric having 70% of PE facing the vesicle interior54 which appear to help in vesicle curvature and membrane fusion. The PC/sphingomyelin (Sph) ratio in the APM and TV are 1.02 and 0.57 respectively. The latter ratio is remarkable since PC provides the natural (annular) environment supporting the H, K-ATPase function whereas the Sph is totally unsuited to this15,53. Consistent with their signaling roles the APM content (µMoles/mg) of phosphatidyl inositol (PI) and phosphatidyl serine (PS) are 23 and 13 which are 300% higher than those in TV26,54. The Sph molecules have over 90% saturated fatty acids including 38% of the rare 14:0 (myristic acid, not found in any other phospholipid), which helps the structural and functional uniqueness of the cholesterol-Sph microdomains54. All of these novel characteristics of the APM lipids would likely be similar to carry out similar functions in the synaptic junction as follows.
Projected involvement of CaR in voltage gated channeling of Ca for synaptic fusion
The CaR is a ubiquitous Ca-sensor protein that plays a vital role in maintaining a stable Ca-environment for the cell. Although the CaR is expressed along the nephron, gastrointestinal tract and numerous regions of the brain, particularly the hippocampus, cerebellum, and hypothalamus40–42 it’s true function and mode of action is still unknown.
The CaR is frequently expressed on the cell surface as a dimer of two identical glycoprotein subunits that exists in several isoforms of varying molecular mass between 130–160 k Da43–45. The homodimer spans across the plasma membrane by seven transmembrane segments (TMS) with a N-terminal glycosylated extracellular (sensor) module for sensing the outside Ca environment, and a C-terminal intracellular (transmitter) component for relaying the changes to cell for intracellular adjustments. The transbilayer layouts of the two CaR monomers would be parallel to each other with two closely apposed TMS segments serving as a GPCR (G-protein coupled receptor) to cope with the fast changing membrane environment at the synapses. The glycosylated sensor module of CaR would sense the Ca-level at the extracellular synaptic junction bringing appropriate changes in membrane-phase with the help of the GPCR. Simultaneously the transmitter module of CaR will make the cells aware of such changes in order to make necessary intracellular adjustments for the Ca-targeted (voltage gated) fusion of docked neurotransmitter vesicles. The outer leaflet of the synaptic plasma membrane neighboring the CaR sensor module would possess the rigid Sph-Cholesterol micro-domains that appear important for Ca-channeling and associated membrane fusion55 which will be analogous to the fusion of intracellular TV with the APM after hormonal stimulation of the parietal cells54. In parietal cells the unsaturated PE molecules (70% facing the TV interior), having high degree (70%) of unsaturated fatty acids and a remarkable amount (20%) of 20:4, arachidonic acid26 appear to play major roles during fusion with the APM. Such PE molecules will have similar function in synaptic fusion. These highly unsaturated PE would help the rapid mingling between the inner-leaflet of the docked synaptic-vesicles and the outer-leaflet of the synaptic plasma-membrane during fusion, as well as during reuptake, whilst also being the active source of necessary eicosanoids (especially 20:4) for the ongoing membrane events.
According to this hypothesis the CaR helps in the preparation of the voltage gated Ca-channel. Using the sudden power of depolarization for sucking positively charged Ca (from CaR’s sensor zone), the CaR somehow helps the neighboring voltage-gated Ca-channel to direct its surge of Ca to the docked vesicle-fusion site at or very near the inner leaflet of the membrane bilayer. Thus, the GPCR-administered signals from CaR would be executed rapidly (< 1 ms) to enable voltage gated Ca-channeling, thereby providing a rational basis for the presence of CaR at the nerve terminus.
The validity of the CaR model, described above, as a component of the rapid voltage-gated channeling of Ca to the synaptic fusion sites facing cell interior remains to be tested.
NaAF seems to control its own intracellular level by a self-regulation scheme involving gene expression
The phosphorylation of Ser10 of histone H3 at the N-terminal tail has been strongly implicated to be involved in gene expression and has recently been reviewed46. As mentioned earlier, the HAF possesses high NH2OH-insensitive protein kinase activity as revealed by the ability of HAF to phosphorylate histone, and at same time it is not auto-phosphorylated or phosphorylated by endogenous protein kinase27,31. These observations suggest that the HAF (and its analogous NaAF) is capable of sending a direct signal to the nucleus for gene expression enabling it to control its own intracellular level. Since NaAF is the principal actor and main intracellular regulator of the P-2 ATPase system, it is most likely that NaAF would regulate its own synthesis and turnover to maintain safe housekeeping. Hence, this possibility of histone phosphorylation by NaAF as a means of self-regulation, so that it can perform its core activities through gene expression in various brain areas, must be carefully explored.
Conclusion
The tetrameric (α²β²) dual topology model (with built-in ion channels) for the functional P-2 ATPase system explains various functions of the P2-ATPase within a cell. The ubiquitous cytosolic protein regulator (NaAF) regulates P-2 ATPase function in close concert with µM Ca where Ca acts as the terminal regulator of an intracellular signaling cascade. The Na, K-ATPase system also seems to function in altered states as a Ca-ATPase based on local, high Ca environments in the stomach, kidney and brain. The possibility of the GPCR-equipped CaR playing a role in the voltage-gated-channeling of Ca for the fusion of neurotransmitter vesicles to the synaptic membrane has been raised. The dual channel P-2 ATPase model well accommodates numerous hard-to-explain reports in the literature of P-2 ATPase function; hence should now be able to explain the physiochemical nature of the channeling of various cations.
Competing interests
No competing interests were disclosed.
Grant information
The author was a recipient of Research Career Development Award (AM00623) from NIH during the course of this investigation at Upstate Medical Center, Syracuse, NY 13210. This work was supported in part by a grant from U. S. Public Health Service AM 19531, and the Surgical Research Fund of the Upstate Medical Center. TKR is currently involved in mind-body research with Vivekananda Mind-Body Research Institute (VMBRI) and Ramakrishna Sarada Vedanta Center of Phoenix, Tempe, Az 85281.
Faculty Opinions recommendedReferences
- 1.
Skou JC, Essman M:
The Na K-ATPase.
J Bioenergetics and Biomembrane.
1992; 24(3): 249–261. PubMed Abstract
- 2.
Hansen O:
Half-of-the-sites reactivity of the Na K-ATPase examined by the accessibility of vanadate and ATP into enzyme ouabain complex.
Curr Top Membr Transp.
1983; 19: 219–222. Publisher Full Text
- 3.
Askari A:
Na+ + K+-ATPase: on the number of the ATP sites of the functional unit.
J Bioenerg Biomembr.
1987; 19(4): 359–374. PubMed Abstract
| Publisher Full Text
- 4.
Antolovic R, Hamer E, Serpersu EH, et al.:
Affinity labelling with MgATP analogues reveals coexisting Na+ and K+ forms of the α-subunits of Na+/K+-ATPase.
Eur J Biochem.
1999; 261(1): 181–189. PubMed Abstract
| Publisher Full Text
- 5.
Pauls H, Serpersu EH, Kirch U, et al.:
Chromium(III)ATP inactivating (Na+ + K+)-ATPase supports Na+-Na+ and Rb+-Rb+ exchanges in everted red blood cells but not Na+,K+ transport.
Eur J Biochem.
1986; 157(3): 585–595. PubMed Abstract
| Publisher Full Text
- 6.
Hall C, Ruoho A:
Ouabain-binding-site photoaffinity probes that label both subunits of Na+, K+-ATPase.
Proc Natl Acad Sci.
1980; 77(8): 4529–4533. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 7.
Ray TK, Das PK, Zinkievich M, et al.:
In Gastrointestinal Disorders: Symptoms, Treatment and Prevention, Nova Science Publications, N. Y. (Editors, M. Battik and N. Grimaldi).2011; 2012: 175–200.
- 8.
Ray TK, Nandi J:
K-stimulated p-nitrophenyl phosphatase is not a partial reaction of the gastric (H+ + K+)-transporting ATPase: Evidence supporting a new model for the univalent-cation-transporting ATPase systems.
Biochem J.
1986; 233(1): 231–238. PubMed Abstract
| Free Full Text
- 9.
Nandi J, Ray TK:
Mechanism of action of gastric secretory inhibitors: Effects of SCN- OCN-, NO-2, and NH+4 on (H+ + K+)-ATPase-mediated transport of H+ inside gastric microsomal vesicles.
Arch Biochem Biophys.
1982; 216(1): 259–271. PubMed Abstract
| Publisher Full Text
- 10.
Ray TK, Nandi J, Pidhorodeckyi N, et al.:
Polyamines are inhibitors of gastric acid secretion.
Proc Natl Acad Sci U S A.
1982; 79(5): 1448–1452. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 11.
Das PK, Chakrabarti R, Bandopadhyay S, et al.:
Demonstration of an endogenous activator for the Na, K-ATPase system.
Mol Cell Biochem.
1989; 91(1–2): 123–129. PubMed Abstract
| Publisher Full Text
- 12.
Ray T:
The parietal cell gastric H, K-ATPase also functions as the Na, K-ATPase and Ca-ATPase in altered states.
F1000Research.
2013; 2: 165.V2. Publisher Full Text
- 13.
Ivanov AV, Modyanov NN, Askari A:
Role of the self-association of β-subunits in the oligomeric structure of Na+/K+-ATPase.
Biochem J.
2002; 364(Pt 1): 293–299. PubMed Abstract
| Free Full Text
- 14.
Xie Z, Cai T:
Na+-K+--ATPase-mediated signal transduction: From protein interaction to cellular function.
Mol Interv.
2003; 3(3): 157–168. PubMed Abstract
| Publisher Full Text
- 15.
Nandi J, Wright MV, Ray TK:
Effects of phospholipase A2 on gastric microsomal H+, K+-ATPase system: Role of "boundary lipids" and the endogenous activator protein.
Biochemistry.
1983; 22(25): 5814–5821. PubMed Abstract
| Publisher Full Text
- 16.
Bandopadhyay S, Das PK, Wright MV, et al.:
Characteristics of a pure endogenous activator of the gastric H+,K+-ATPase system: Evaluation of the role as a possible intracellular regulator.
J Biol Chem.
1987; 262(12): 5664–5670. PubMed Abstract
- 17.
Facciotti MT, Rouhani-Manshadi S, Glaeser RM:
Energy transduction in transmembrane ion pumps.
Trends Biochem Sci.
2004; 29(8): 445–451. PubMed Abstract
| Publisher Full Text
- 18.
Bublitz M, Poulsen H, Morth JP, et al.:
In and out of the cation pumps: P-type ATPase structure revisited.
Curr Opin Struc Biol.
2010; 20(4): 431–439. PubMed Abstract
| Publisher Full Text
- 19.
Blanco G:
Na, K-ATPase subunit heterogeneity as a mechanism for tissue-specific ion regulation.
Semin Nephrol.
2005; 25(5): 292–303. PubMed Abstract
| Publisher Full Text
- 20.
Beggah AT, Hasler U, Crambert G, et al.:
Transport and pharmacological properties of nine different human Na,K-ATPase isozymes.
J Biol Chem.
2000; 275(3): 1976–1986. PubMed Abstract
| Publisher Full Text
- 21.
Segall L, Daly SE, Blostein R:
Mechanistic basis for kinetic differences between the rat alpha 1, alpha 2, and alpha 3 isoforms of the Na,K-ATPase
J Biol Chem.
2001; 276(34): 31535–31541. PubMed Abstract
| Publisher Full Text
- 22.
Shyjan AW, Gottardi C, Levenson R:
The Na,K-ATPase beta 2 Subunit Is Expressed in Rat Brain and Copurifies with Na,K-ATPase Activity.
J Biol Chem.
1990; 265(9): 5166–5169. PubMed Abstract
- 23.
Lutsenko S, Kaplan JH:
Organization of P-type ATPases: significance of structural diversity.
Biochemistry.
1995; 34(48): 15607–15613. PubMed Abstract
| Publisher Full Text
- 24.
Brini M, Carafoli E:
Calcium pumps in health and disease
Physiol Rev.
2009; 89(4): 1341–1378. PubMed Abstract
| Publisher Full Text
- 25.
Bandopadhyay S, Ray TK:
Purification and characterization of a cytosolic activator protein for the gastric H+,K+-ATPase system from dog fundic mucosa, Prep Biochem.
Prep Biochem.
1986; 16(1): 21–32. PubMed Abstract
| Publisher Full Text
- 26.
Ray TK, Das PK, Nandi J, et al.:
Characteristics of the isolated apical plasmalemma and intracellular tubulovesicles of the gastric acid secreting cells: Demonstration of secretagogue-induced membrane mobilization.
Biochemistry.
1988; 27(25): 8958–8968. PubMed Abstract
| Publisher Full Text
- 27.
Ray TK, Chakrabarti R:
Regulation of active transport of monovalent cations across the animal cell plasma membranes by cytosolic regulatory proteins.
Indian J Biochem Biophys.
1988; 25(3): 219–225. PubMed Abstract
| Publisher Full Text
- 28.
Nandi J, Meng-Ai Z, Ray TK:
Purification and partial characterization of the (H+,K+)-transporting adenosine triphosphatase from fundic mucosa.
Biochemistry.
1987; 26(14): 4264–4272. PubMed Abstract
| Publisher Full Text
- 29.
Ray TK, Nandi J:
Modulation of gastric H+,K+-transporting ATPase function by sodium.
FEBS Lett.
1985; 185(1): 24–28. PubMed Abstract
| Publisher Full Text
- 30.
Ray TK, Bandopadhyay S, Das PK:
Regulation of the gastric H+, K+-transporting ATPase by a cytosolic 80 kDa activator protein and calcium: Ca2+ as a possible physiological switch.
Indian J Biochem Biophys.
1988; 25(1–2): 43–51. PubMed Abstract
- 31.
Das PK, Bandopadhyay S, Banerjee A, et al.:
Fed Proc.1987; 46(3): 261 (abstract).
- 32.
Liang P, Martin-Vasallo P, Sweadner KJ:
Isoforms of Na,K-ATPase alpha and beta subunits in the rat cerebellum and in granule cell cultures
J Neuroscience.
1997; 17(10): 3488–3502. PubMed Abstract
- 33.
Greenlee MM, Lynch IJ, Gumz ML, et al.:
The renal H, K-ATPases.
Curr Opin Nephrol Hypertens.
2010; 19(5): 478–482. PubMed Abstract
| Publisher Full Text
- 34.
Donato R:
S100 proteins in tissue development, repair and regeneration World.
J Biol Chem.
2013; 26: 1–12.
- 35.
Dodge FA Jr, Rahamimoff R:
Co-operative action of calcium ions in transmitter release at the neuromuscular junction.
J Physiol (Lond).
1967; 193(2): 419–432. PubMed Abstract
| Free Full Text
- 36.
Llinas R, Sugimori M, Silver RB:
Microdomains of high calcium concentration in a presynaptic terminal.
Science.
1992; 256(5057): 677–679. PubMed Abstract
| Publisher Full Text
- 37.
Demuro A, Parker I:
Imaging single-channel calcium microdomains.
Cell Calcium.
2006; 40(5–6): 413–422. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 38.
Blanco G, Mercer RW:
Isozymes of the Na-K-ATPase: Heterogeneity in structure, diversity in function.
Am J Physiol.
1998; 275(5 Pt 2): F633–F650. PubMed Abstract
- 39.
Beauge L, Campos MA:
Calcium inhibition of the ATPase and phosphatase activities of (Na1 1 K1)-ATPase.
Biochim Biophys Acta.
1983; 729: 137–149.
- 40.
Magno AL, Ward BK, Ratajczak T:
The calcium sensing receptors: A molecular perspective.
Endocr Rev.
2011; 32(1): 3–30. PubMed Abstract
| Publisher Full Text
- 41.
Xiong ZG, Lu WY, Macdonald, JF:
Extracellular calcium sensed by a novel cation channel in hippocampal neurons.
Proc Natl Acad Sci U S A.
1997; 94(13): 7012–7017. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 42.
Lu B, Zhang Q, Wang H, et al.:
Extracellular Calcium Controls Background Current and Neuronal Excitability via an UNC79-UNC80-NALCN Cation Channel Complex.
Neuron.
2010; 68(3): 488–499. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 43.
Bai M, Trivedi S, Brown EM:
Dimerization of the extracellular calcium-sensing receptor (CaR) on the cell surface of CaR-transfected HEK293 cells.
J Biol Chem.
1998; 273(36): 23605–23610. PubMed Abstract
| Publisher Full Text
- 44.
Pidasheva S, Grant M, Canaff L, et al.:
Calcium-sensing receptor dimerizes in the endoplasmic reticulum: biochemical and biophysical characterization of CASR mutants retained intracellularly.
Hum Mol Genet.
2006; 15(14): 2200–2209. PubMed Abstract
| Publisher Full Text
- 45.
Ward DT, Brown EM, Harris HW:
Disulfide bonds in the extracellular calcium-polyvalent cation-sensing receptor correlate with dimer formation and its response to divalent cations in vitro.
J Biol Chem.
1998; 273(23): 14476–14483. PubMed Abstract
| Publisher Full Text
- 46.
Nowak SJ, Corces VG:
Phosphorylation of histone H3: a balancing act between chromosome condensation and transcriptional activation.
Trends Genet.
2004; 20(4): 214–220. PubMed Abstract
| Publisher Full Text
- 47.
Gumz ML, Lynch JI, Greenlee MM, et al.:
The renal H+-K+-ATPases: Physiology, regulation and structure.
Am J Physiol Renal Physiol.
2010; 298(1): F12–F21. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 48.
Cougnon M, Bouyer P, Planelles G, et al.:
Does the colonic H,K-ATPase also act as an Na,K-ATPase?
Proc Natl Acad Sci.
1998; 95(11): 6516–6520. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 49.
Padan E, Kozachkov L, Herz K, et al.:
NhaA crystal structure: functional-structural insights.
J Exp Biol.
2009; 212(Pt 11): 1593–1603. PubMed Abstract
| Publisher Full Text
- 50.
Caroppo R, Gerbino A, Debellis L, et al.:
Asymmetrical, agonist-induced fluctuations in local extracellular [Ca2+] in intact polarized epithelia.
The Embo J.
2001; 20(22): 6316–6326. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 51.
Sands JM, Naruse M, Baum M, et al.:
Apical extracellular calcium/polyvalent cation sensing receptor regulates vasopressin-elicited water permeability in rat kidney inner medullary collecting duct.
J Clin Invest.
1997; 99(6): 1399–1405. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 52.
Urushidani T, Forte JG:
Signal transduction and activation of acid secretion in the parietal cell.
J Membr Biol.
1997; 159(2): 99–111. PubMed Abstract
| Publisher Full Text
- 53.
Sen PC, Ray TK:
Control of the potassium ion-stimulated adenosine triphosphatase of pig gastric microsomes: effects of lipid environment and the endogenous activator.
Arch of Biochem Biophys.
1980; 202(1): 8–17. PubMed Abstract
| Publisher Full Text
- 54.
Ray TK, Das PK, Sen PC, et al.:
Current outlooks on the lipids of gastric membranes and beyond.
Trends in Cell and Molecular Biol.
2008; 3: 69–78. Reference Source
- 55.
Rogasevskaia T, Coorssen JR:
Sphingomyelin-enriched microdomains define the efficiency of native Ca(2+)-triggered membrane fusion.
J Cell Sci.
2006; 119(Pt 13): 2688–94. PubMed Abstract
| Publisher Full Text


Comments on this article Comments (0)