Keywords
gastrin, glucagon-like peptides, somatostatin
gastrin, glucagon-like peptides, somatostatin
Gastrin is initially synthesized as a larger precursor protein and subsequently processed, via a multi-step pathway, to the classical active carboxyl-terminal amidated peptide1. It has become apparent that some of the processing intermediates may have biological activities of their own. Significant biological effects have been reported for both the larger progastrin precursor peptide and the shorter carboxyl-terminal glycine-extended gastrin (G-Gly), suggesting that these are important pathophysiological mediators. The majority of studies have examined the pathophysiological roles of gastrin precursors in gastrointestinal cancers and considerable data have implicated these peptides as stimulants of proliferation and/or inhibitors of apoptosis in a variety of tissues and cell lines, including Barrett’s oeosphagus and oesophageal adenocarcinoma2,3, stomach4,5, pancreas6,7 and normal and malignant colonic epithelium8–12. Growth promoting effects of G-Gly on lung cancer have also been reported13.
Although the precise cell signaling pathways activated by gastrin-processing intermediates have not been definitively described, it seems in most cases that mechanisms distinct from the classical gastrin (CCK2) receptor are involved3,6,10. It is not yet clear whether these gastrin-processing intermediates have distinct physiological, as opposed to pathophysiological roles. Glycine-extended gastrin is produced and stored in significant amounts in the gastric antrum, has gastrointestinal trophic effects and interacts with amidated gastrin to modulate gene expression and gastric acid secretion14–16. Gastrin stimulates both acid secretion and somatostatin release as a feedback inhibitory mechanism17. Similarly, release of cholecystokinin (CCK) from duodenal I-cells is believed to be an important negative feedback mechanism, leading to the inhibition of acid secretion via the release of somatostatin from D-cells in the gastric body and fundus17. Somatostatin release is also stimulated by several other peptide hormones released from the proximal and distal small bowel (including glucagon-like peptide-1 (GLP-1), secretin and oxyntomodulin18 and these form part of the physiological feedback mechanisms that decrease gastric acid in the post-prandial period. The current study was designed to assess the effects of G-Gly on somatostatin release from D-cells and compare these effects with those of amidated gastrin.
New Zealand White rabbits (2–2.5 kg) (Charles River Ltd, Margate, UK) were housed singly in 120 x 60 x 60 cm cages and fed ad libitum on rabbit chow (Special Diets Services, Witham, UK) with a standard 16/8 hour light/dark cycle according to standard Royal Postgraduate Medical School policy. Rabbits were humanely euthanized with 100 mg/kg pentobarbitone intravenously according to institutional policy. One rabbit was used per cell preparation procedure. Post-mortem, the stomach was removed and primary rabbit fundic D-cells were isolated by EDTA-collagenase digestion and enriched by centrifugal elutriation as described previously18–20. The D-cell enriched fraction was suspended in culture medium (DMEM: Ham’s F12 50:50 containing 4% foetal calf serum (Gibco, Paisley, UK), 10 mM HEPES pH 7.4, 2 mM glutamine, 8 mg/l bovine insulin, 1 mg/l hydrocortisone, 100 mg/l penicillin, 100 mg/l streptomycin, 100 mg/l gentamicin (all from Sigma, Poole, UK) and plated at 1 x 106 cells/well onto 12 well tissue culture plates coated with growth factor-reduced Matrigel (diluted 1:7 with water) (Universal Biologicals, London, UK). Cells were cultured for 48 hours after which either somatostatin release experiments were performed or the culture medium was changed and supplemented with 10 nM gastrin or 10 nM G-Gly as appropriate for a further 24 hours, until release experiments were performed.
Somatostatin release experiments were performed as previously described18–20: the culture medium was removed, the cells washed, with release medium (Earl’s balanced salt solution containing 0.1% bovine serum albumin and 10 mM HEPES, pH 7.4) and basal somatostatin, as well as 10 nM cholecystokinin (CCK) , and 10 nM glucagon-like peptide-1 (7-36 amide) (GLP-1)-stimulated somatostatin release was assessed over 2 hours18–20. Cellular somatostatin was extracted by boiling the adherent cells in 3% (final vol/vol) glacial acetic acid in distilled water20. Both released and cellular somatostatin were assessed by radioimmunoassay using K2 anti-somatostatin serum (kindly provided by Professor SR Bloom and Dr M Ghatei, Royal Postgraduate Medical School, Hammersmith Hospital, using 125I somatostatin-14 as tracer and human somatostatin-14 as standard (Bachem, St Helens, UK)) as previously described18,20. Each experimental condition was tested in duplicate and compared with control, untreated wells on the same plate. Results were compared by analysis of variance and Student’s t-test and represent mean ± SEM of 8 different cell preparations. Gastrin (1–17)-Gly (G-Gly) was purchased from NeoMPS (Strasbourg, France), human gastrin-17, sulfated CCK-8 and GLP-1 (7–36) amide were from Bachem.
Cell viability following prolonged gastrin and G-Gly treatment was assessed using the modified 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolinium bromide (MTT) (Sigma) as previously described20.
Initial experiments with only the standard 2-hour stimulation period (without any prolonged pretreatment with any peptides) confirmed that gastrin increased basal but not CCK-stimulated somatostatin release. G-Gly over the 2 hour stimulation period did not alter basal, gastrin or CCK-stimulated release (Figure 1 and Table 1). Gastrin alone did stimulate somatostatin release but was less effective than CCK and neither gastrin nor the gastrin plus G-Gly combination had any effect on CCK-stimulated gastrin release.

D-cells were cultured for 48 hours and then stimulated with peptides for 2 hours as shown, Somatostatin-like immunoreactivity released into the media was quantified by radioimmunoassay. Results expressed and mean ± SEM, compared to untreated control cells, n = 8, * p<0.05 compared to basal control, *P<0.01 compared to basal control.
Experimental data from 8 separate stomach preparations showing somatostatin-like immunoreactivity released from cultured rabbit fundic D-cells stimulated for 2 hours with gastrin, glycine-extended gastrin or both peptides (all 10 nM) +/- CCK (10 nM). SLI results expressed as% of basal, unstimulated release in the relevant stomach preparation.
Twenty four hours pretreatment with gastrin enhanced subsequent basal somatostatin release by 13% and CCK-stimulated release by 10% (both P<0.05). G-Gly enhanced basal somatostatin release by 22% and CCK-stimulated release by 24% (both p<0.05) (Figure 2). The combination of gastrin and G-Gly synergistically increased both basal somatostatin release (35%) and subsequent CCK-stimulated somatostatin release (53%) (p<0.05 compared to the effect of either peptide alone) (Figure 2 and Table 2).

D-cells were cultured for 48 hours and then treated for a further 24 hours with peptides as shown, before stimulation with either CCK or control culture medium for 2 hours. Somatostatin-like immunoreactivity released into the media was quantified by radioimmunoassay. Results expressed and mean ± SEM, compared to untreated control cells, n = 8, * p<0.05 compared to relevant basal control, **p<0.05 compared to gastrin or G-Gly as sole pre-treatment.
Experimental data from 8 separate stomach preparations showing somatostatin-like immunoreactivity released from cultured rabbit fundic D-cells stimulated for 2 hours with CCK or GLP-1 (both 10 nM), following a 24-hour pretreatment period with gastrin, glycine-extended gastrin or both peptides (all 10 nM). SLI results expressed as% of basal, unstimulated and untreated release in the relevant stomach preparation.
To further examine the effects of G-Gly pretreatment on agonist-stimulated release, an alternative direct stimulant of rabbit fundic D-cells, GLP-1, was used18. In keeping with previous studies, GLP-1 did stimulate somatostatin release but was markedly less potent than CCK. As shown in (Figure 3 and Table 2), again 24 hours pretreatment with gastrin alone significantly but relatively modestly potentiated subsequent GLP-1-stimulated somatostatin release (a 25% increase compared to the control GLP-1 treated cells), whilst G-Gly was more effective in enhancing GLP-1-stimulated somatostatin release (a 37% increase compared to the control GLP-1 treated cells). The combination of G-Gly and gastrin was significantly more potent than either peptide alone in enhancing GLP-1 stimulated somatostatin release (a 70% increase compared to the control GLP-1 stimulated cells).
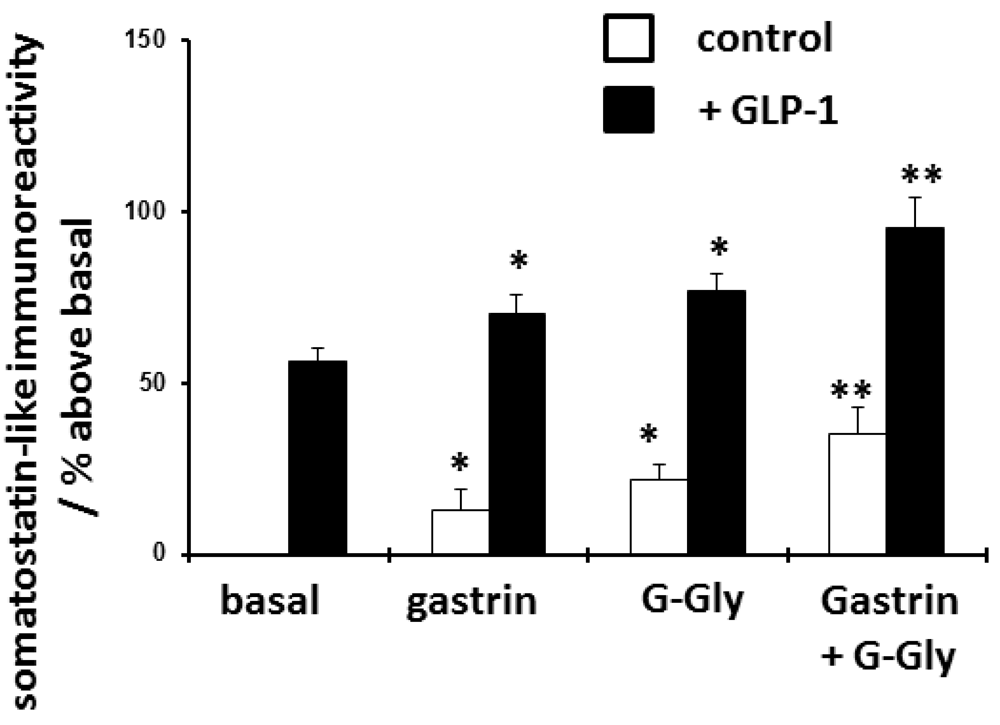
D-cells were cultured for 48 hours and then treated for a further 24 hours with peptides as shown, before stimulation with either GLP-1 or control culture medium for 2 hours. Somatostatin-like immunoreactivity was extracted from cells and quantified by radioimmunoassay. Results expressed and mean ± SEM, compared to untreated control cells, n = 8, * p<0.05 compared to relevant basal control, **p<0.05 compared to gastrin or G-Gly as sole pretreatment.
Twenty four hour pretreatment with both gastrin peptides individually increased D-cell somatostatin content (Figure 4 and Table 3). The dual peptide combination was again synergistic in enhancing cellular somatostatin content, the combination increasing total somatostatin levels by 57% compared to untreated basal levels.

D-cells were cultured for 48 hours and then treated for a further 24 hours with peptides as shown, Somatostatin-like immunoreactivity was extracted from cells and was quantified by radioimmunoassay. Results expressed and mean ± SEM, compared to untreated control cells, n = 8, *p<0.05 compared to untreated basal control. **p<0.05 compared to gastrin or G-Gly as sole treatment.
Experimental data from 8 separate stomach preparations showing cellular somatostatin-like immunoreactivity contained in cultured rabbit fundic D-cells treated for a 24-hour pretreatment period with gastrin, glycine-extended gastrin or both peptides (all 10 nM). SLI results expresses as% of untreated control cells from the relevant stomach preparation.
| Preparation no. | Basal | Gastrin | G-Gly | Gastrin & G-Gly |
|---|---|---|---|---|
| 1 | 100 | 108 | 127 | 149 |
| 2 | 100 | 128 | 135 | 193 |
| 3 | 100 | 105 | 118 | 160 |
| 4 | 100 | 109 | 122 | 152 |
| 5 | 100 | 112 | 148 | 192 |
| 6 | 100 | 108 | 110 | 129 |
| 7 | 100 | 110 | 120 | 150 |
| 8 | 100 | 105 | 116 | 143 |
This study demonstrates that both gastrin and G-Gly enhance the subsequent basal and CCK or GLP-1 stimulated release of somatostatin from rabbit fundic D-cells. No acute stimulatory effects of G-Gly were demonstrated but the 24 hour exposure of D-cells to G-Gly significantly increased somatostatin release. It is clear that hormone release is regulated at multiple points (transcription, translation, processing)21 and that different agents may regulate overall function with different temporal patterns. However, it is not yet clear at which point(s) G-Gly regulates somatostatin release. The increase in cellular somatostatin seen after treatment with G-Gly suggests that upregulation of transcription or translation could be involved. An alternative but not mutually exclusive hypothesis is that the gastrin peptides are specific trophic factors for D-cells and the increased somatostatin release in cultured cells reflects these effects. There was no difference in cell viability, assessed by the modified MTT assay between gastrin or G-Gly treated and non-treated cells, but this does not exclude more subtle enhancement of function. Further studies will be required to elucidate the mechanisms underlying these effects of G-Gly and gastrin.
Previous studies have suggested that G-Gly has important roles in cell proliferation and regulation of acid secretion14–16, although specific effects on regulation of the gastric endocrine system have not been investigated previously. The effect of G-Gly in increasing acid secretion from cultured parietal cells is only seen with more prolonged exposure15, similar to the results reported here, suggesting this peptide may have longer term regulatory actions on transcription or processing instead of just stimulating hormone release.
It is known that multiple feedback loops regulate gastric acid secretion. Gastrin stimulates both acid secretion and negative feedback inhibition of acid secretion via the simultaneous release of somatostatin17. G-Gly is also released from G-cells following meals and may enhance acid secretion22. The results reported here suggest that longer term exposure of D-cells to gastrin and G-Gly also stimulates a further negative feedback loop by enhancing subsequent somatostatin release thus providing a means to restrain acid hypersecretion caused by hypergastrinaemia and control longer term acid secretion. Several studies have confirmed that G-Gly is produced in gastric antral G-cells22–25 and this study adds further support to the suggestion that these peptide processing intermediates may have a role in the normal physiological control of the gastric secretions.
The mechanisms of action of both gastrin and G-Gly in enhancing somatostatin release in these circumstances remain to be elucidated. Interestingly, the combination of these peptides had a synergistic effect on the release of somatostatin as has been noted in the control of acid secretion and cell growth2–4,6,26–28. Gastrin and G-Gly have separate but complimentary actions on cell signaling pathways and gene transcription2,3,14,27. This further supports the notion that both gastrin and G-Gly produced by the gastric antrum and duodenum have important roles in the regulation of gastric homeostasis.
This study was funded by the MRC (Research Training Fellowship awarded to ILPB) and The Royal Society (Small Project Grant Awarded to ILPB).
The author would like to acknowledge the late Professor J Calam for helpful discussions and Professor S Bloom and Dr M Ghatei for the gift of the somatostatin antiserum.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 20 Feb 13 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)