Introduction
End-stage renal disease (ESRD) is a debilitating condition resulting in death unless treated. Kidney transplantation, dialysis, and conservative therapy supported by palliative care are the three primary treatment options for patients with ESRD1,2. Although kidney transplantation is the most cost-effective therapy, the supply of donor kidneys is limited3. In addition, patients who are eligible for and choose kidney transplantation usually have to wait months to years for a kidney to become available. During this waiting period, patients are treated with dialysis. The two main types of dialysis are hemodialysis (HD) and peritoneal dialysis (PD). In HD, the removal of wastes and excess fluid is accomplished by circulating blood outside the body through an external filter (dialyzer). HD is usually performed in a hospital or clinic (in-center HD or ICHD), but can also be performed at home (HHD). Conventional HD is typically performed three times per week, with each treatment lasting 3 to 5 hours. In PD, the removal of wastes and excess fluid is accomplished inside the body, in the abdominal or peritoneal cavity. A dialysis solution is placed in the peritoneal cavity, which allows wastes to move from the blood to this solution. After a period of time, the solutions are drained from the body4. Unlike HD5, PD is usually a continuous therapy, in which dialysis solutions are repeatedly drained from and instilled into the peritoneal cavity so there is a possibility of dialysate dwell (and hence solute removal) up to 24 hours/day 7 days a week4. However, some patients are treated with intermittent PD, which is either performed manually (the patient uses gravity to fill and drain the abdominal cavity) through continuous ambulatory PD (CAPD) treatment, or with a cycler (a machine that automatically fills and drains the abdominal cavity, usually while the patient sleeps), in automated PD (APD) treatment6.
The prevalence of ESRD (estimated to be 2.8 million globally at the end of 20117) and, consequently, the demand for dialysis, continues to increase due to an aging population, extended life expectancies, the increased prevalence of precipitating conditions such as diabetes6. Worldwide, hemodialysis continues to be the most common mode of therapy for individuals with ESRD6 and the need to provide dialysis treatments for a large and growing patient population places a heavy economic burden on the global health care system8. In the United States, (US), for example, 1.3% of Medicare patients with ESRD accounted for 7.5% of Medicare spending in 20105.
In response to such pressures, initiatives aimed specifically at controlling dialysis-related costs have already been implemented in many countries, including Australia, Portugal, Thailand, United Kingdom (UK) and US9–14. For example, the Australian government implemented a range of national health reforms in 2012, including a national efficient price (NEP) for hospital services, which was based on the average costs of best practice pathways14. Similarly, Portugal implemented a bundled payment program for dialysis, bundling dialysis services and products into one rate11. In addition, the Centers for Medicare and Medicaid Services (CMS) in the US enacted an expanded bundled prospective payment system (PPS) for dialysis services in order to control for the unpredictable and escalating costs associated with the provision of dialysis, and to combine pharmaceuticals and lab services that were previously charged separately13. These and other initiatives are similar in striving for maintaining healthcare costs and identifying interventions that are less resource intensive and/or cost-effective. Given this changing economic environment, where payers and providers around the world are facing pressure to control healthcare costs while improving patient outcomes, we conducted a systematic review of economic evaluations of PD and ICHD published since 2004. Our purpose was to assess whether the recent literature continues to support the use of PD to be a less resource intensive therapy compared with HD from payer, provider and societal perspectives15.
Methods
We conducted a systematic literature search for peer-reviewed economic evaluations of dialysis modalities published between January 1, 2004 and March 31, 2012 in the second quarter of 2012. To ensure that we captured articles that might not have been included in the database for the prior literature synthesis which assessed literature from 1996–2006, our literature search included an overlap of three years of inclusion (2004–2012) with the prior literature synthesis. We applied our search strategies in PubMed; EMBASE; Cochrane Collaboration; Database of Abstracts of Reviews of Effects; UK National Health System Economic Evaluations Database; and Health Technology Assessment Database. Medicine indexing terms for dialysis therapies, including renal replacement therapy, peritoneal dialysis, renal dialysis, and hemodialysis units, hospital that linked to the subheading economics, and free-text search terms, including hemodialysis, peritoneal dialysis, renal dialysis, continuous ambulatory peritoneal dialysis, conventional hemodialysis, automated peritoneal dialysis, cost, costs, expenditures, cost analysis, cost effectiveness, cost benefit analysis, pharmacoeconomics, health economics, and economics, were used in the database search.
Our search identified 498 publications (Figure 1). Two investigators (FXL and TPQ) screened 40% of all the found abstracts independently based on our research objective. With 96% of the selected articles matched, one investigator (TPQ) continued the remaining selection. Articles were excluded (n=444) if they were of the following publication types: Editorial, Letter, Case Report, Comment, Interview, Lecture, News, Newspaper Article, or Patient Education Handout, or if the title/abstract did not suggest that a comparison was made between the costs of at least one sub-modality of PD to at least one sub-modality of ICHD. Articles were included (n=54) if they were original research studies with text in English, and if they compared costs and/or cost effectiveness associated with PD and ICHD or subtypes of PD and ICHD. To be comprehensive, additional articles (n=15) were identified through a review of references from the selected papers. These 69 articles were subjected to full-text review and further inclusion criteria were applied. Studies that did not focus on an economic evaluation of dialysis, or did not differentiate between the costs of PD and ICHD (or their subtypes) were excluded, as were review articles with no original data. Twenty-four articles (18 cost studies and 6 cost-effectiveness studies) met all of these inclusion criteria. Relevant study data were manually extracted (TPQ) and cross-checked for accuracy (FXL).
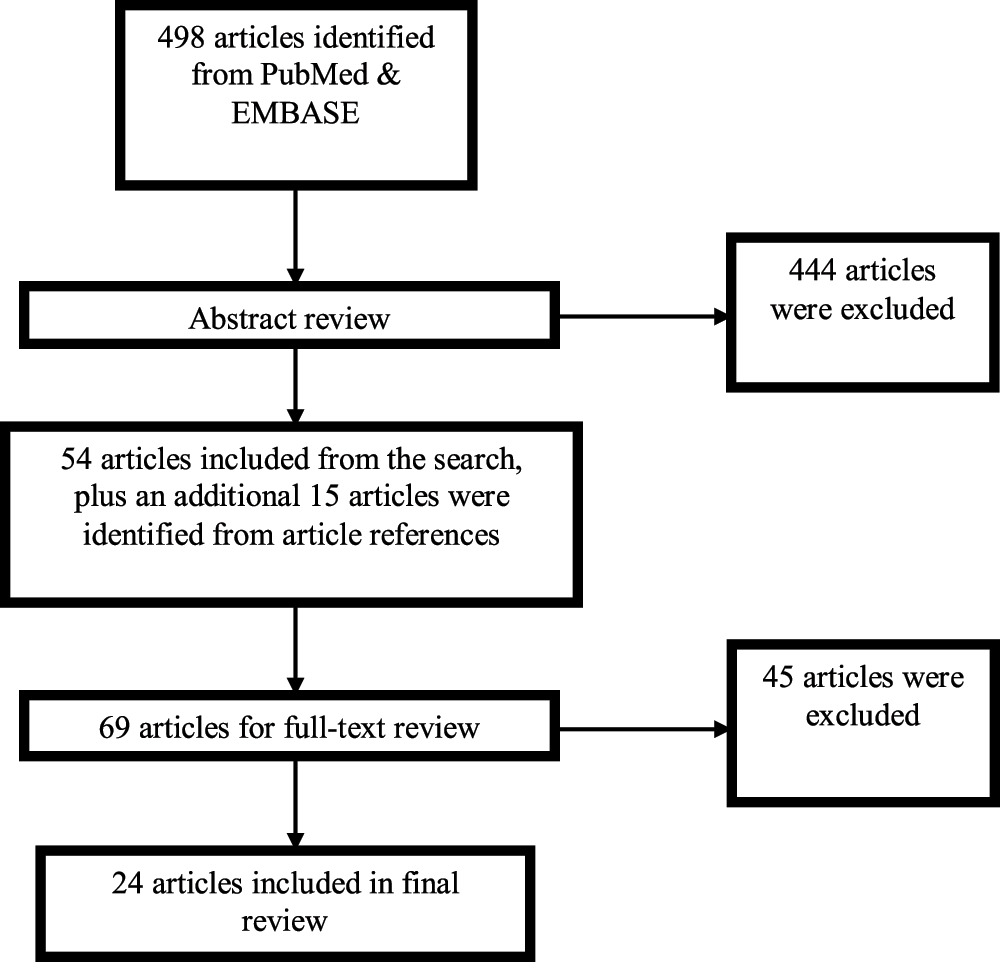
Figure 1. Flow chart of study selection.
Results
The 24 articles included in our review provide economic and cost-utility data from 13 high-income (Australia, Austria, Finland, France, Greece, Italy, Japan, New Zealand, Singapore, Spain, Switzerland, UK, US), three upper-middle income (Chile, Malaysia, Mexico), and six lower-middle income (China, Columbia, India, Romania, Thailand, Turkey) countries. Designations of high, upper-middle, and lower-middle income countries are based on World Bank definitions whereby economies are divided according to the 2011 gross national income (GNI) per capita, which is calculated using the World Bank Atlas method. Defined groups are: low income, $1,025 or less; lower-middle income, $1,026–$4,035; upper-middle income, $4,036–$12,475; and high income, $12,476 or more16. Table 1 outlines the costs of ICHD and PD found in 19 countries from our 18 cost studies.
Cross-country comparisons of economic data are challenging to perform well under the best of circumstances, but perhaps especially in the case of dialysis, where ICHD-related costs are comprised primarily of labor and capital expenses, whereas PD-related costs are associated primarily with disposables, such as dialysate solutions and supplies. In this review, the usual concerns were compounded by the variability of the data compared within individual studies and by incomplete transparency within individual reports. Cost perspectives, where specified, ranged from patients, service providers, and payers to government health systems. With different perspectives, different costs are considered relevant and different methods of apportioning direct and overhead expenses may influence the reported results17. The currency years reported in these studies, where specified, spanned more than a decade (1997 to 2010), demanding significant assumptions to adjust for price inflation, international currency valuations, and purchasing power parity in order to arrive at a common-currency metric. Furthermore, differences in provider payment and healthcare financing systems, variation in rates of HD versus PD use affecting economies of scale across different countries, and potential differences in treatment settings intensify the challenges for cross-country comparisons of these studies. Data sources were similarly varied, including literature-derived secondary sources, hospital records, insurance information, disease registries, government publications, expert opinions, surveys, and semi-structured interviews. Finally, the 19 countries from which dialysis costs were reported are at different levels of economic development. As such, they have widely varying infrastructures available to support dialysis services, as well as different barriers to the use of each dialysis modality18,19. Each of these factors further magnify the danger of cross-country comparisons20. Nevertheless, the data shown in Table 1 remain valuable insofar as they highlight the magnitude of dialysis costs and the relative (not absolute) cost differences between the dialysis modalities across countries. Ratios of costs for ICHD compared to PD are included in Table 1 to assist with this exercise. Ratios less than 1.00 (parity) denote lower cost of ICHD compared to PD.
Table 1. Annual cost of ICHD and PD in 19 countries.
| Country | Year/
Currency | Perspective
(I) = implied
(S) = stated | Costs
included | Costs (in local currency) | ICHD:PD
Cost
ratio |
|---|
| PD | CAPD | APD | Satellite
HD | ICHD |
|---|
High
Income | Finland21 | 2004 €a | Payer (S) | Medical direct,
Total medical | 52,608
55,743 | - | - | - | 65,912
77,126 | 1.25–1.38 |
| France38 | 2005 € | Payer, National
Health System
(S) | Medical direct,
Total medical | - | 49,366
49,853 | 49,089
49,676 | - | 71,893
81,449 | 1.46–1.64 |
| France39 | 2003 € | National Health
System, Society
(S) | Medical direct | 23,926 | - | - | - | Public:
97,696
Private:
61,959 | 2.59–4.08 |
| Greece30 | €, year not
specified | Payer, National
Health System
(S) | Total medical | 38,359 | - | - | - | 45,479 | 1.19 |
| Italy40 | 2001 € | National Health
System, Society
(S) | Medical direct | - | 16,790 | 20,075 | 21,880b | 25,941c | 1.09–1.55 |
| Japan22 | 2003 Yen
converted
to 2003 US$
using PPP | Payer, National
Health System
(S) | Medical direct | 49,215 | - | - | - | 42,098 | 0.86 |
New
Zealand41 | 2002–2004
NZ$ | National Health
System, Society
(S) | Medical direct | - | 36,615 | 45,686 | 48,172 | 64,318 | 1.05–1.76 |
| Singapore32 | 2006 SGD | Government (S) | Medical direct | | 14,880 | 21,336 | - | 24,720 | 1.16–1.66 |
| Spain28 | 2010 € | - | Total medical | 25,664 | - | - | - | 24,918–
27,933 | 0.97–1.09 |
| Spain42 | 2010 € | Public
Administration (S) | Total medical
plus
indirect | 25,826
33,255 | - | - | - | 37,968
46,897 | 1.41–1.47 |
| Switzerland43 | 2001 CHF | Health
Insurance Funds
(S) | Total medical | 59,049 | - | - | - | 83,683 | 1.42 |
| UK32 | 2007 £ | Hospital (S) | Medical direct | - | 16,355 | 22,350 | - | 35,023 | 1.57–2.14 |
| UK44 | £, year not
specified | Payer, National
Health Service
(S) | Medical direct | - | 15,570 | 21,655 | 32,669 | 35,023 | 1.51–2.25 |
| US45 | US$,
year not
specified | Payer, Insurer
(I) | Medical direct | 129,997 | - | - | - | 173,507 | 1.33 |
| US46 | 2005 US$ | Payer, Medicare
(S) | Medical direct | 50,847 | - | - | - | 69,758 | 1.37 |
| US47 | 2004 US$ | Payer, Medicare
(S) | Medical direct | 53,277 | - | - | - | 72,189 | 1.35 |
Upper-
Middle
Income | Chile32 | 2005 US$ | Government (I) | Total medical | 17,031 | - | - | - | 18,885 | 1.12 |
| Chile33 | 2005 US$ | Payer, Society (I) | Medical direct,
Total medical
plus indirect | 16,666
20,742 | - | - | - | 14,884
20,803 | 0.89–1.00 |
| Mexico32 | 2006 US$ | Government
(S) | Medical direct | 15,724 | - | - | - | 24,032 | 1.53 |
Lower-Middle
Income | China32 | 2005 RMB | Hospital (S) | Total medical | 84,141 | - | - | - | 98,204 | 1.17 |
| Columbia32 | 2005 US$ | Government
(I) | Hospitalization
costs only | - | 775 | 884 | - | 2,144 | 2.43–2.77 |
| India48 | 2010
Rupees | Patients (I) | Medical direct,
Total medical | 19,528
28,763 | - | - | - | 14,669
29,252 | 0.75–1.02 |
| Romania32 | 2006 € | Government
(S) | Medical direct | 12,700 | - | - | - | 18,400 | 1.45 |
| Thailand32 | 2005 Baht | Government
(I) | Total medical | 461,541 | - | - | - | 519,047 | 1.12 |
| Turkey49 | 2001 US$ | Payer, Various
(I) | Medical direct | - | 22,350 | - | - | 22,759 | 1.02 |
The findings from our review demonstrate that ICHD costs, whether they are medical direct-, total medical-, or total medical plus indirect costs, are generally (but not always) higher than PD costs. A 2009 Finnish study21 of the total medical costs (payer perspective) of PD and ICHD reported that the cost of ICHD tended to be about 25%–38% higher than the cost of PD (ICHD:PD ratio = 1.25-1.38). In contrast, a 2007 study evaluating the direct costs of PD and ICHD (payer perspective) in Japan22, found that the cost of ICHD tended to be about 86% of the cost of PD (ICHD:PD ratio = 0.86). Nevertheless, data from 82% (9/11) of the high-income, 50% (1/2) of the upper-middle income, and 83% (5/6) of the lower-middle income countries represented in our review show the cost of ICHD to be more than that of PD. Figure 2 arrays the country-specific cost ratios for ICHD compared to PD.
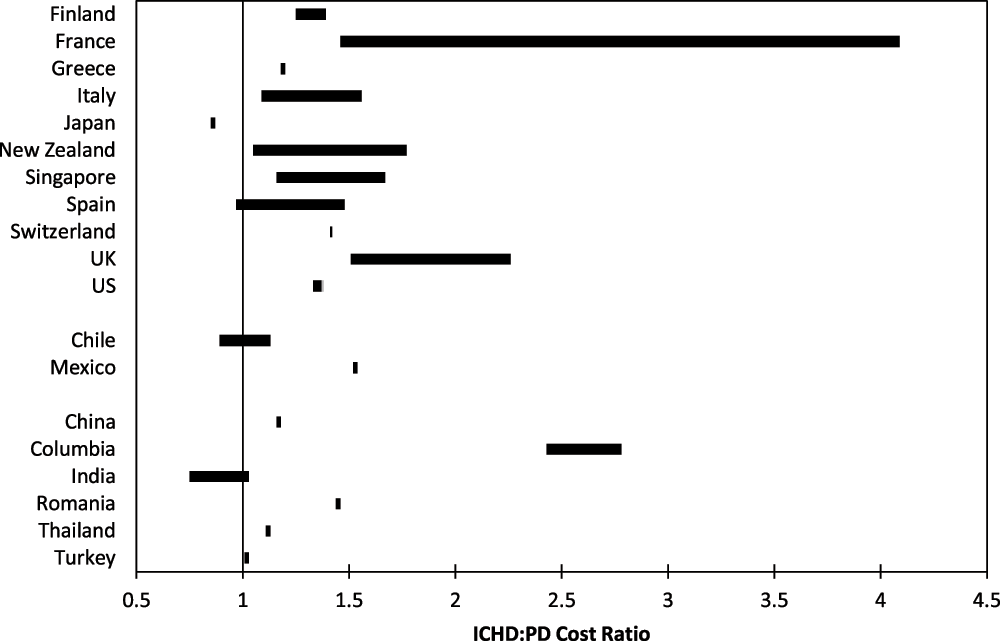
Figure 2. PD (peritoneal dialysis): ICHD (in-center hemodialysis) cost ratios in 19 countries.
Six of the 24 studies in our review were cost-effectiveness or cost-utility studies; that is, they considered the outcomes of each dialysis modality in terms of patient life-years, and/or healthy life-years (typically measured as quality-adjusted life years) in addition to costs. Despite wide variability in methodologies, outcome measures, perspectives, time horizons, costs, and analytic approaches used in these six studies, each came to a similar conclusion: the cost-effectiveness of PD is equivalent to (1 study), or better than (5 studies) the cost-effectiveness of ICHD (Table 2).
Table 2. Cost-effectiveness/cost-utility of ICHD and PD.
| Country | Outcome
measure* | Conclusion | Comments |
|---|
| Australia35 | LY, QALY | Home-based dialysis
options (including PD)
are more cost-effective
compared to ICHD | Increasing rate of PD by switching patients from ICHD saves
A$122.1 million annually (central health-care payer perspective). The
reported model lacked good quality data on changes in utility-based
quality of life resulting in changes to dialysis modality; however, the
authors conclude that it is reasonable to assume improved quality of
life with PD (vs. ICHD) |
| Austria34 | QALY | PD is more cost-effective
compared to ICHD | Increasing assignment to PD of 20% incident patients saves €26
million (payer perspective) and gains 839 QALY over 10 years |
| Greece36 | QALY | PD is more cost-effective
compared to ICHD | Health system payer perspective. PD produces a lower cost per
QALY (€54,504) compared to ICHD (€60,353) |
| Finland50 | LY | CAPD is more cost-
effective compared to
ICHD | From the provider perspective, where costs represent 3-year
cumulative costs and effectiveness represents 3-year survival.
The ratio of cost-effectiveness for CAPD:ICHD ranges from .64 to
.94 (depending on the analysis strategy), indicating that CAPD is
consistently more cost-effective than ICHD |
| Malaysia51 | LY | CAPD is marginally more
cost-effective compared
to ICHD | Ministry of Health perspective. Cost per life-year gained is RM
31,635 for CAPD vs. RM 33,642 for ICHD. The authors conclude
that CAPD and ICHD are both viable options in Malaysia, and that
both modalities can be delivered efficiently (i.e., CAPD center
serving approximately 100 patients annually; HD center providing
approximately 15,000 HD treatments annually) |
| Thailand31 | LY, QALY | PD is more cost-effective
compared to ICHD,
especially in younger
patients with ESRD | In models with adjusted and unadjusted survival, the incremental
costs of providing ‘PD first’ ranged from 466,000 Baht per LY saved
(667,000 Baht per QALY gained) for patients aged 20 years to
497,000 Baht per LY saved (7000,000 Baht per QALY gained) for
patients aged 70 years from a societal perspective. Slightly higher
(i.e., less favorable) cost-effectiveness and cost-utility ratios were
observed with a policy of ‘HD first’ |
The 24 economic analyses reviewed in the context of this study were of variable quality. This is a particular concern because the individuals who need to use these analyses to guide decisions may not be experts at evaluating them23. To document the quality differences between studies in the context of this review, we characterized the reports using criteria adapted from the Quality of Health Economics Studies (QHES) instrument23, which was specifically developed to emphasize appropriate methods; and valid, transparent, and comprehensive results reporting in health economic analyses23.
Characteristics of high-quality studies not only clearly specified the objective, perspective, and data source, but also included a detailed description of the study methodologies; total direct, non-direct, indirect, and intangible medical costs; and addressed both limitations and biases. While the majority of cost-effectiveness studies clearly described study objective, perspective, and study estimates (83%–100%), fewer studies addressed the validity of outcome measures and biases (67%). Similarly, cost studies frequently reported study objective and direct medical costs, but none of the cost studies included intangible costs and only a few studies (17%) included indirect costs. Intangible costs may be difficult to assess well in the context of dialysis due to the lack of a firm value and the challenge in quantifying pain and suffering within a heterogeneous population.
Discussion
We conducted a systematic search for peer-reviewed economic evaluations of dialysis modalities published between January 2004 and March 2012, with the goal of evaluating the economic literature within the context of recent global dialysis payment policies encouraging greater use of PD, while building on an earlier systematic review of the literature15 that was published between 1996 and 2006. The results of our review reiterate that ICHD is generally more costly, and less cost-effective than PD in the majority of studies and countries that examined costs and/or cost effectiveness associated with the different dialysis modalities. These results are consistent with previously published systematic reviews of the literature15,24. In fact, there is a growing body of evidence suggesting greater use of PD or the use of PD as first treatment modality for appropriate ESRD patients25–27. Our findings show that existing reimbursement incentives for delivering dialysis therapies have implications for both clinical practice and health policy. The burden of rising healthcare costs globally, coupled with competing economic priorities, highlight the importance of establishing policies that encourage the use of PD over ICHD, whenever feasible and clinically appropriate.
There are exceptions where PD has been reported to be more costly than ICHD; these are noted in Table 1. The reasons behind these exceptions are myriad, but chief among them may be methodological differences between studies, as well as variations in practice patterns, cost estimations, and the inclusion/exclusion of specific cost elements. For example, a study conducted in Spain28 included staff wages for direct PD-related medical costs, whereas ICHD costs excluded these costs in the calculation of direct medical costs. In effect, this particular study reported PD to be more costly than ICHD.
Most of the studies in our review consider only direct medical and non-medical costs in their analyses. Direct medical costs include physician and other staff costs (fees; salaries); capital costs of hemodialysis machines and PD cyclers; costs associated with establishing and maintaining vascular access; ongoing use of dialysis-related supplies such as solutions, medications, and tubing; as well as costs associated with radiology, laboratory and hospitalization. Direct non-medical costs include overheads for administering and maintaining buildings and facilities but they may also include patients’ travel to a dialysis center or physician’s office. It is important to keep in mind that PD and ICHD rely on very different direct cost components. The direct cost of ICHD is driven primarily by the cost of staff, buildings, infrastructure, and capital equipment (i.e., fixed costs), whereas the direct cost of PD is driven primarily by the supply costs of dialysis solutions, tubing and other disposables (i.e., variable costs). Thus, it is not enough to compare dialysis modalities based on differences in one or two cost components. Rather, cost comparisons of PD and ICHD must be based on the total costs of therapy, as direct medical costs alone do not have the same relevance from all economic perspectives. For instance, in the developing world where labor is relatively inexpensive and the cost of imported dialysis solutions and supplies is high, it is easy to perceive PD as being more expensive than ICHD29. The reverse may be true in developed economies where labor is relatively more costly than disposable supplies.
Indirect costs, or productivity losses of patients and caregivers were rarely included in the studies we reviewed, even in the four studies focusing on six countries for which the stated perspective of the analysis was societal30–33. Of these, the most comprehensive array of indirect costs is represented in a Thai study that considers all resources used by dialysis patients and/or caregivers, including capital, labor and materials costs of health-care providers, as well as the real and opportunity costs associated with patient treatment time, informal patient care, and sick leave31. Of course, the inclusion of indirect and/or productivity costs will substantially increase the reported cost of any dialysis modality, but these costs have a particular impact on the cost of ICHD which requires regular, lengthy, dialysis treatments that are provided outside of patients’ homes.
Intangible costs denote those consequences of an intervention that are particularly challenging to measure, such as the value of improved health per se, or the pain, suffering, and impairment in quality of life associated with treatment. Only five30,31,34–36 of the 24 studies included in our review considered intangible costs, all of them via the utility scores used to arrive at quality-adjusted life years.
Our review has several limitations. First, our review focused on economic evidence comparing PD and HD; articles comparing the clinical and patient outcomes associated with PD and HD were not included in this review. Second, we included all articles we found that compared the costs and/or cost effectiveness of PD vs. ICHD. These articles are of varying quality and our report did not differentiate the quality of these findings. As such, it is difficult to make generalizations about the published literature of dialysis economics owing to the limited number of studies available and differences among them in terms of study methods, geography, scope, and quality. Some studies rely on decades-old data that may not be relevant to present-day economies; other studies fail to indicate the currency-year used in analysis. Between-country differences in economic development, practice patterns, reimbursement structures, provider payment, healthcare system financing, and social norms often make it difficult to interpret the findings from one country in the context of another country. In addition, the proportion of patients on HD vs. PD and the treatment settings (provider demographics such as freestanding/satellite vs. hospital-based dialysis clinics) vary across countries. Even studies that report ‘direct costs’ may include/exclude different components of direct cost and, thus, may not necessarily produce comparable results. Further, some authors clearly specify the perspective taken in their analyses (e.g., societal, payer, provider); in other papers the perspective is only implied. Reported study methods, too, may be insufficiently transparent to evaluate study quality; for example, the calculations used to derive costs or the elements of costs (e.g., frequency of dialysis, prescription drug use) are often lacking or unclear. Data sources may vary among studies as well; data sources may include disease registries; hospital, insurer, or government databases; secondary literature; government publications; dialysis product distributors; dialysis center data; and/or individual patient interviews. Just one of these between-study differences introduces uncertainty when drawing comparisons among study results. Because published studies of the economics of dialysis often vary on many of these parameters, specific, robust between-study comparisons cannot be made.
Despite the methodological differences and variable quality across the studies within our review, ICHD clearly emerged as a more expensive modality than PD in most of the 19 countries represented in our review. These results are consistent with earlier systematic reviews of the dialysis literature15,37. Similarly, the cost-effectiveness studies presented in Table 2 begin to show a nascent picture of cost-savings related to PD; the limited number of cost-effectiveness studies and the methodological differences between them (e.g., differences in the variables considered within each modelled analysis) preclude the drawing of specific conclusions.
Conclusion
The magnitude of costs associated with providing dialysis therapies globally is large and growing. Our findings echo those of prior published reviews and add to the growing body of evidence finding PD is significantly cost-saving compared to ICHD therapy in most developed countries and some developing countries. Additionally, evidence continues to suggest that PD is a cost-effective strategy relative to ICHD. Therefore, it appears that increasing the use of clinically-appropriate PD would substantially reduce healthcare costs and help health systems meet ever-tightening budget constraints.
Author contributions
FXL and TQ conceived, designed, and implemented the review. JB contributed to the data extraction of the study from reviewed papers and provided expertise in nephrology. FXL, TQ, and GI prepared the first draft of the manuscript. LN contributed to the design and preparation of the manuscript. All authors were involved in reviewing and revising the draft manuscript and have agreed to the final content.
Competing interests
Frank Xiaoqing Liu is an employee and stockholder of Baxter Healthcare Corporation, Deerfield, IL USA; At the time this research was conducted, Tiffany Quock and Les Noe were employees of ICON, which received research funding from Baxter Healthcare Corporation for the systematic review. Tiffany is now at Covance Market Access Services Inc. and Les is now at InVentiv Health Clinical; John Burkart is faculty at Wake Forest University, Winston-Salem, NC, USA and has been an ad hoc consultant for Baxter Healthcare Corporation; Gary Inglese was an employee of Baxter Healthcare Corporation at the time of this manuscript development and is now at Hollister Incorporated.
Grant information
The study was supported by funding from Baxter Healthcare Corporation, Deerfield, IL 60015 USA. The publication of the study results was not contingent on the sponsor's approval or censorship of the manuscript.
Acknowledgements
The authors acknowledge Nancy Neil, Ph.D. from Chordata Consulting, LLC and University of Washington, Seattle, WA, USA, for her help on the preparation of the manuscript and Kathryn Webb, MS, for her help on the literature search.
Faculty Opinions recommendedReferences
- 1.
Fassett RG, Robertson IK, Mace R, et al.:
Palliative care in end-stage kidney disease.
Nephrology (Carlton).
2011; 16(1): 4–12. PubMed Abstract
| Publisher Full Text
- 2.
Jassal SV, Kelman EE, Watson D:
Non-dialysis care: an important component of care for elderly individuals with advanced stages of chronic kidney disease.
Nephron Clin Pract.
2011; 119(suppl 1): c5–c9. PubMed Abstract
| Publisher Full Text
- 3.
Abecassis M, Bartlett ST, Collins AJ, et al.:
Kidney transplantation as primary therapy for end-stage renal disease: a National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQITM) conference.
Clin J Am Soc Nephrol.
2008; 3(2): 471–480. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 4.
Renal Resource Center 2010. An Introduction to Peritoneal Dialysis. Reference Source.
- 5.
Ghaffari A, Kalantar-Zadeh K, Lee J, et al.:
PD First: Peritoneal Dialysis as the Default Transition to Dialysis Therapy.
Semin Dial.
2013; 26(6): 706–713. PubMed Abstract
| Publisher Full Text
- 6.
U.S. Renal Data System. USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. 2012.
- 7.
Fresenius Medical Care. ESRD Patients in 2011. Reference Source.
- 8.
Zelmer JL:
The economic burden of end-stage renal disease in Canada.
Kidney Int.
2007; 72(9): 1122–1129. PubMed Abstract
| Publisher Full Text
- 9.
Praditpornsilpa K, Lekhyananda S, Premasathian N, et al.:
Prevalence trend of renal replacement therapy in Thailand: impact of health economics policy.
J Med Assoc Thai.
2011; 94(suppl 4): s1–s6. PubMed Abstract
- 10.
Sirivongs DKV, Wangsiripaisal A, et al.:
Fact about PD First Policy in Thailand. Vol ISBN 978-616-223-129-2. Nonthaburi Thailand: Sahamitrprinting & publishing.co, Ltd.
- 11.
Rocha MJ, Ferreira S, Martins LS, et al.:
Cost analysis of renal replacement therapy by transplant in a system of bundled payment of dialysis.
Clin Transplant.
2012; 26(4): 529–531. PubMed Abstract
| Publisher Full Text
- 12.
Iglehart JK:
Bundled Payment for ESRD - including ESAs in Medicare’s Dialysis Package.
N Engl J Med.
2011; 364(7): 593–595. PubMed Abstract
| Publisher Full Text
- 13.
Golper TA, Guest S, Glickman JD, et al.:
Home dialysis in the new USA bundled payment plan: implications and impact.
Perit Dial Int.
2011; 31(1): 12–16. PubMed Abstract
| Publisher Full Text
- 14.
Council of Australian Governments. National Healthcare Agreement. 2011. Australia. 2011; Reference Source.
- 15.
Just PM, Riella MC, Tschosik EA, et al.:
Economic evaluations of dialysis treatment modalities.
Health Policy.
2008; 86(2–3): 163–180. PubMed Abstract
| Publisher Full Text
- 16.
The world bank. how we classify countries. 2013. Reference Source.
- 17.
Moore R, Marriott N:
Cost and price in the NHS: the importance of monetary value in the decision-making framework--the case of purchasing renal replacement therapy.
Health Serv Manage Res.
1999; 12(1): 1–14. PubMed Abstract
- 18.
Nissenson A, Prichard SS, Cheng IK, et al.:
Non-medical factors that impact on ESRD modality selection.
Kidney Int Suppl.
1993; 40: s120–127. PubMed Abstract
- 19.
Little J, Irwin A, Marshall T, et al.:
Predicting a patient's choice of dialysis modality: experience in a United Kingdom renal department.
Am J Kidney Dis.
2001; 37(5): 981–986. PubMed Abstract
| Publisher Full Text
- 20.
Wordsworth S, Ludbrook A:
Comparing costing results in across country economic evaluations: the use of technology specific purchasing power parities.
Health Econ.
2005; 14(1): 93–99. PubMed Abstract
| Publisher Full Text
- 21.
Hallinen T, Soini EJ, Martikainen JA, et al.:
Costs and quality of life effects of the first year of renal replacement therapy in one finnish treatment centre.
J Med Econ.
2009; 12(2): 136–140. PubMed Abstract
| Publisher Full Text
- 22.
Fukuhara S, Yamazaki C, Hayashino Y, et al.:
The organization and financing of end-stage renal disease treatment in Japan.
Int J Health Care Finance Econ.
2007; 7(2–3): 217–231. PubMed Abstract
| Publisher Full Text
- 23.
Ofman JJ, Sullivan SD, Neumann PJ, et al.:
Examining the value and quality of health economic analyses: implications of utilizing the QHES.
J Manag Care Pharm.
2003; 9(1): 53–61. PubMed Abstract
- 24.
MacLeod A, Grant A, Donaldson C, et al.:
Effectiveness and efficiency of methods of dialysis therapy for end-stage renal disease: systematic reviews.
Health Technol Assess.
1998; 2(5): 1–166. PubMed Abstract
| Publisher Full Text
- 25.
Burkart J:
The future of peritoneal dialysis in the united states: optimizing its use.
Clin J Am Soc Nephrol.
2009; 4(suppl 1): s125–131. PubMed Abstract
| Publisher Full Text
- 26.
Chaudhary K, Sangha H, Khanna R:
Peritoneal dialysis first: rationale.
Clin J Am Soc Nephrol.
2011; 6(2): 447–456. PubMed Abstract
| Publisher Full Text
- 27.
Jose MD, Johnson DW, Mudge DW, et al.:
Peritoneal dialysis practice in Australia and New Zealand: a call to action.
Nephrology (Carlton).
2011; 16(1): 19–29. PubMed Abstract
| Publisher Full Text
- 28.
Lamas Barreiro J, Alonso Suarez M, Saavedra Alonso JA, et al.:
Costs and added value of haemodialysis and peritoneal dialysis outsourcing agreements.
Nefrologia.
2011; 31(6): 656–663. PubMed Abstract
| Publisher Full Text
- 29.
Chugh KS, Jha V, Chugh S:
Economics of dialysis and renal transplantation in the developing world.
Transplant Proc.
1999; 31(8): 3275–3277. PubMed Abstract
| Publisher Full Text
- 30.
Kontodimopoulos N, Niakas D:
A socio-economic comparison of hemodialysis and peritoneal dialysis in greece.
Int J Healthcare Technology and Management.
2005; 6(3): 296–306. Publisher Full Text
- 31.
Teerawattananon Y, Mugford M, Tangcharoensathien V:
Economic evaluation of palliative management versus peritoneal dialysis and hemodialysis for end-stage renal disease: evidence for coverage decisions in Thailand.
Value Health.
2007; 10(1): 61–72. PubMed Abstract
| Publisher Full Text
- 32.
Neil N, Walker DR, Sesso R, et al.:
Gaining efficiencies: resources and demand for dialysis around the globe.
Value Health.
2009; 12(1): 73–79. PubMed Abstract
| Publisher Full Text
- 33.
Pacheco A, Saffie A, Torres R, et al.:
Cost/Utility study of peritoneal dialysis and hemodialysis in Chile.
Perit Dial Int.
2007; 27(3): 359–363. PubMed Abstract
- 34.
Haller M, Gutjahr G, Kramar R, et al.:
Cost-effectiveness analysis of renal replacement therapy in Austria.
Nephrol Dial Transplant.
2011; 26(9): 2988–2995. PubMed Abstract
| Publisher Full Text
- 35.
Howard K, Salkeld G, White S, et al.:
The cost-effectiveness of increasing kidney transplantation and home-based dialysis.
Nephrology (Carlton).
2009; 14(1): 123–132. PubMed Abstract
| Publisher Full Text
- 36.
Kontodimopoulos N, Niakas D:
An estimate of lifelong costs and QALYs in renal replacement therapy based on patients' life expectancy.
Health Policy.
2008; 86(1): 85–96. PubMed Abstract
| Publisher Full Text
- 37.
Menzin J, Lines LM, Weiner DE, et al.:
A review of the costs and cost effectiveness of interventions in chronic kidney disease: implications for policy.
Pharmacoeconomics.
2011; 29(10): 839–861. PubMed Abstract
| Publisher Full Text
- 38.
Benain JP, Faller B, Briat C, et al.:
[Cost of dialysis in France].
Nephrol Ther.
2007; 3(3): 96–106. PubMed Abstract
| Publisher Full Text
- 39.
Durand-zaleski I, Combe C, Lang P:
International Study of Health Care Organization and Financing for end-stage renal disease in France.
Int J Health Care Finance Econ.
2007; 7(2–3): 171–183. PubMed Abstract
| Publisher Full Text
- 40.
Pontoriero G, Pozzoni P, Vecchio LD, et al.:
International study of health care organization and financing for renal replacement therapy in Italy: an evolving reality.
Int J Health Care Finance Econ.
2007; 7(2–3): 201–215. PubMed Abstract
| Publisher Full Text
- 41.
Ashton T, Marshall MR:
The organization and financing of dialysis and kidney transplantation services in New Zealand.
Int J Health Care Finance Econ.
2007; 7(4): 233–252. PubMed Abstract
| Publisher Full Text
- 42.
Villa G, Rodriguez-carmona A, Fernandez-ortiz l, et al.:
Cost analysis of the Spanish renal replacement therapy programme.
Nephrol Dial Transplant.
2011; 26(11): 3709–3714. PubMed Abstract
| Publisher Full Text
- 43.
Sandoz MS, Ess SM, Keusch GW, et al.:
Prevalence and direct medical costs of end-stage renal disease in patients with type 2 diabetes mellitus in Switzerland for 2001.
Swiss Med Wkly.
2004; 134(31–32): 448–458. PubMed Abstract
- 44.
Baboolal K, McEwan P, Sondhi S, et al.:
The cost of renal dialysis in a UK setting--a multicentre study.
Nephrol Dial Transplant.
2008; 23(6): 1982–1989. PubMed Abstract
| Publisher Full Text
- 45.
Berger A, Edelsberg J, Lnglese GW, et al.:
Cost comparison of peritoneal dialysis versus hemodialysis in end-stage renal disease.
Am J Manag Care.
2009; 15(8): 509–518. PubMed Abstract
- 46.
Neil N, Guest S, Wong L, et al.:
The financial implications for Medicare of greater use of peritoneal dialysis.
Clin Ther.
2009; 31(4): 880–888. PubMed Abstract
| Publisher Full Text
- 47.
Shih YC, Guo A, Just PM, et al.:
Impact of initial dialysis modality and modality switches on Medicare expenditures of end-stage renal disease patients.
Kidney Int.
2005; 68(1): 319–329. PubMed Abstract
| Publisher Full Text
- 48.
Jeloka TK, Upase S, Chitikeshi S, et al.:
Monthly cost of three exchanges a day peritoneal dialysis is same as of thrice a week hemodialysis in self-paying Indian patients.
Indian J Nephrol.
2012; 22(1): 39–41. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 49.
Erek E, Sever MS, Akoglu E, et al.:
Cost of renal replacement therapy in Turkey.
Nephrology (Carlton).
2004; 9(1): 33–38. PubMed Abstract
| Publisher Full Text
- 50.
Salonen T, Reina T, Oksa H, et al.:
Alternative strategies to evaluate the cost-effectiveness of peritoneal dialysis and hemodialysis.
Int Urol Nephrol.
2007; 39(1): 289–298. PubMed Abstract
| Publisher Full Text
- 51.
Hooi IS, Lim TO, Goh A, et al.:
Economic evaluation of centre haemodialysis and continuous ambulatory peritoneal dialysis in Ministry of Health hospitals, Malaysia.
Nephrology (Carlton).
2005; 10(1): 25–32. PubMed Abstract
| Publisher Full Text


Comments on this article Comments (0)