Case report
A 22 year old Hispanic woman who had a history of cerebral palsy, seizure disorder with tracheostomy and required night-time mechanical ventilation for chronic respiratory insufficiency, was admitted to the hospital with a gradually worsening shortness of breath, fever, chills and rigor that persisted over the course of 1 week.
The patient had been on assist control ventilation through a tracheostomy and portable home-ventilator (Pulmonetics LTV 1000; Carefusion Corp. San Diego, CA) for 12 months prior to this hospitalization. Although she had suffered from frequent respiratory tract infections in the past, with the last one being 4 months prior to this admission, she required mechanical ventilation only at night-time, as she breathed spontaneously during the day, and received feeding through a gastro-enterostomy tube. There was no previous history of seizures. The patient’s family denied any recent weight loss, night sweats or skin rashes. Her home medications included levetiracetam 250 mg per G tube twice daily, multivitamins and inhaled albuterol as needed for bronchospasm. Her vital signs upon admission were as follows: blood pressure of 84/50 mmHg, heart rate of 109 beats per minute, respiratory rate of 20 breaths per minute, oral temperature 38.3°C, and oxygen saturation of 98%. Her home ventilatory setting included assist-control mode at a rate of 10/minute, tidal volume of 400 ml and positive end-expiratory pressure of 5 cm H2O through a positive end-expiratory pressure (PEEP) valve built into the lambda ventilator circuit. She was immediately admitted to the intensive care unit (ICU).
Physical examination revealed a frail, non-verbal female with habitus and posture suggestive of spasticity, connected to a ventilator via tracheostomy. Physical examination of the chest revealed scattered crepitations over bibasilar lung fields, and predominantly over the right axillary area. She had severe spasticity affecting both the upper and lower extremities. Her initial chest X-ray showed a multifocal pneumonia predominantly confined to the right upper lobe. Admission labs revealed hemoglobin 12.5 gm/dL, white count 8.8 × 103/L, platelets 197 × 103/L. Her serum sodium levels were 138 mEq/dL, and the blood urea nitrogen (BUN) and creatinine values were 20 mg/dL and 0.9 mg/dL respectively. Arterial blood gas showed a pH of 7.30 and PaO2 of 70 mmHg.
In addition to fluid resuscitation with normal saline infusions, given her history of pneumonia caused by Pseudomonas aeruginosa 3 months before the hospitalization, the patient was started on intravenous piperacillin and tazobactam at 3.75 g every 8 hours. Her blood cultures were drawn and were subsequently reported as negative for both bacterial and fungal organisms. Respiratory cultures obtained from tracheal aspirate grew P. aeruginosa sensitive to meropenem and hence she was started on meropenem 500 mg intravenously every 6 hours, after discontinuing piperacillin and tazobactam as well as vancomycin. Despite being on antibiotics for 7 days and showing negative culture for Pseudomonas, she continued to be febrile with temperature spikes of 38.6°C with night sweats. Oxygen saturation progressively worsened and subsequent chest X-rays showed a progressive increase in pulmonary infiltrates bilaterally, involving all four lung quadrants. Further laboratory cultures for respiratory pathogens including a viral panel, Legionella, Mycoplasma and cocci serology were negative. Her sputum obtained from the tracheal aspirate grew Mycobacterium abscessus, initially identified as M. chelonae-abscessus complex, on day 10 of admission to the ICU. By this time, the patient showed a progressive decline in her respiratory status with computerized tomographic (CT) scans of the chest revealing findings consistent with a ‘tree-in-bud’ appearance on the initial images (Figure 1). Intravenous cefoxitin 2 g every 6 hours along with intravenous azithromycin 500 mg daily was started. Her progressive deterioration in respiratory status and worsening of the pulmonary infiltrates were consistent with acute respiratory distress syndrome (ARDS) characterized by bilateral four quadrant infiltrates and PaO2/FiO2 ratio <200 in the absence of features of left atrial hypertension (Figure 2). A 2D echocardiogram showed left ventricular ejection fraction of 65% without any evidence of atrial or ventricular dysfunction. The patient required a fractional inspired oxygen (FiO2) concentration of 100%, PEEP of 10 cm H2O, and low tidal volume ventilation with permissive hypercapnia. She then received low tidal volume “lung protective” ventilation keeping the tidal volume of 400 ml and careful fluid management to prevent fluid overload for the underlying ARDS. The patient was also maintained on respiratory support therapy including chest physical therapy and inhaled bronchodilators including albuterol.
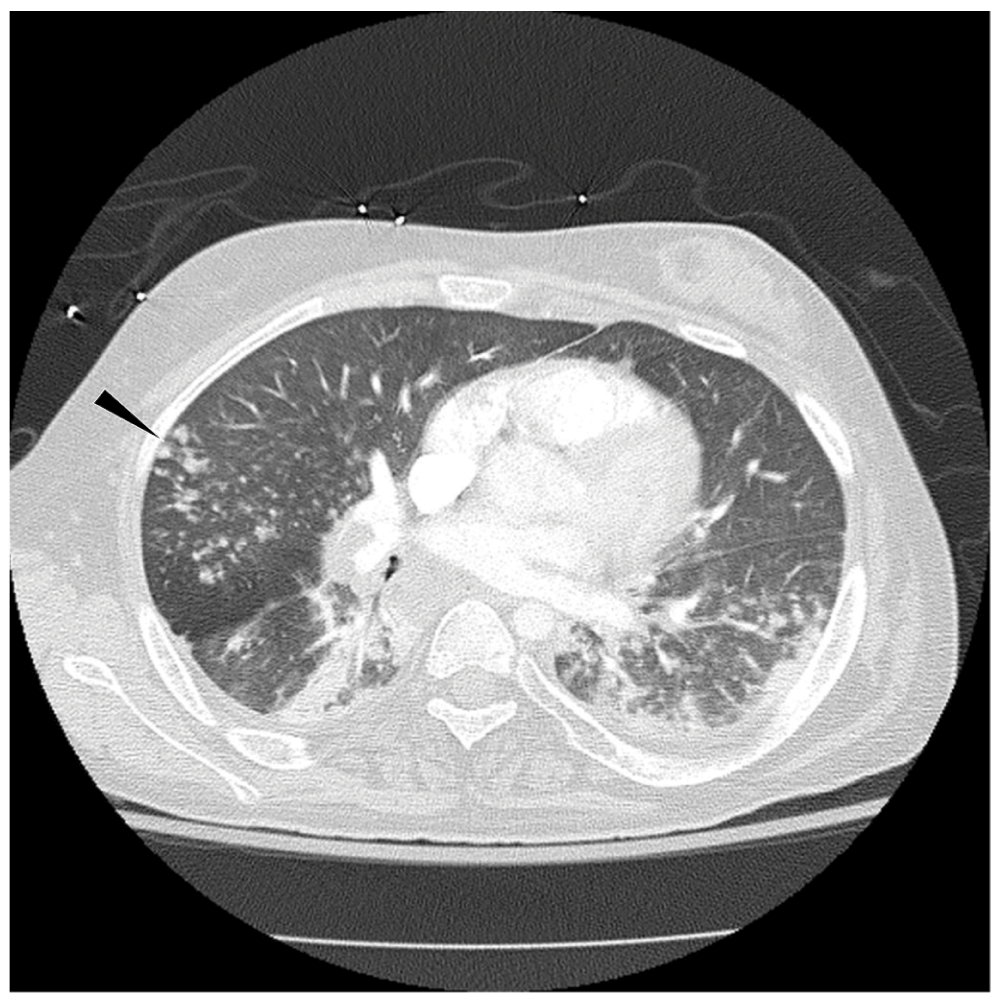
Figure 1. Axial CT of chest shows scattered tree-in-bud opacities (arrow head) in bilateral lower lobes.
There is also a trace left pleural effusion.
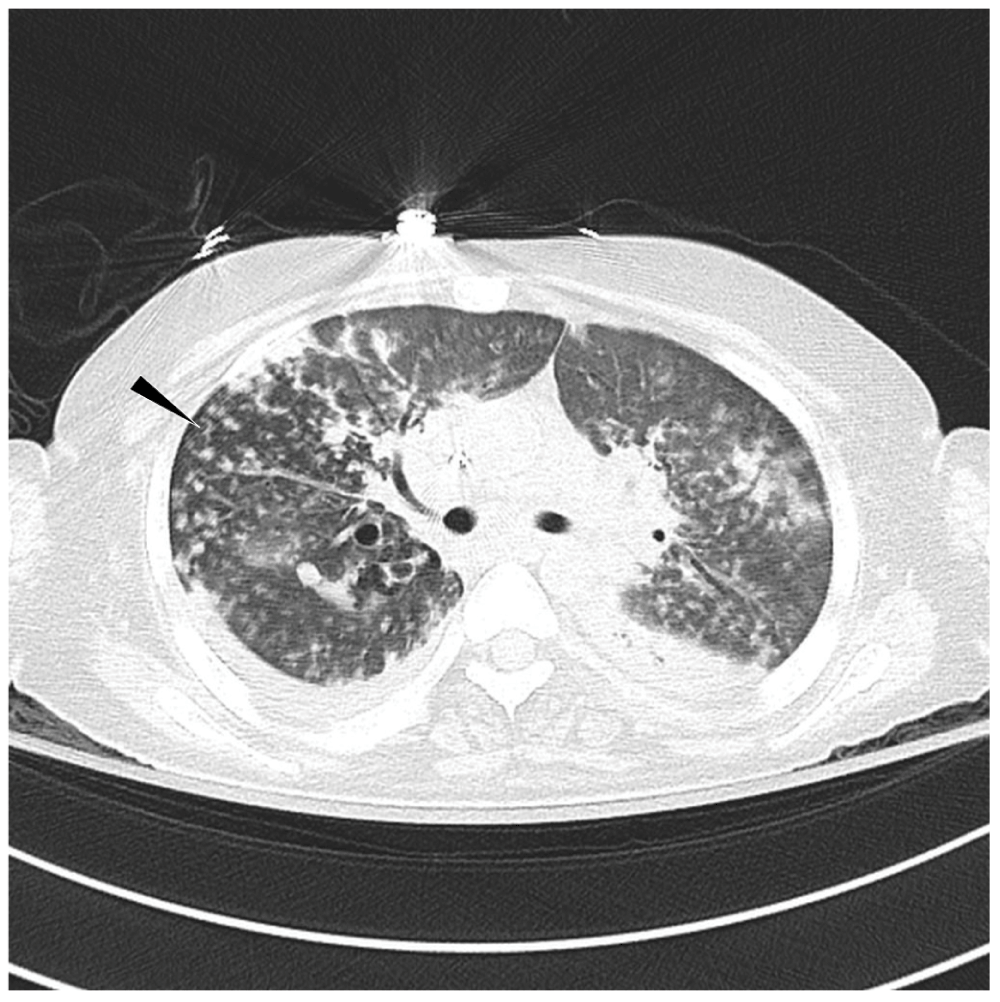
Figure 2. Axial CT of chest shows multifocal airspace opacities including scattered areas of tree-in-bud opacities (arrow head).
There are also bilateral small pleural effusions.
On day 15 of hospital admission, the drug sensitivity report revealed that the infection was caused by a multidrug resistant M. abscessus strain, sensitive only to tigecycline and amikacin. Therefore the patient was started on intravenous (IV) tigecycline 50 mg twice daily and inhaled amikacin 500 mg twice daily. After being on these regimen for 7 days, there was a progressive improvement in oxygenation and radiological evidence for resolution of pulmonary infiltrates. After a prolonged hospital stay for 6 weeks, the patient was subsequently discharged to a long-term acute care facility and inhaled amikacin for 4 weeks and IV tigecycline for a total of 12 weeks. Sputum cultures were repeated at the 8th and 12th week of treatment with tigecycline and revealed no mycobacterial growth (“bacteriological cure”). The patient’s bacteriological cure from this atypical mycobacterium correlated with her clinico-radiological course, as evidenced by the improvement in the pulmonary infiltrates. A subsequent chest CT scan that was performed 8 weeks after commencement of tigecycline confirmed these findings (Figure 3). She returned to her home ventilator settings of assist-control mode with minimal oxygen requirements (fractional inspire oxygen of 30%). Subsequent outpatient follow-up at 3 months up revealed a stable cardiorespiratory status at her baseline.
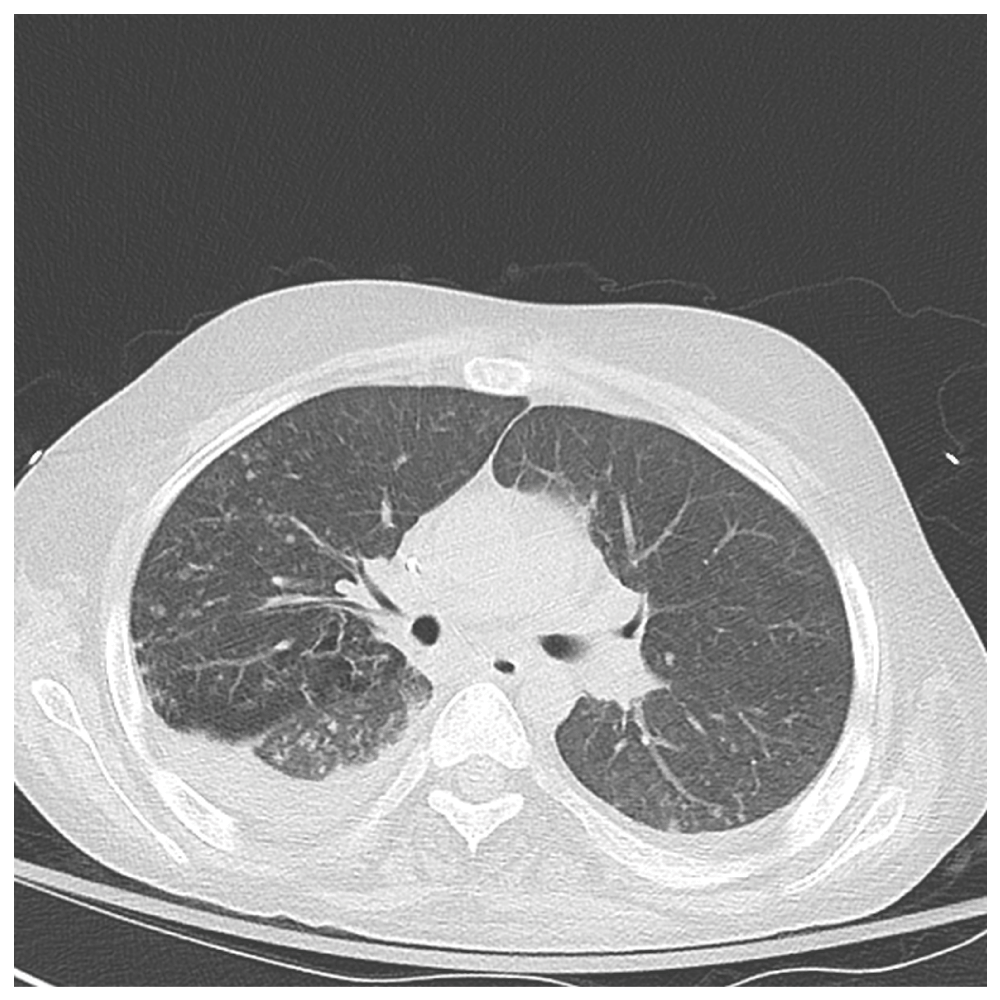
Figure 3. Axial CT chest shows near complete resolution of airspace disease in the lower lobes.
There are bilateral residual trace pleural effusions.
Discussion
M. chelonae-abscessus complex, subspecies abscessus accounts for 85% of the pulmonary diseases caused by M. chelonae isolates1. M. abscessus is a rapidly growing, aerobic acid fast bacillus (AFB) that produces non-pigmented colonies on most types of solid medium in less than 7 days2. Although M. abscessus is a rapid grower, complete speciation of the organism is important but not often done in most clinical laboratory settings. Hence, M. abscessus is often confused with M. chelonae. The organism isolated from our patient was initially identified as M. chelonae but was subsequently confirmed as M. abscessus. This is important for several reasons as both organisms have different clinical manifestations. M. chelonae typically affects patients who are immunosuppressed and are on chronic steroid therapy. The majority of these patients present with disseminated skin and soft tissue infections, predominantly affecting the extremities3. However M. abscessus is more nosocomial and virulent and it causes disease in patients regardless of their immune status. Regarding antibiotic sensitivity, both M. chelonae and M. abscessus are resistant to most antibiotics, except to clarithromycin and amikacin3. M abscessus is usually sensitive to cefoxitin whereas cefoxitin sensitivity of M. chelonae is variable3. Our patient was empirically started on cefoxitin based on this clinical observation. However, M. abscessus isolated from our patient was resistant to most antibiotics except tigecycline and amikacin. In fact the patient showed a rapid improvement of her symptoms and clinico-radiological findings after being started on tigecycline and amikacin. Subsequent sputum cultures were negative for M. abscessus.
Patients with localized M. abscessus infections respond well to appropriate treatment. The treatment approach to disseminated disease varies and is typically based on in vitro susceptibility testing. The goals of therapy should be more realistic and should include symptomatic improvements, radiographic regression of infiltrates, improvements in sputum culture positivity and conversion to negativity. A combination therapy of amikacin and cefoxitin or imipenem for 2 to 4 weeks is recommended, although the cost of therapy and morbidity has an effect on the treatment outcome. In cases of failure to treat with the above regimen or in cases of drug resistance, drugs showing efficacy in vitro against M. abscessus, such as linezolid, tigecycline (a tetracycline derivative) and telithromycin (a ketolide) have been shown to be effective4. M. abscessus isolated from our patient was resistant to most of the drugs and hence was treated with tigecycline. The patient presented a significant clinical, radiological improvement and also had a negative sputum conversion. Although M. abscessus species are rapid growers, the usual clinical course is indolent. To the best of our knowledge, no cases of rapid progression of pulmonary disease to ARDS secondary to M. abscessus have been reported in the literature. Fulminant, rapidly progressive diseases with M. abscessus have been associated with gastroesophageal disorders and cystic fibrosis5. The diagnosis of M. abscessus is based on the diagnostic criteria formulated by the American Thoracic Society4. Although not considered as confirmatory tests, imaging studies and especially high resolution CT scans (HRCT) play an important role in the diagnosis and follow-up of M. abscessus infection. Non-tuberculous mycobacterial (NTM) infections are classified in two groups based on HRCT presenting either a cavitary pattern or a nodular bronchiectasis pattern with tree-in-bud appearance6. M. abscessus cases show widely scattered tree-in-bud appearance on CT chest7. Our patient presented the same characteristic appearance on her chest CT scan. Small centrilobular nodules of soft tissue attenuation interlinked to linear structures of similar size originating from a single stalk gives the characteristic tree-in-bud appearance on imaging studies8. Although this pattern was classically reported in mycobacterial infections, it has also been described in other infectious, inflammatory, immunologic and pulmonary vascular disorders9. Differential diagnosis of tree-in-bud appearance on CT scans in the setting of acute rapidly progressive disease should also include bacterial pneumonia, Haemophilus influenza infection, tumor emboli in the pulmonary vasculature and inhalation of toxic fumes9. Upon identifying this pattern on HRCT scans; work-up for the broad differentials should include a thorough medical history and clinical examination. The histopathological features contributing to this pattern in mycobacterial infection are the accumulation of caseous material within or around the bronchioles, with stalk and terminal tufts, a manifestation of caseous material in the terminal bronchioles and alveolar ducts, respectively8. The tree-in-bud sign is important in discriminating between phases of unfavorable progression and phases of quiescence or resolution10. Chest CT scan is important when surgical intervention is planned or when assessing the effect of chemotherapy, as the resolution of the infection can be demonstrated sooner and quickly by radiological evidence.
In summary, our case report highlights the importance of identifying M. abscessus as a cause of severe respiratory failure requiring intense respiratory support and aggressive medical management. We also emphasize the importance of monitoring drug sensitivity in these cases as it would improve the chances of successful treatment of these potentially fatal infections.
Consent
Written informed consent for publication of clinical details and clinical images was obtained from the patient’s mother.
Author contributions
All authors have seen and approved the text of the manuscript and taken responsibility for its contents. Dr John has collected the relevant clinical data and drafted the article which was critically revised and edited by the co-authors, Dr Zangeneh and Dr Parthasarathy.
Competing interests
No competing interests were disclosed.
Grant information
The author(s) declared that no grants were involved in supporting this work.
Faculty Opinions recommendedReferences
- 1.
Singh N, Yu VL:
Successful treatment of pulmonary infection due to Mycobacterium chelonae: case report and review.
Clin Infect Dis.
1992; 14(1): 156–161. PubMed Abstract
| Publisher Full Text
- 2.
Jeon K, Kwon OJ, Lee NY, et al.:
Antibiotic treatment of Mycobacterium abscessus lung disease: a retrospective analysis of 65 patients.
Am J Respir Crit Care Med.
2009; 180(9): 896–902. PubMed Abstract
| Publisher Full Text
- 3.
Mueller PS, Edson RS:
Disseminated Mycobacterium abscessus infection manifesting as fever of unknown origin and intra-abdominal lymphadenitis: case report and literature review.
Diagn Microbiol Infect Dis.
2001; 39(1): 33–37. PubMed Abstract
| Publisher Full Text
- 4.
Griffith DE, Aksamit T, Brown-Elliott BA, et al.: ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America.
An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases.
Am J Respir Crit Care Med.
2007; 175(4): 367–416. PubMed Abstract
| Publisher Full Text
- 5.
Griffith DE, Girard WM, Wallace RJ Jr:
Clinical features of pulmonary disease caused by rapidly growing mycobacteria: an analysis of 154 patients.
Am Rev Respir Dis.
1993; 147(5): 1271–8. PubMed Abstract
| Publisher Full Text
- 6.
Polverosi R, Guarise A, Balestro E, et al.:
High-resolution CT of nontuberculous mycobacteria pulmonary infection in immunocompetent, non-HIV-positive patients.
Radiol Med.
2010; 115(2): 191–204. PubMed Abstract
| Publisher Full Text
- 7.
Kurashima A:
[Radiographic findings of pulmonary nontuberculous mycobacteriosis other than Mycobacterium avium complex].
Kekkaku.
2009; 84(8): 577–83. PubMed Abstract
- 8.
Rossi SE, Franquet T, Volpacchio M, et al.:
Tree-in-bud pattern at thin-section CT of the lungs: radiologic-pathologic overview.
Radiographics.
2005; 25(3): 789–801. PubMed Abstract
| Publisher Full Text
- 9.
Bastawrous S, Hirschmann JV:
A 71–year-old man with fever, productive cough, and tree-in-bud pattern on chest CT scan.
Chest.
2013; 144(2): 700–703. PubMed Abstract
| Publisher Full Text
- 10.
Ferrara I, Cappabianca S, Brunese L, et al.:
HRCT in detection of pulmonary infections from nontuberculous mycobacteria: personal experience.
Radiol Med.
2009; 114(3): 376–389. PubMed Abstract
| Publisher Full Text



Comments on this article Comments (0)