Keywords
Solitary Fibrous Tumor, Haemangiopericytoma, pterygomaxillary and infratemporal fossa, neoplasms
Solitary Fibrous Tumor, Haemangiopericytoma, pterygomaxillary and infratemporal fossa, neoplasms
Solitary fibrous tumors (SFTs) were first described by Klempere and Rabin7 in 1931 as spindle-cell tumors originating from the pleura. Until recently, there was some debate with regards to the name and origin of these tumors11. The term haemangiopericytomas (HPCs) was used by Stout and Murray10 in 1942 when they described a type of vascular tumor derived from the capillary pericytes, previously described by Zimmermann in 1923.
However, over the years, the use of the term HPC has raised many issues. Morphological, immunohistochemical and clinical features of HPCs are not specific for one entity3,8. With the exception of myopericytoma, infantile myofibromatosis and HPC-like lesions of the sinonasal tract showing myoid differentiation, all other HPC-like lesions are best considered as subtypes of SFT3. Due to their mesenchymal origin5, we now are aware that SFTs may involve several extrapleural sites including soft tissues or meninges5,8.
Only a few cases of SFT have been described in the literature involving the skull base9,14, but a massive malignant SFT of the soft tissue involving the infratemporal and the pterygomaxillary fossa is extremely rare and represents a multidisciplinary clinical and surgical challenge. The vast extent of the tumor and its localisation near vital structures makes it an important challenge for the surgical team, especially when the tumor shows malignant histological characteristics. Classical transfacial surgical approaches may leave unsightly scars with high morbidity. However, a combined approach with nasoendoscopy provides excellent access for tumor resection without any concerns over the aesthetic result.
To our knowledge, this is the first case reported of a massive SFT involving this region, treated with minimal invasive surgery without any facial osteotomies.
Written informed consent for publication of clinical details and clinical images was obtained from the patient.
Early December 2010, a 54-year-old caucasian man was referred to our otolaryngologist head and neck surgery department. He presented no relevant personal or family history. He had noticed progressive loss of vision and facial pain over the last year. The ophthalmologist, who saw him for decreased vision in the right eye, found a mass on the axial CT that was causing his compressive optic neuropathy.
The clinical examination revealed subtle proptosis of the right eye. Using flexible nasolaryngoscopy, we saw small bulging in the nasal cavity on the right side. All cranial nerves were intact and no lymph node enlargement was found. The patient was not complaining of epistaxis or nasal obstruction.
The first computer tomography scan with intravenous iodine contrast, requested by the ophthalmologist, indicated a lesion in the right pterygomaxillary fossa eroding the base of the skull to the temporal lobe and to the right cavernous sinus. There was also an extension in the orbital apex and erosion of the pterygoid process with bulging of the posterior wall of the right nostril. The mass was visible with contrast gadolinium enhancement on the MRI. Its dimensions were 4.6cm anterioposterior × 3.3cm transverse × 4.7cm cranio-caudal (Figure 1–Figure 3).
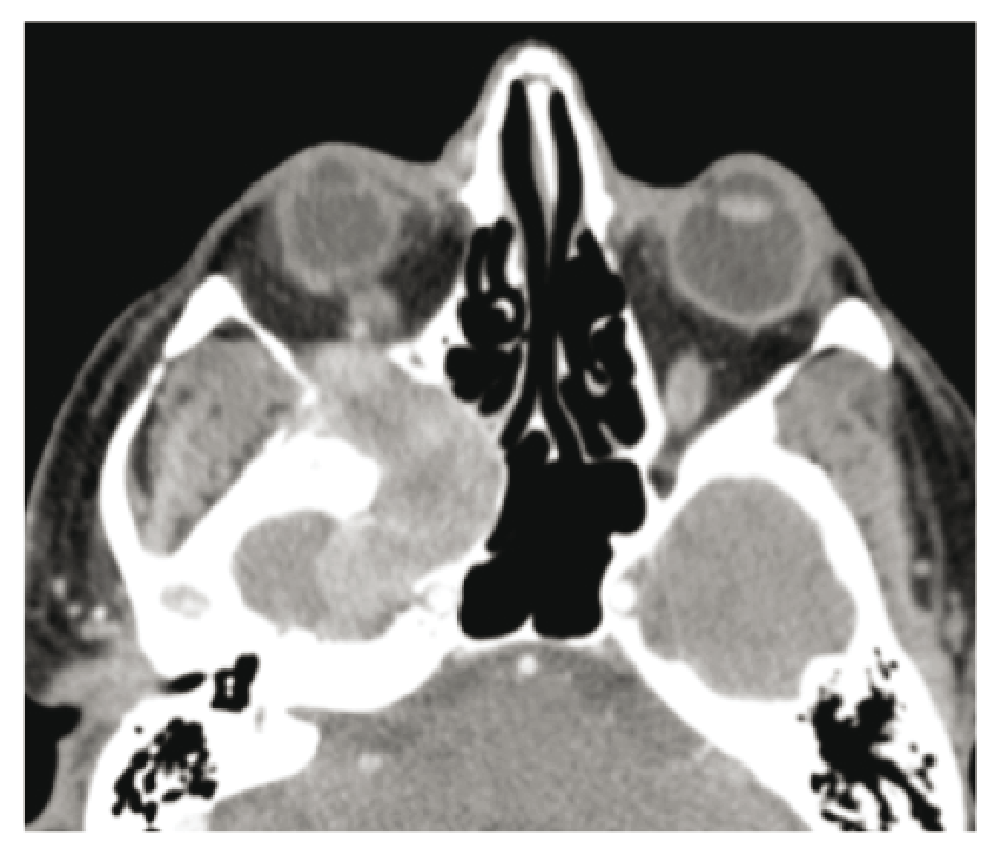
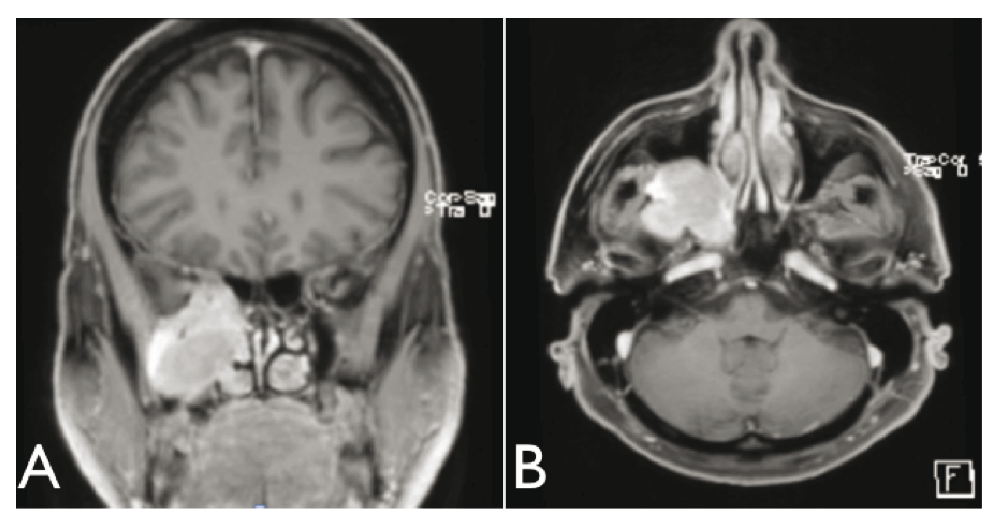
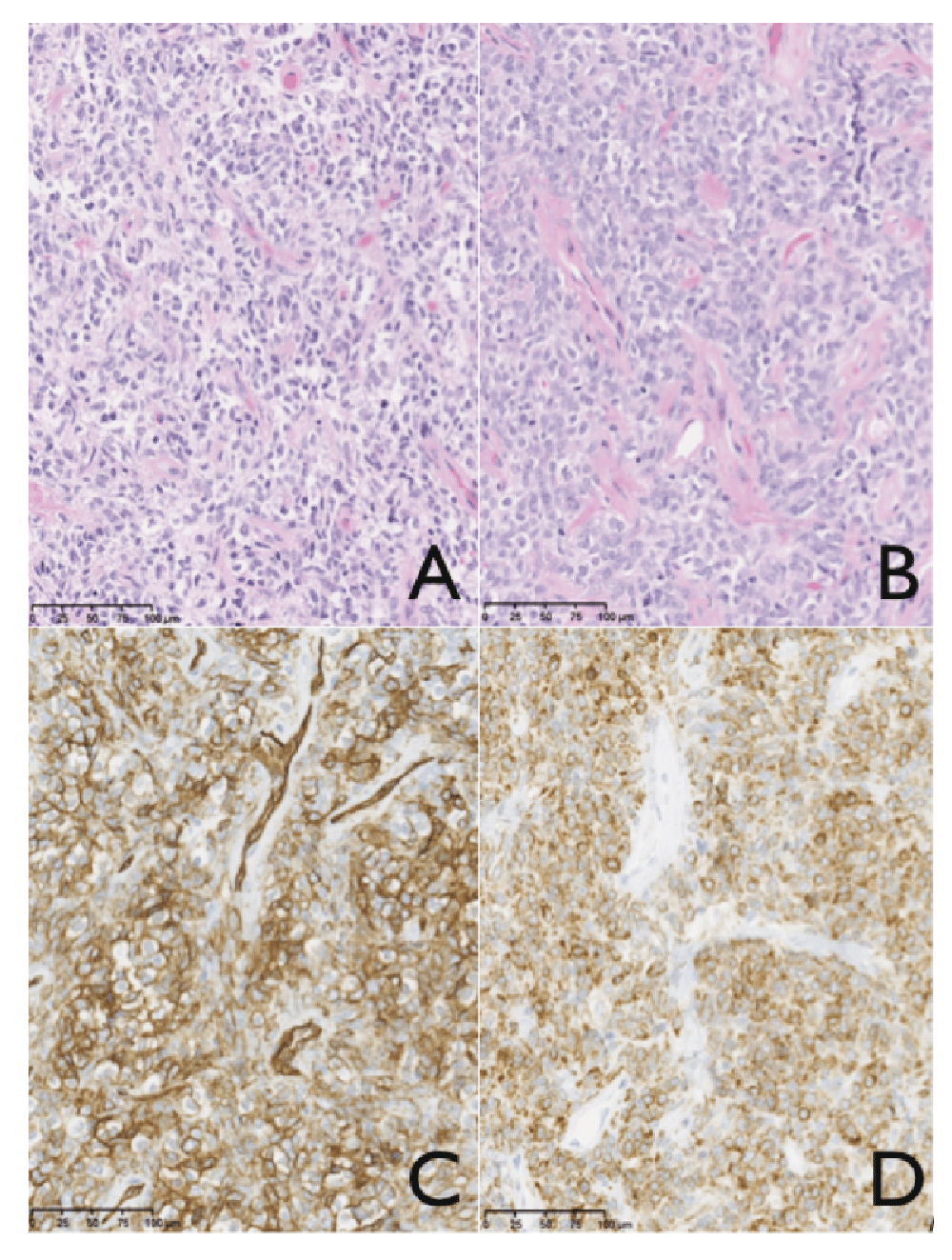
Shortly after the MRI, a transmaxillary endoscopic biopsy was performed. The result of the frozen tissue examination was compatible with a diagnosis of neuroendocrine carcinoma or hemangiopericytoma. The final pathology indicated a solitary fibrous tumor and this was confirmed by an expert otolaryngologist pathologist in Toronto. There was hypercellularity and a mitotic index of 4 mitoses per field, a sign of a highly aggressive tumor8 (Figure 4).
The case was discussed at the multidisciplinary oncology clinic of our otolaryngology department. With neurosurgical and radio-oncology advice, it was decided to schedule surgery. A preoperative angiography was made with embolization of the tumoral branches by the right internal maxillary artery. It was made the day before surgery to minimize the risks of severe intraoperative bleeding.
The surgery began with the removal of the inferior and superior right turbinate. After that, we removed the median portion of the maxillary sinus. The tumor was bulging at this location and increased at the level of the sphenoid sinus.
To attain greater exposure, a Caldwell-Luc incision was made with minimal osteotomy of the anterior maxillary sinus. Using endoscopy, we witnessed the fibrous tumor covering the complete posterior wall of the maxillary sinus.
Laterally, the resection was made until we saw the fatty tissue of the infratemporal fossa. The vidian nerve had to be removed.
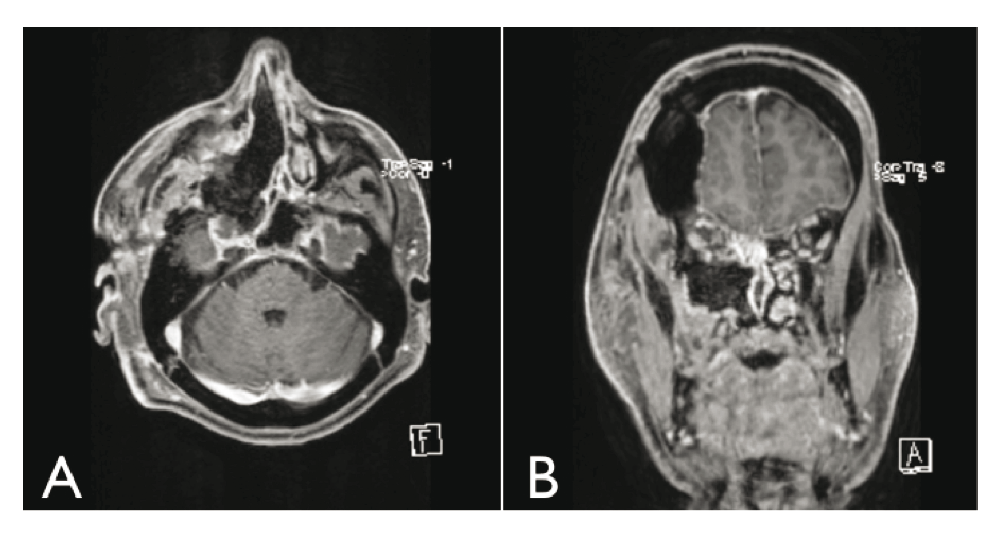
We excised the tumor with the capsule as much as possible. We had to terminate the surgery with a subtotal resection (STR), because it was noted that some nodularity persisted on the dura mater on the lateral-superior side of the tumor (Figure 3a,b). It was left there, because the sealing at this location would make it very difficult to reach and remove. In addition, it was considered a benign tumor with aggressive potential. As a result, radiotherapy was determined to be necessary, in any case.
The neurosurgeon performed frontotemporal craniotomy with right infratemporal fossa exploration. The debulking was made using ultrasonic suction-irrigation (Cavitron).
One month later, the patient started external beam radiation (60 Gy by 30 fractions) of the paranasal sinus with infra temporal fossa and pterygomaxillary fossa.
On monthly follow-up, it appeared that the scars were healing well. There were no problems with the sinuses and no further complaints of facial pain. However, there is no improvement of the visual acuity in the patient’s right eye. Subsequent MRI follow-up showed a continual small asymptomatic residual tumor, but no signs of recurrence more than one year later.
SFTs are rare spindle-cell neoplasms of variable histological grades. Despite some confusion in the past regarding HPC and SFT, the relationship between the two is now more obvious. It is also known that SFT of the soft tissue can occur in the head and neck region and throughout the body4,6,12.
Most cases of soft tissue SFTs occur in the early fifth decade of life8 with no sex predilection2,3. Its occurrence is less than 2% of all soft tissue tumors8.
Skull base SFTs may include a wide variety of symptoms, although they are usually asymptomatic on presentation13. The symptoms manifest most frequently as a slowly expanding painless mass. Decreased vision, nasal obstruction, local pain, recurrent sinusitis, epistaxis, headaches, dural erosion, cerebrospinal fluid rhinorrhea, anosmia and lower cranial nerve palsies may all be part of the patient symptomatology.
SFTs from any site are usually benign and surgical resection alone is curative4,11. However, malignancy is possible, although the criteria remain imprecise. It may be suspected with the radiology exam (mass >10cm) or by the presence of metastasis13. The presence of infiltrative margins with surrounding tissues, high mitotic count (≥4 mitoses per 10 high-power high fields) of cellular pleomorphism and tumor necrosis also suggests malignancy4,8.
The main treatment is surgical for benign and malignant SFTs. There is little evidence (principally from case reports) from adjuvant radiotherapy and none supporting the use of chemotherapy. But when histopathology suggests malignancy or when there are positive surgical resection margins, radiotherapy must be discussed, as for other sarcomas1,8.
In conclusion, we successfully managed a case of massive solitary fibrous tumor of the soft tissue, involving the infratemporal and pterygomaxillary fossa. With the combination of conventional frontotemporal craniotomy with sinus surgery endoscopy, we were capable of removing most of the tumor while preserving vital structures close to the tumor and without the need for facial osteotomies, leaving the patient without any undesirable scars. With the addition of adjuvant radiotherapy treatment, we were able to achieve no sign of recurrence at the one-year follow-up.
SFT is very uncommon but should be part of the differential diagnosis in patients with a skull base lesion. Diagnosis is difficult but pathologists should be aware of the classical finding of this disease consisting of spindled cells in a disorganized pattern, with alternating hypocellular and hypercellular areas separated by hyalinized collagen and branching HPC-like vessels. Also the immunophenotyping staining positive for the presence of CD34 and Bcl-2 can be useful. The surgical approach needs to weigh risks and benefits of subtotal vs. total resection because of the surrounding vital structures.
François Cloutier: first author, acquisition of data, study design, participated in drafting the article and revising it critically for important intellectual content.
Gave final approval of the version to be submitted.
Geneviève Lapointe: Second author, substantial contribution to conception and design of this study.
participated in drafting the article and revising it critically for important intellectual content, gave final approval of the version to be submitted and any revised version.
Sylvie Nadeau: Third author, substantial contribution to conception and design, participated in drafting the article and revising it critically for important intellectual content, and gave final approval of the version to be submitted and any revised version.
We would like to thank the entire otolaryngology and neurosurgery department from the Hôpital de l’Enfant-Jesus.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 17 Dec 13 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)