Introduction
Research over the past 15 years has demonstrated the importance of protein arginine methylation in various biological processes including transcriptional regulation, DNA repair, RNA processing, and signal transduction1–5. The fact that hundreds of cellular proteins have been identified as the targets of protein arginine methylation supports the idea that arginine methylation regulates diverse cellular processes6. Arginine methylation is catalyzed by protein arginine methyltransferases (Prmts) that transfer a methyl group from S-adenosyl methionine (AdeMet) to protein substrates. To date, nine protein arginine methyltransferases have been identified in the mammalian genome and have been classified as type I, type II and type III enzymes by the activity of producing ω-NG,NG-asymmetric dimethylarginine (ADMA), ω-NG,NG-symmetric dimethylarginine (SDMA), and ω-NG-monomethylarginine (MMA), respectively2,4,7.
Among the family of Prmts, Prmt7 is unique in that it possesses two AdoMet-binding domains, which may have resulted from a gene duplication event8. As for many of the Prmts, histones are among the identified substrate molecules, suggesting a functional role for Prmt7 in regulating gene expression as a chromatin modifier. Initial work indicated that H2A and H4 were symmetrically dimethylated by Prmt7 in vitro9, whereas several studies have reported that Prmt7 only produces MMA on histones and other substrates8,10,11. A recent study indicated that Prmt7 symmetrically dimethylates H4R3 and H2AR3 in a manner that contributes to the repression of expression of genes involved in DNA repair12. Another study revealed that Prmt7-mediated H4R3 symmetric dimethylation antagonizes MLL4-catalyzed H3K4 methylation on neuron-specific gene promoters during differentiation13, suggesting that Prmt7 might negatively regulate tissue differentiation by its chromatin-modifying activity. Aside from the role in neuronal differentiation, the regulatory function of Prmt7 in the differentiation of other lineages has not been reported.
Adipocyte differentiation is one of the most intensively studied differentiation processes. Both human and mouse mesenchymal stem cells (MSCs) that reside in fat pads and bone marrow undergo lineage commitment and terminal differentiation to become mature adipocytes14–16. The adipogenic differentiation process is controlled by a number of tissue-specific transcription factors, such as the CCAAT/enhancer binding proteins (C/EBPs), peroxisome proliferator-activated receptor gamma (PPARγ), and numerous chromatin remodeling and modifying enzymes including the ATP-dependent SWI/SNF complex and Prmts17–20. It has been shown that Prmt5 interacts with Brg1-based SWI/SNF complex21 and facilitates the binding of Brg1 to the PPARγ and to PPARγ target promoters to facilitate the activation of adipogenic genes19. Furthermore, Prmt4, which also has been shown to interact with Brg1-based SWI/SNF complex22, acts as a co-activator of PPARγ to promote adipocyte gene expression20. Prmt7 had been recently shown to interact with Brg1-based SWI/SNF complex12, but whether Prmt7 has functional roles in adipocyte differentiation remains unclear.
In the present study, we examined the role of Prmt7 in adipocyte differentiation in mouse C3H10T1/2 embryonic mesenchymal cells and in C/EBPα-reprogrammed murine NIH3T3 fibroblasts. By knocking down and over-expressing Prmt7, we showed that Prmt7 has no effect on lipid accumulation and adipogenic gene expression in differentiating cells. Based on the results, we concluded that Prmt7 is not required for differentiation in tissue culture models of adipogenesis.
Methods
Cell culture
Mouse C3H10T1/2 and NIH3T3 cells were obtained from the ATCC. C3H10T1/2 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) high glucose (Invitrogen) supplemented with 10% fetal calf serum (FCS) (Sigma) and 100U/ml of penicillin/streptomycin (Invitrogen). NIH3T3 cells were maintained in DMEM high glucose with 10% calf serum (Sigma). 293T and BOSC23 cells were obtained from S.N. Jones (UMass Medical School) and R.E. Kingston (Massachusetts General Hospital), respectively, and were grown in the same medium as C3H10T1/2 cells. For adipogenic differentiation, two-day postconfluent cells were differentiated with DMEM medium containing 10% FCS, 10μg/ml insulin, 0.5mM 3-isobutyl-1-methyxanthine, 1μM dexamethasone, and 10μM troglitazone (Sigma). After 48 hours incubation, media on the differentiating cells was replaced with media containing 5μg/ml insulin. Subsequently, the media was changed every other day until harvest. To evaluate cell proliferation, 1×105 cells were seeded in 6-well plates (Corning Inc.), and the number of viable cells was counted under a microscope (CK2, Olympus) each day from day 1 to day 4 with a hemocytometer (Hausser Scientific).
Plasmid DNA constructs
pENTR/pTER+ vector and pLentiX2 Dest vector were gifts from Dr. Eric Campeau (UMass Medical School). The preparation of lentiviral small hairpin RNA (shRNA) constructs was done as previously described12,23. Briefly, shPrmt7-1, shPrmt7-2 and scrambled control oligonucleotides were cloned into a pENTR/pTER+ vector. These constructs were individually incubated with the pLentiX2 DEST vector and LR clonase II enzyme mix (Invitrogen) to generate pLentiX2 DEST/ shPrmt7-1, pLentiX2 DEST/shPrmt7-2 and pLentiX2 DEST/shCtrl constructs. These lentiviral constructs were amplified in Stbl3 competent cells (Invitrogen) for generating lentiviruses. The pBABE puromycin empty vector (pBABE vector), FLAG-tagged PRMT7 construct (pBABE PRMT7) and pBABE CEBPα construct were previously described12,24 and were individually amplified in TOP10 competent cells (Invitrogen) for generating retroviral DNA as previously described12,24.
Virus production and infection
The preparation of viruses was performed as previously described23,25. Briefly, for lentiviruses, the packaging vectors pLP1, pLP2, pVSVG (Invitrogen) and pLentiX2 DEST/shRNA constructs were co-transfected into 293T cells with Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s instructions. BOSC23 cells were used for pBABE-based retrovirus production. After 48 hours incubation, the supernatant was harvested and filtered through 0.45μm syringe filter (Millipore). For viral infection, 1ml of the filtered supernatant and 4μg/ml of polybrene (Sigma) were used to infect one million cells. After 48 hours incubation, virus infected cells were selected in 2.5μg/ml puromycin (Invitrogen).
Protein expression analysis
Cells were washed twice with cold PBS and were harvested in RIPA buffer (50mM Tris-HCl pH7.4, 150mM NaCl, 1mM EDTA, 1% Nonidet P-40 (Thermo Scientific) and 0.25% sodium deoxycholate) supplemented with protease inhibitor cocktail (Roche). The samples were sonicated at high intensity setting for 3 minutes with 30sec on/off cycle in a Bioruptor (UCD-200, Diagenode). After quantifying the protein concentration by means of a Bio-Rad protein assay, the protein samples were then mixed with 4× SDS loading buffer (240mM Tris-HCl pH6.8, 8% SDS, 40% glycerol, 0.01% bromophenol blue and 10% β-mercaptoethanol) and boiled at 95°C for 10min. 30μg protein samples were separated on 10% SDS-PAGE and transferred onto PVDF membrane (Bio-Rad). The blots were blocked overnight in 3% non-fat milk (Essential Everyday). The next day, proteins were detected using specific antibodies (1:1000 dilution) and HRP-conjugated secondary antibodies (1:2000 dilution). The rabbit polyclonal antibodies against human PRMT7 (sc-98882) and rat C/EBPα (sc-61) were purchased from Santa Cruz Biotechnology. The mouse monoclonal antibody against mouse PPARγ (sc-7273) and the goat polyclonal antibody against human PRMT5 (sc-22132) were also purchased from Santa Cruz Biotechnology. Rabbit polyclonal anti-PI3K (ABS233) antibody was from Millipore. The secondary antibodies (NA9340 and NA9310) were purchased from GE Healthcare Life Sciences. The blots were developed on X-ray films with ECL Western Blotting Detection Reagents (GE Healthcare Life Sciences). The signal intensity was quantified by ImageJ.
Gene expression analysis
Total RNA was isolated from samples using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. cDNA was prepared from 1μg of total RNA by Superscript III reverse transcriptase kit (Invitrogen). Quantitative PCR was performed on StepOne Plus real-time PCR machine with Fast SYBR Green Master mix (Applied Biosystems). The specific primers for gene expression analysis were:
Fasn forward 5′-CGTGTTGGCCTACACCCAGAGCT-3′;
Fasn reverse 5′-GGCAGCAGGGCCTCCAGCACCTT-3′;
AdipoQ forward 5′-CAGTGGATCTGACGACACCA-3′;
AdipoQ reverse 5′-CGAATGGGTACATTGGGAAC-3′;
Fabp4 forward 5′-GCGTGGAATTCGATGAAATCA-3′;
Fabp4 reverse 5′-CCCGCCATCTAGGGTTATGA-3′;
5S rRNA forward 5′-GTCTACGGACATACCACCCTG-3′;
5S rRNA reverse 5′-TACAGCACCCGGTATTCCCAG-3′.
Relative expression levels were determined by the comparative Ct method26.
Oil Red O staining
Differentiating cells were washed once with PBS and fixed in 10% phosphate-buffered formalin (Fisher Scientific) overnight. The next day, the fixed cells were washed with 60% isopropanol and air-dried completely. The cells were then stained with 60% Oil Red O (AMRESCO) for 10 minutes and washed repeatedly with tap water to remove excess stain. To quantify staining, Oil Red O was extracted from the cells with 100% isopropanol, and the optical density was measured at 500nm (OD500).
Results
The protein levels of Prmt7 remain constant during adipogenic differentiation of C3H10T1/2 cells
The C3H10T1/2 cell line was established from C3H mouse embryos and has served as a faithful cell culture model for mesenchymal lineage differentiation27–29. C3H10T1/2 cells can be differentiated into mature adipocytes by treating the confluent cells with a cocktail that contains insulin, dexamethasone, 3-isobutyl-1-methyxanthine (IBMX) and PPARγ ligands30. Using this model, we first examined Prmt7 protein levels during adipogenic differentiation by Western blot analysis. We found that Prmt7 protein levels are relatively constant from the onset of differentiation (day 0) through the day 6 post-differentiation (Figure 1A and Figure 1B) (Dataset 1). We concluded that Prmt7 protein levels were not altered in differentiating C3H10T1/2 cells.
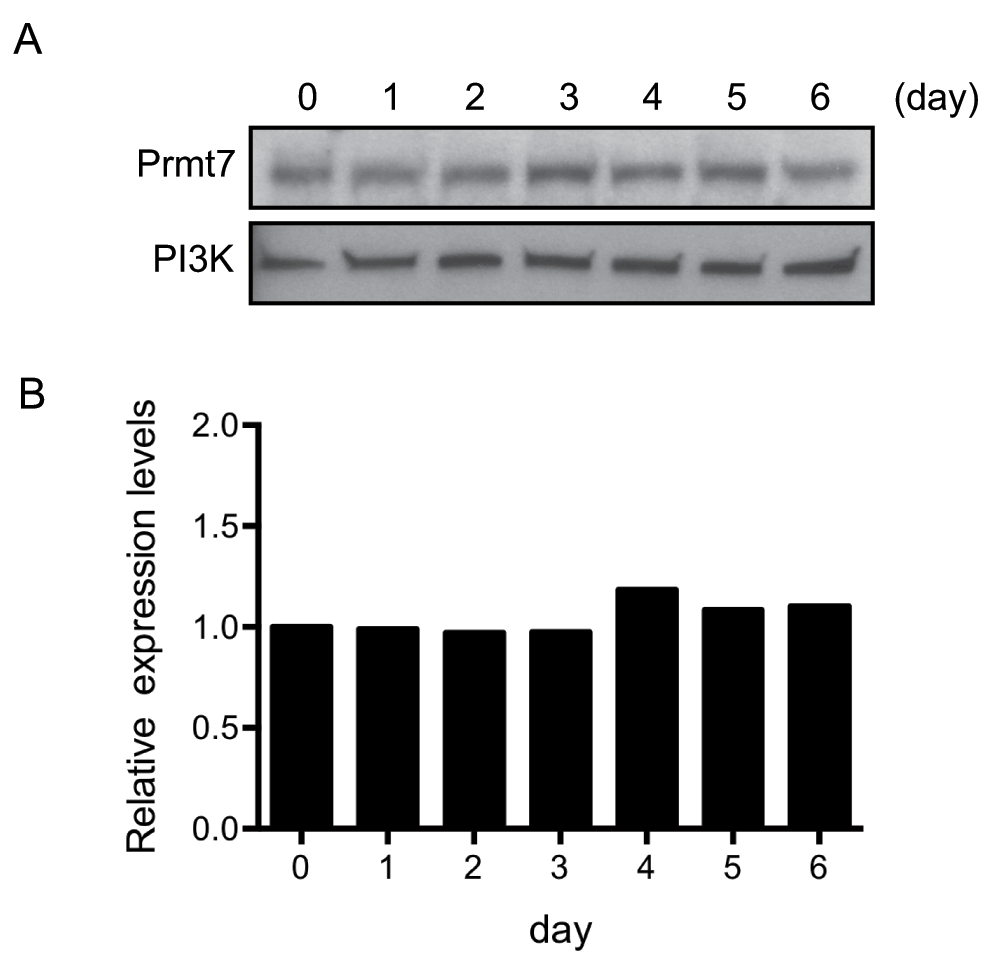
Figure 1. Prmt7 protein levels are constant in differentiating C3H10T1/2 cells.
(A) Western blot analysis of the protein extracts from day 0 to day 6 post-differentiated C3H10T1/2 cells. Duplicate blots were probed with anti-Prmt7 antibody and with anti-PI3K antibody as a loading control. (B) The quantification of (A) by ImageJ. The levels of Prmt7 were normalized to PI3K loading control and presented as the relative expression levels to the day 0 sample (day 0=1). The data represent the average of two independent experiments (n=2).
Virus-mediated knockdown and over-expression of Prmt7 in C3H10T1/2 cells
To study the function of Prmt7, we used viral vectors to knock down or over-express Prmt7 in C3H10T1/2 cells. Two lentiviral constructs (pLentiX2 DEST/shPrmt7-1 and pLentiX2 DEST/shPrmt7-2) that encode shRNAs against Prmt7 mRNA were used for knocking down endogenous Prmt7 in proliferating C3H10T1/2 cells. A pBABE retroviral construct (pBABE-PRMT7) encoding FLAG-tagged PRMT7 was used to over-express PRMT7. The virus-infected cells were selected with puromycin and the levels of Prmt7 in the selected cells were examined by Western blot analysis (Figure 2A). Endogenous Prmt7 levels were reduced 10-fold or more in the knockdown cells compared to the scrambled shRNA control cells (Figure 2B). Prmt7 levels were increased more than 5-fold in the FLAG-tagged PRMT7 over-expression cells compared to the pBABE empty vector control (Figure 2B) (Dataset 2). Since Prmt5 is the major type II arginine methyltransferase and is also associated with SWI/SNF complexes21, we examined Prmt5 protein levels in our samples. We observed no changes in Prmt5 levels in Prmt7 knockdown and over-expression C3H10T1/2 cells (Figure 2A).
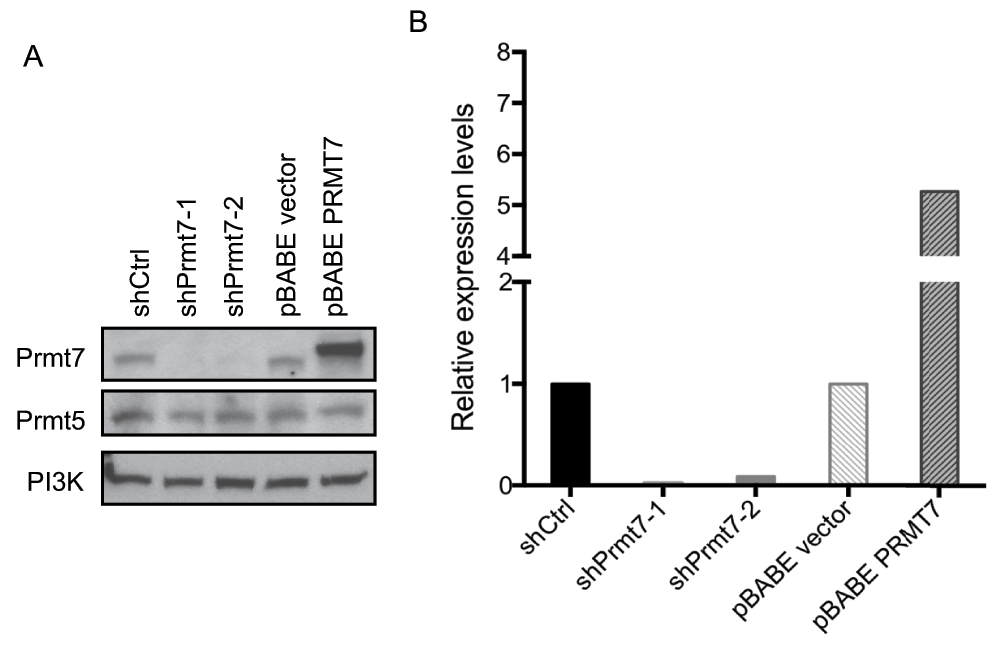
Figure 2. Prmt7 was specifically knocked down or over-expressed in C3H10T1/2 cells.
(A) A representative Western blot analysis from proliferating C3H10T1/2 cells with Prmt7 knockdown and over-expression. Endogenous Prmt7 was specifically depleted by two different lentiviral shRNA constructs (shPrmt7-1 and shPrmt7-2). The scrambled shRNA lentiviral construct (shCtrl) was used as a control. The pBABE retroviral construct encoding FLAG-tagged PRMT7 (pBABE - PRMT7;12) was used to ectopically express PRMT7, and the pBABE empty vector (pBABE vector) was used as a control. Duplicate blots were probed with anti-Prmt7 and anti-Prmt5 antibodies and with anti-PI3K antibody as a loading control. (B) The quantification of Prmt7 in (A) by ImageJ. The levels of Prmt7 were normalized to the PI3K loading control and are presented as expression levels relative to the scrambled shRNA control or pBABE empty vector control. The data represent the average of two independent experiments (n=2).
Prmt7 has no effect on cell proliferation of C3H10T1/2 cells
Both Prmt7 and Prmt5 exhibit type II arginine methyltransferase activity. However, unlike Prmt5, Prmt7 has no effect on cell proliferation in NIH3T3 fibroblasts12. We measured the cell proliferation rate of Prmt7 knockdown and over-expression C3H10T1/2 cells, and found that neither the reduction of Prmt7 nor the over-expression of Prmt7 affected the proliferation of C3H10T1/2 cells (Figure 3) (Dataset 3). This result is consistent with the results from the previous study on NIH3T3 fibroblasts12.
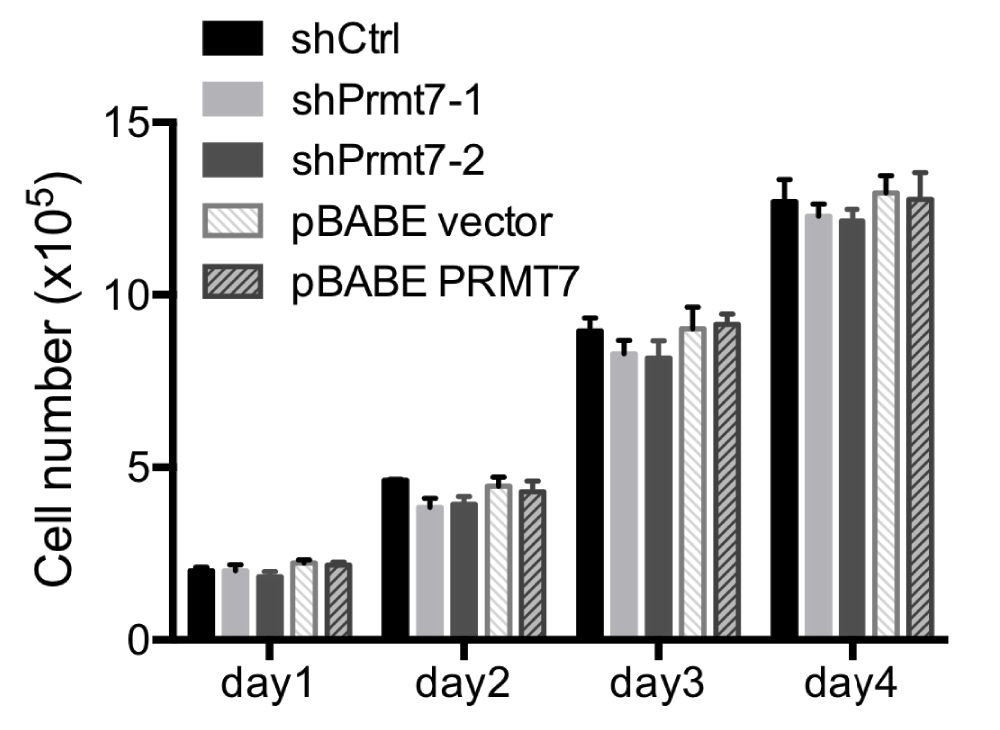
Figure 3. Knockdown or over-expression of Prmt7 does not affect C3H10T1/2 cell proliferation.
The growth rates of control (shCtrl and pBABE vector), Prmt7 knockdown (shPrmt7-1 and shPrmt7-2) and Prmt7 over-expression (pBABE PRMT7) C3H10T1/2 cells were measured by seeding 1×105 cells in 6-well plates, and the viable cells were counted each day for 4 days after seeding. The data represent the average of two independent experiments (n=2) counted in duplicate. Error bars show the standard deviation.
Prmt7 is not required for adipogenic differentiation of C3H10T1/2 cells
To determine whether Prmt7 affects adipogenesis, the Prmt7 knockdown and over-expression C3H10T1/2 cells were grown to confluence and treated with the differentiation cocktail. At day 6 post-differentiation, the accumulation of intracellular neutral lipids was measured by Oil Red O staining. The Oil Red O staining showed similar levels of lipid accumulation in Prmt7 knockdown as well as Prmt7 over-expression cells as compared to the control cells. (Figure 4A and Figure 4B) (Dataset 4). This result suggests that the changes in Prmt7 levels did not affect lipid accumulation in differentiating C3H10T1/2 cells.
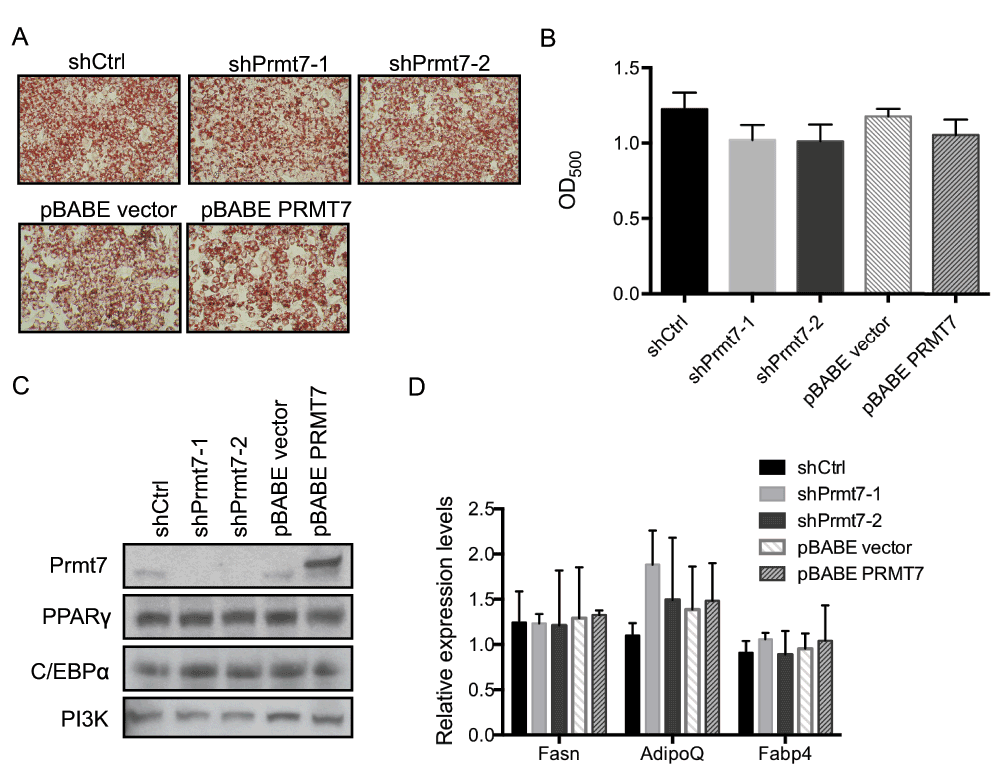
Figure 4. Knockdown or over-expression of Prmt7 has no effect on C3H10T1/2 adipogenic differentiation.
(A) Representative Oil-Red O staining images of day 6 post-differentiated C3H10T1/2 cells in which Prmt7 was either knocked down (shPrmt7-1 and shPrmt7-2) or over-expressed (pBABE PRMT7). The Prmt7 knockdown and over-expression C3H10T1/2 cells were grown to confluence and differentiated 48 h later. At day 6 post-differentiation, the cells were fixed with 10% formalin and stained with Oil-Red O. (B) The quantification of (A). The Oil-Red O stain was extracted with 100% isopropanol and the optical density at 500nm (OD500) was determined. The data represent the average of two independent experiments (n=2) assayed in duplicate. Error bars show standard deviation. (C) A representative Western blot analysis of endogenous Prmt7, PPARγ and C/EBPα expression in day 6 post-differentiated C3H10T1/2 cells. The levels of PI3K are presented as a loading control. (D) Gene expression analysis on day 6 post-differentiated C3H10T1/2 cells. The mRNA levels of fatty acid synthase (Fasn), adiponectin (AdipoQ) and fatty acid binding protein 4 (Fabp4) were measured by RT-qPCR. The individual mRNA levels were normalized to 5S rRNA. The normalized expression levels of the control cells in one of the experiments were set as 1. The data are presented as the average of relative expression levels from two independent experiments (n=2) assayed in duplicate. Error bars show standard deviation.
Adipogenic gene and protein expression in C3H10T1/2 cells was not affected by Prmt7 knockdown or over-expression
PPARγ and C/EBPα are the key transcription factors for adipogenic differentiation and for the maintenance of the adipocyte phenotype31–34. We examined the protein levels of PPARγ and C/EBPα in day 6 post-differentiation cells by Western blot analysis and found no significant difference in either Prmt7 knockdown or Prmt7 over-expression cells compared to the corresponding controls (Figure 4C). In addition, to rule out the possibility that Prmt7 functions as a cofactor of PPARγ and C/EBPα, we measured the mRNA expression levels of PPARγ and C/EBPα target genes in day 6 post-differentiation samples by real-time quantitative PCR (Figure 4D) (Dataset 5). We found that Prmt7 had no significant impact on fatty acid synthase (Fasn), adiponectin (AdipoQ), and fatty acid binding protein 4 (Fabp4) gene expression in the differentiating cells. These results suggest that Prmt7 is dispensable for adipogenic gene expression.
Prmt7 has no effect on C/EBPα-reprogrammed NIH3T3 fibroblasts
Previous studies had shown that ectopic expression of PPARγ or C/EBPα alone in non-adipogenic NIH3T3 cell line is able to reprogram NIH3T3 fibroblasts into adipocyte-like cells33,34. To test if Prmt7 is required for the reprogramming of NIH3T3 fibroblasts, we first knocked down and over-expressed Prmt7 in NIH3T3 fibroblasts by using the same viral constructs that we used in C3H10T1/2 cells, and confirmed the knockdown and over-expression of Prmt7 by Western blot analysis (Figure 5A). These cells were further infected with retroviruses encoding C/EBPα at 70% confluence. After the cells reached confluence, the differentiation cocktail was added to stimulate adipogenic differentiation. At day 6 post-differentiation, the accumulated lipid was evaluated by Oil Red O staining (Figure 5B). We found that neither knockdown nor over-expression of Prmt7 caused a significant difference in Oil Red O staining in C/EBPα-reprogrammed NIH3T3 fibroblasts, which is consistent with the results in C3H10T1/2 cells.
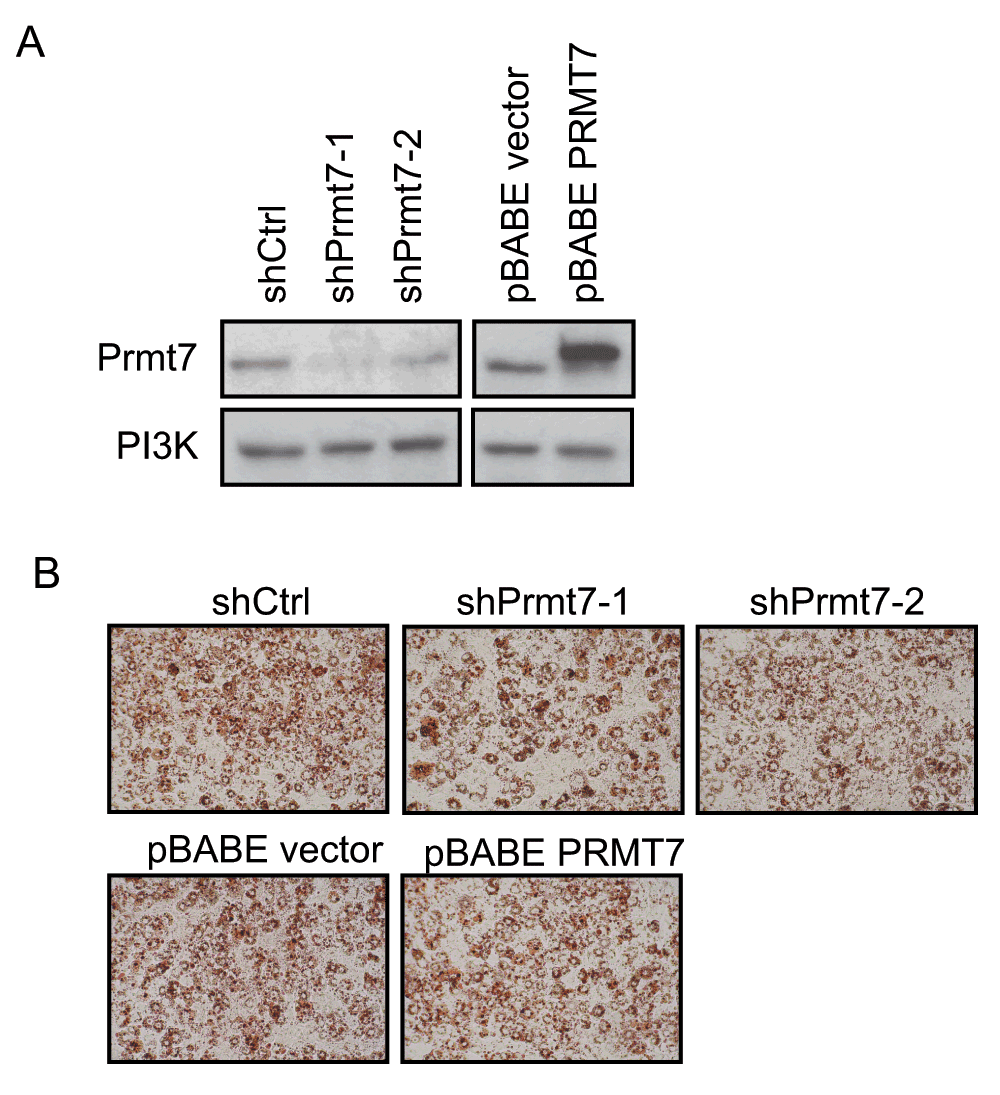
Figure 5. Knockdown or over-expression of Prmt7 has no effect on C/EBPα-mediated reprogramming of NIH3T3 fibroblasts.
(A) Western blot analysis on Prmt7 knockdown and over-expression NIH3T3 fibroblasts. Endogenous Prmt7 was specifically depleted by the lentiviral shRNA constructs (shPrmt7-1 and shPrmt7-2). The scrambled shRNA lentiviral construct (shCtrl) was used as a control. The pBABE retroviral construct encoding FLAG-tagged PRMT7 (pBABE PRMT7) was used to ectopically express PRMT7 and the pBABE empty vector was used as a control. The blot was probed with anti-Prmt7 antibody, and the PI3K levels are presented as a loading control. (B) Oil-Red O staining images of C/EBPα-reprogrammed NIH3T3 fibroblasts at day 6 post-differentiation. Prmt7 knockdown and over-expression NIH3T3 fibroblasts were infected with retroviruses encoding C/EBPα at 70% confluence. Two day post-confluent cells were differentiated. At day 6 post-differentiation, the cells were fixed with 10% formalin and stained with Oil-Red O.
Discussion
Changes in gene expression during cell differentiation require alterations in higher-order chromatin organization as well as in local chromatin structure. Cells possess histone modifying enzymes and ATP-dependent chromatin remodeling enzymes to facilitate chromatin changes. The interplay between these two families of enzymes has been shown to be crucial for both transcription activation and repression18,21,35. Prmt7 was identified as a histone arginine methylating enzyme9–11 and was shown to associate with Brg1-based SWI/SNF ATP-dependent chromatin remodeling complex12. These findings led us to investigate the possible roles of Prmt7 in adipogenic differentiation, which is a process that requires the function of Brg1-based SWI/SNF complex24. Our data clearly showed that Prmt7 levels were significantly changed in the knockdown or over-expression cells, but manipulation of Prmt7 levels did not cause a differentiation deficiency. It is established that Brg1-based SWI/SNF complex is recruited to the adipogenic promoters upon differentiation24. However, whether Prmt7 associates with Brg1-based SWI/SNF complex at adipogenic promoters is still unknown. Since Prmt7 has no effect on adipogenic gene expression, we expect that Prmt7 is not recruited to adipogenic promoters. Alternatively, even if there is binding, the function of Prmt7 is dispensable at these loci.
Functional redundancy within the Prmt family has not been characterized. Prmt7 was classified as a type II and a type III arginine methyltransferase by characterization of its in vitro catalytic activity8–11. Whether other Prmts functionally compensate for Prmt7 is still unknown. The predominant type II arginine methyltransferase Prmt5 catalyzes the formation of MMA and SDMA in a nonprocessive fashion36,37. Type I arginine methyltransferases also produce MMA38–40. It is possible that Prmt5 or type I Prmts partially or fully compensate for the loss of Prmt7 in the cells. Our data showed that Prmt7 knockdown or over-expression has no effect on Prmt5 protein levels in C3H10T1/2 cells. This observation is consistent with the results from the previous study in HeLa cells41. However, we still cannot rule out the possibility that Prmt5 compensates for Prmt7 enzyme activity, even though Prmt5 protein levels remain constant. Further investigation is needed to address the possible crosstalk between Prmt5 and Prmt7 in chromatin regulation.
To our knowledge, Prmt7 knock-out or transgenic mice have not been reported. Whether changes in Prmt7 levels cause any developmental deficiencies in vivo remains unknown. However, several studies using cell lines or tissues have revealed regulatory roles for Prmt7 in tissue-specific gene expression. For example, PRMT7 negatively regulates neuronal differentiation of a human embryonal carcinoma cell line by repressing the expression of differentiation-specific genes13. In mouse germ cells, Prmt7 was recruited to the imprinting control region through physical interaction with CTCFL, a testis-specific nuclear protein, and repressed imprinted gene expression42. Furthermore, mouse embryonic stem cells and germ cells have relative high levels of Prmt7 compared with mouse embryonic fibroblasts43,44. This evidence suggests that Prmt7 might have important functions in the maintenance of stem cell pluripotency and that the down-regulation of Prmt7 might be required for early cell fate determination. Taken together, these data suggest that Prmt7 might have a role during early development.
Author contributions
ANI and SS conceived the study. Y-JH, SS and ANI designed the experiments and reviewed the data. Y-JH and ANI wrote the manuscript. All authors were involved in the revision of the draft manuscript and have agreed to the final content.
Competing interests
No competing interests were disclosed.
Grant information
This work was supported by NIH grants DK084278 to ANI and SS and GM56244 to ANI. ANI is a member of the UMass Medical School Diabetes and Endocrine Research Center, supported by the National Institutes of Health grant DK32520.
Acknowledgements
We thank AR Barutcu and KM Imbalzano for comments on the manuscript.
Faculty Opinions recommendedReferences
- 1.
Bedford MT, Richard S:
Arginine methylation an emerging regulator of protein function.
Mol Cell.
2005; 18(3): 263–272. PubMed Abstract
| Publisher Full Text
- 2.
Bedford MT, Clarke SG:
Protein arginine methylation in mammals: who, what, and why.
Mol Cell.
2009; 33(1): 1–13. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 3.
Lee YH, Stallcup MR:
Minireview: protein arginine methylation of nonhistone proteins in transcriptional regulation.
Mol Endocrinol.
2009; 23(4): 425–433. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 4.
Di Lorenzo A, Bedford MT:
Histone arginine methylation.
FEBS Lett.
2011; 585(13): 2024–2031. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 5.
Yang Y, Bedford MT:
Protein arginine methyltransferases and cancer.
Nat Rev Cancer.
2013; 13(1): 37–50. PubMed Abstract
| Publisher Full Text
- 6.
Boisvert FM, Cote J, Boulanger MC, et al.:
A proteomic analysis of arginine-methylated protein complexes.
Mol Cell Proteomics.
2003; 2(12): 1319–1330. PubMed Abstract
| Publisher Full Text
- 7.
Karkhanis V, Hu YJ, Baiocchi RA, et al.:
Versatility of PRMT5-induced methylation in growth control and development.
Trends Biochem Sci.
2011; 36(12): 633–641. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 8.
Miranda TB, Miranda M, Frankel A, et al.:
PRMT7 is a member of the protein arginine methyltransferase family with a distinct substrate specificity.
J Biol Chem.
2004; 279(22): 22902–22907. PubMed Abstract
| Publisher Full Text
- 9.
Lee JH, Cook JR, Yang ZH, et al.:
PRMT7, a new protein arginine methyltransferase that synthesizes symmetric dimethylarginine.
J Biol Chem.
2005; 280(5): 3656–3664. PubMed Abstract
| Publisher Full Text
- 10.
Zurita-Lopez CI, Sandberg T, Kelly R, et al.:
Human protein arginine methyltransferase 7 (PRMT7) is a type III enzyme forming ω-NG-monomethylated arginine residues.
J Biol Chem.
2012; 287(11): 7859–7870. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 11.
Feng Y, Maity R, Whitelegge JP, et al.:
Mammalian Protein Arginine Methyltransferase 7 (PRMT7) Specifically Targets R-X-R Sites in Lysine and Arginine-Rich Regions.
J Biol Chem.
2013; (in press). PubMed Abstract
| Publisher Full Text
- 12.
Karkhanis V, Wang L, Tae S, et al.:
Protein arginine methyltransferase 7 regulates cellular response to DNA damage by methylating promoter histones H2A and H4 of the polymerase δ catalytic subunit gene, POLD1.
J Biol Chem.
2012; 287(35): 29801–29814. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 13.
Dhar SS, Lee SH, Kan PY, et al.:
Trans-tail regulation of MLL4-catalyzed H3K4 methylation by H4R3 symmetric dimethylation is mediated by a tandem PHD of MLL4.
Genes Dev.
2012; 26(24): 2749–2762. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 14.
Tang W, Zeve D, Suh JM, et al.:
White fat progenitor cells reside in the adipose vasculature.
Science.
2008; 322(5901): 583–586. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 15.
Rodeheffer MS, Birsoy K, Friedman JM:
Identification of white adipocyte progenitor cells in vivo.
Cell.
2008; 135(2): 240–249. PubMed Abstract
| Publisher Full Text
- 16.
Gesta S, Tseng YH, Kahn CR:
Developmental origin of fat: tracking obesity to its source.
Cell.
2007; 131(2): 242–256. PubMed Abstract
| Publisher Full Text
- 17.
Farmer SR:
Transcriptional control of adipocyte formation.
Cell Metab.
2006; 4(4): 263–273. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 18.
de la Serna IL, Ohkawa Y, Imbalzano AN:
Chromatin remodelling in mammalian differentiation: lessons from ATP-dependent remodellers.
Nat Rev Genet.
2006; 7(6): 461–473. PubMed Abstract
| Publisher Full Text
- 19.
LeBlanc SE, Konda S, Wu Q, et al.:
Protein arginine methyltransferase 5 (Prmt5) promotes gene expression of peroxisome proliferator-activated receptor γ2 (PPARγ2) and its target genes during adipogenesis.
Mol Endocrinol.
2012; 26(4): 583–597. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 20.
Yadav N, Cheng D, Richard S, et al.:
CARM1 promotes adipocyte differentiation by coactivating PPARgamma.
EMBO Rep.
2008; 9(2): 193–198. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 21.
Pal S, Yun R, Datta A, et al.:
mSin3A/histone deacetylase 2- and PRMT5-containing Brg1 complex is involved in transcriptional repression of the Myc target gene cad.
Mol Cell Biol.
2003; 23(21): 7475–7487. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 22.
Xu W, Cho H, Kadam S, et al.:
A methylation-mediator complex in hormone signaling.
Genes Dev.
2004; 18(2): 144–156. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 23.
Campeau E, Ruhl VE, Rodier F, et al.:
A versatile viral system for expression and depletion of proteins in mammalian cells.
PLoS One.
2009; 4(8): e6529. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 24.
Salma N, Xiao H, Mueller E, et al.:
Temporal recruitment of transcription factors and SWI/SNF chromatin-remodeling enzymes during adipogenic induction of the peroxisome proliferator-activated receptor gamma nuclear hormone receptor.
Mol Cell Biol.
2004; 24(11): 4651–4663. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 25.
Morgenstern JP, Land H:
Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line.
Nucleic Acids Res.
1990; 18(12): 3587–3596. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 26.
Wong ML, Medrano JF:
Real-time PCR for mRNA quantitation.
Biotechniques.
2005; 39(1): 75–85. PubMed Abstract
- 27.
Taylor SM, Jones PA:
Multiple new phenotypes induced in 10T1/2 and 3T3 cells treated with 5-azacytidine.
Cell.
1979; 17(4): 771–779. PubMed Abstract
| Publisher Full Text
- 28.
Konieczny SF, Emerson CP Jr:
5-Azacytidine induction of stable mesodermal stem cell lineages from 10T1/2 cells: evidence for regulatory genes controlling determination.
Cell.
1984; 38(3): 791–800. PubMed Abstract
| Publisher Full Text
- 29.
Tang QQ, Otto TC, Lane MD:
Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage.
Proc Natl Acad Sci U S A.
2004; 101(26): 9607–9611. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 30.
Cho YC, Zheng W, Yamamoto M, et al.:
Differentiation of pluripotent C3H10T1/2 cells rapidly elevates CYP1B1 through a novel process that overcomes a loss of Ah Receptor.
Arch Biochem Biophys.
2005; 439(2): 139–153. PubMed Abstract
| Publisher Full Text
- 31.
Lefterova MI, Zhang Y, Steger DJ, et al.:
PPARgamma and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale.
Genes Dev.
2008; 22(21): 2941–2952. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 32.
Schmidt SF, Jorgensen M, Chen Y, et al.:
Cross species comparison of C/EBPα and PPARγ profiles in mouse and human adipocytes reveals interdependent retention of binding sites.
BMC Genomics.
2011; 12: 152. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 33.
Freytag SO, Paielli DL, Gilbert JD:
Ectopic expression of the CCAAT/enhancer-binding protein alpha promotes the adipogenic program in a variety of mouse fibroblastic cells.
Genes Dev.
1994; 8(14): 1654–1663. PubMed Abstract
| Publisher Full Text
- 34.
Tontonoz P, Hu E, Spiegelman BM:
Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor.
Cell.
1994; 79(7): 1147–1156. PubMed Abstract
| Publisher Full Text
- 35.
Musri MM, Gomis R, Parrizas M:
Chromatin and chromatin-modifying proteins in adipogenesis.
Biochem Cell Biol.
2007; 85(4): 397–410. PubMed Abstract
| Publisher Full Text
- 36.
Branscombe TL, Frankel A, Lee JH, et al.:
PRMT5 (Janus kinase-binding protein 1) catalyzes the formation of symmetric dimethylarginine residues in proteins.
J Biol Chem.
2001; 276(35): 32971–32976. PubMed Abstract
| Publisher Full Text
- 37.
Wang M, Xu RM, Thompson PR:
Substrate specificity, processivity, and kinetic mechanism of protein arginine methyltransferase 5.
Biochemistry.
2013; 52(32): 5430–5440. PubMed Abstract
| Publisher Full Text
- 38.
Lin WJ, Gary JD, Yang MC, et al.:
The mammalian immediate-early TIS21 protein and the leukemia-associated BTG1 protein interact with a protein-arginine N-methyltransferase.
J Biol Chem.
1996; 271(25): 15034–15044. PubMed Abstract
| Publisher Full Text
- 39.
Osborne TC, Obianyo O, Zhang X, et al.:
Protein arginine methyltransferase 1: positively charged residues in substrate peptides distal to the site of methylation are important for substrate binding and catalysis.
Biochemistry.
2007; 46(46): 13370–13381. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 40.
Frankel A, Yadav N, Lee J, et al.:
The novel human protein arginine N-methyltransferase PRMT6 is a nuclear enzyme displaying unique substrate specificity.
J Biol Chem.
2002; 277(5): 3537–3543. PubMed Abstract
| Publisher Full Text
- 41.
Gonsalvez GB, Tian L, Ospina JK, et al.:
Two distinct arginine methyltransferases are required for biogenesis of Sm-class ribonucleoproteins.
J Cell Biol.
2007; 178(5): 733–740. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 42.
Jelinic P, Stehle JC, Shaw P:
The testis-specific factor CTCFL cooperates with the protein methyltransferase PRMT7 in H19 imprinting control region methylation.
PLoS Biol.
2006; 4(11): e355. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 43.
Buhr N, Carapito C, Schaeffer C, et al.:
Nuclear proteome analysis of undifferentiated mouse embryonic stem and germ cells.
Electrophoresis.
2008; 29(11): 2381–2390. PubMed Abstract
| Publisher Full Text
- 44.
Girardot M, Hirasawa R, Kacem S, et al.:
PRMT5-mediated histone H4 arginine-3 symmetrical dimethylation marks chromatin at G + C-rich regions of the mouse genome.
Nucleic Acids Res.
2013; (in press). PubMed Abstract
| Publisher Full Text





Comments on this article Comments (0)