Vitamin D - a multifunctional hormone
Vitamin D was discovered in the early 20th century when it was described that rickets could be cured by sunlight or cod liver oil. This fat-soluble vitamin was the fourth vitamin described and therefore designated as vitamin D. Vitamin D was found to regulate intestinal absorption and renal reabsorption of calcium and bone metabolism. Later research has demonstrated that vitamin D can be obtained from the diet or be de novo synthesized from 7-dehydrocholesterol. Further, the active form of vitamin D, 1α,25-dihydroxyvitamin D3, interacts with a receptor in the target cells, showing that vitamin D should be biochemically characterized as a hormone rather than as a vitamin1.
During the last decades, the outlook on vitamin D has widened, from being a vitamin solely involved in bone metabolism and calcium homeostasis, to being a multifunctional hormone known to affect a broad range of physiological processes. This includes effects on the immune system, brain and fetal development, insulin secretion, cancer, apoptosis, cell proliferation and differentiation as well as the cardiovascular system via the vitamin D receptor (VDR)1–4. The vitamin D receptor is widely expressed and it has been suggested that 1α,25-dihydroxyvitamin D3 may have other roles yet undiscovered3,5,6. The aim of this paper is to review the literature on effects of vitamin D and vitamin D analogs on steroidogenic enzymes.
Bioactivation and metabolism of vitamin D
There are two forms of vitamin D, vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). Ergocalciferol is synthesized in plants, yeast and fungi while cholecalciferol is synthesized in animals. Vitamin D3 is synthesized in the skin from 7-dehydrocholesterol upon exposure to UV-B radiation. Vitamin D3 is then bioactivated in two subsequent steps to gain the biologically active form of vitamin D (Figure 1). In the first step, vitamin D3 is 25-hydroxylated to 25-hydroxyvitamin D3 (calcidiol). 25-Hydroxylation of vitamin D is a reaction that can be catalyzed by the mitochondrial CYP27A1 and the microsomal CYP3A4, CYP2R1 and CYP2J2 in humans. 25-Hydroxylation of vitamin D is mainly performed in the liver and calcidiol is then excreted into the circulation. Calcidiol is converted to 1α,25-dihydroxyvitamin D3 (calcitriol) by 1α-hydroxylation, mainly performed in the kidneys. The principal human 1α-hydroxylase for 25-hydroxyvitamin D3 is CYP27B1. The 1α,25-dihydroxyvitamin D3 produced is excreted into the circulation and acts as a hormone1,3,4,7–9.
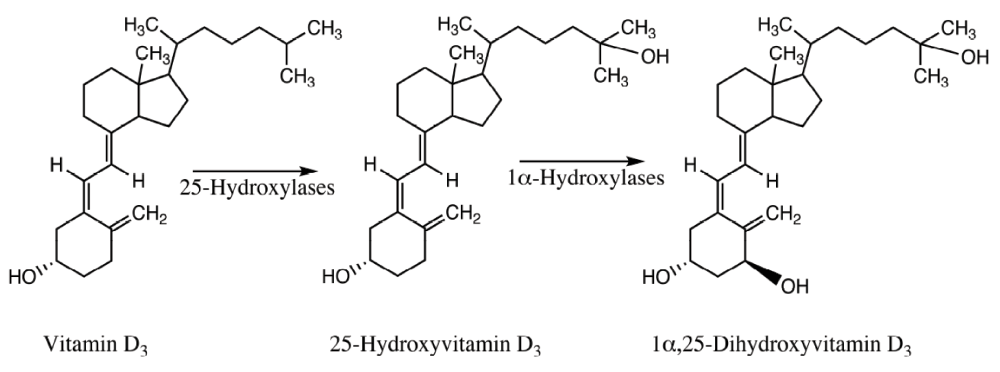
Figure 1. Bioactivation of vitamin D3 to its hormonally active form, 1α,25-dihydroxyvitamin D3.
Extrarenal 1α-hydroxylation of 25-hydroxyvitamin D3 has been reported for a wide range of tissues, including colon, brain, mammary tissue, breast, pancreatic islets, parathyroid glands, placenta, prostate and keratinocytes3,7. These findings suggest that 1α,25-dihydroxyvitamin D3 may be produced locally and act in an intracrine or paracrine fashion. It may be speculated that the local concentration of 1α,25-dihydroxyvitamin D3 in these tissues could be higher than the circulating levels.
The normal serum level of calcidiol is 50–100 nM and for calcitriol 50–125 pM3. Calcitriol is the most potent form of vitamin D even though calcidiol can exert some biological effects as well. The circulating levels of 1α,25-dihydroxyvitamin D3 is tightly regulated via a feed-back mechanism where 1α,25-dihydroxyvitamin D3 downregulates the expression of CYP27B1 and upregulates the expression of CYP24A18.
Both calcidiol and calcitriol is metabolized by CYP24A1 to the less active compounds 24,25-dihydroxyvitamin D3 and 1α,24,25-trihydroxyvitamin D3 respectively10. It has recently been reported that CYP11A1 can catalyze the production of 20-hydroxyvitamin D3 from vitamin D3 and the production of 1α,20-dihydroxyvitamin D3 from 1α-hydroxyvitamin D311–15. Both these metabolites have been reported to exert biological effects on cell differentiation and gene expression in a way resembling the one of 1α,25-dihydroxyvitamin D311,12. The physiological role, if any, of this CY11A1-mediated metabolism of vitamin D remains to be clarified.
Mode of action
The bioactivated form of vitamin D alters the gene expression of a large number of genes. It is well known that 1α,25-dihydroxyvitamin D3 can act either to increase the gene expression or decrease the gene expression, depending on the gene in question. For example, 1α,25-dihydroxyvitamin D3 increases the gene expression of CYP24A1 while it increases the gene expression of CYP27B1. Both these effects of the hormonally active form of vitamin D are mediated via a VDR dependent mechanism. The mechanism for 1α,25-dihydroxyvitamin D3-mediated induction of gene expression is well known and based on the interaction between the 1α,25-dihydroxyvitamin D3-activated VDR and a vitamin D responsive element (VDRE) in the gene promoter. These positive VDRE (pVDRE) consist of a hexameric direct repeat of the consensus sequence 5´-RGKTCA (R=A or G, K=G or T)16. The two half sites are separated by a three nucleotide spacer. The ligand-activated VDR-RXR complex interacts with the pVDRE and acts as a transcription factor to increase the transcriptional rate by recruiting coactivators3.
However, the mechanism for 1α,25-dihydroxyvitamin D3-mediated downregulation of gene expression has in large part remained unclear17. Studies have only been performed for a few genes, regarding the molecular mechanism for the vitamin D-mediated downregulation of gene expression. For these genes, it has been suggested that the mechanism could include recruitment or displacement of corepressors, such as VDR interacting repressor (VDIR) and Williams syndrome transcription factor (WSTF), from the promoter sequence. Further, it has been proposed that epigenetic changes such as histone deacetylation and DNA methylation might be involved in the mechanism18–23. The negative vitamin D response elements (nVDRE) described show a very low level of similarity to the pVDRE described21. Furthermore, it has been proposed that the mechanism for vitamin D-mediated downregulation of gene expression is not based on a direct interaction between the ligand-activated VDR and the promoter sequence, but rather an in-direct interaction via comodulator VDIR18.
Recently, it has been shown in genome-wide ChIP-seq experiments that VDR has a large number of binding sites throughout the genome24–26. These binding sites have been found to be located predominantly within introns and intergenic regions and often far away from the transcriptional start site26. The physiological role of these binding sites remains to be elucidated.
Vitamin D has also been reported to exert rapid effects only seconds or minutes after treatment. Due to the quick response, it has been suggested that these effects are non-genomic and mediated by membrane-bound receptors27.
Steroid hormone synthesis
All steroid hormones are synthesized from the common precursor cholesterol, which can be obtained from the diet or de novo synthesized from acetyl CoA. The production of steroid hormones is regulated via a number of enzymes of which a majority belongs to the cytochrome P450 (CYP) superfamily.
Steroid hormones exert a wide range of physiological responses, including functions in the immune system, protein and carbohydrate metabolism, water and salt balance, reproductive system and development of sexual characteristics. Steroid hormones are synthesized in steroidogenic tissues such as the adrenal cortex, breast, ovaries, prostate and testis, either from cholesterol or from steroidogenic precursors secreted from other steroidogenic tissues.
Adrenal steroidogenesis
The adrenal cortex produces steroid hormones such as aldosterone, corticosterone, cortisol, dehydroepiandrosterone (DHEA) and androstenedione. An overview of steroids and enzyme-catalyzed reactions in the adrenal steroidogenesis is shown in Figure 2. The adrenal steroidogenesis is quantitatively regulated by the transcription of CYP11A1 (cholesterol side-chain cleavage enzyme) and the activity of steroidogenic acute regulatory protein (StAR).
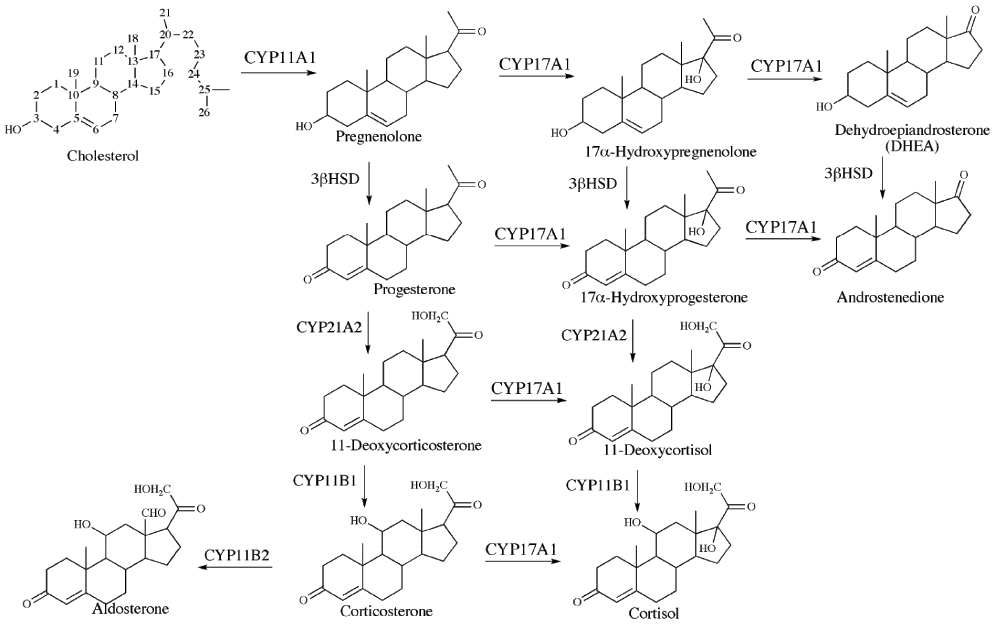
Figure 2. Overview of steroids and enzyme-catalyzed reactions in the adrenal steroidogenesis.
There are three adrenocortical zones, each with a distinct role in the production of steroid hormones; zona glomerulosa produces mineralocorticoids (e.g. aldosterone), zona fasciculata produces glucocorticoids (e.g. cortisol) and zona reticularis is the point of synthesis for adrenal androgens (e.g. DHEA). The qualitative regulation of adrenal steroidogenesis, determining which type of steroid that will be produced, is performed by the transcription and activity of CYP17A1. CYP17A1 catalyzes two different reactions, namely the 17α-hydroxylation and the 17,20-lyase reaction. The expression and activity of CYP17A1 differs between the three adrenal zones28–30. CYP17A1 is not expressed in zona glomerulosa leading to the production of mineralocorticoids. In zona fasciculata, CYP17A1 is expressed and catalyzing 17α-hydroxylation, but not the 17,20-lyase activity, leading to glucocorticoid production. In zona reticularis, CYP17A1 catalyzes both 17α-hydroxylation and 17,20-lyase activities and adrenal androgens are therefore the main product of adrenal steroidogenesis in this adrenal zone28. To control the production of steroid hormones, it is essential to regulate the two activities of CYP17A1 separately. The 17α-hydroxylase activity of CYP17A1 is regulated via the gene expression of CYP17A1. On the other hand, the 17,20-lyase activity of CYP17A1 is regulated via posttranscriptional mechanisms28–34. The adrenal zone-specific activities of CYP17A1 are summarized in Figure 3.
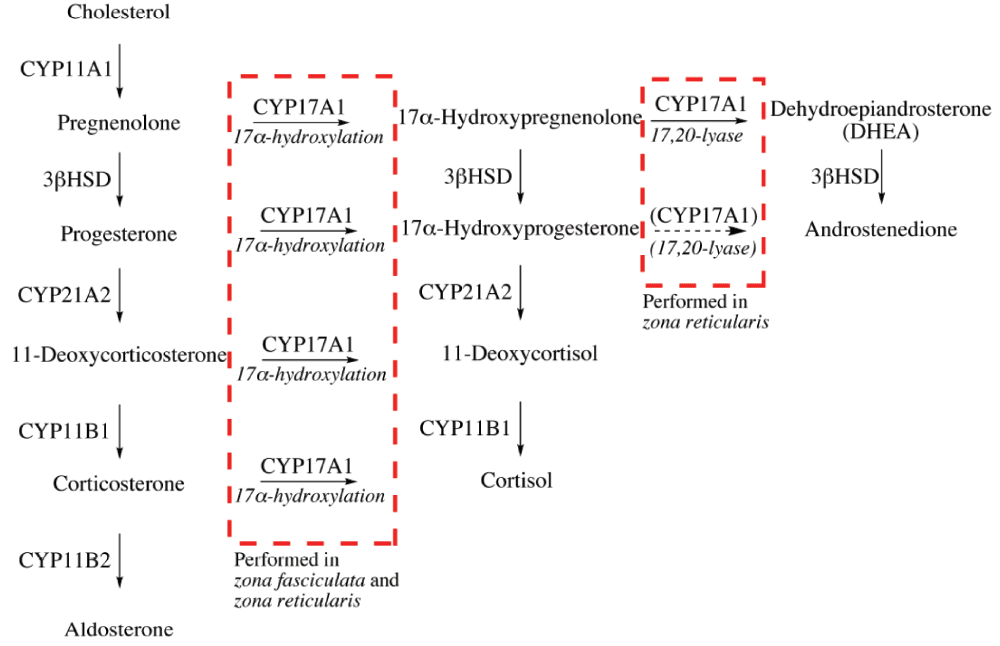
Figure 3. Adrenal zone-specific activities of CYP17A1.
The 17,20-lyase activity has been reported to be regulated via three posttranscriptional mechanism; the abundance of P450 oxidoreductace (POR)35,36, allosteric action of cytochrome b537 and serine phosphorylation of CYP17A138–41. In 1972, Zachman et al.42 described the first case of isolated 17,20-lyase deficiency, which is a rare condition. 17,20-Lyase deficiency could be a result of a mutated cytochrome b5, according to a recent report43. CYP17A1 deficiency, or impaired CYP17A1 activity due to altered posttranscriptional mechanisms, may lead to hypertension, hypokalemia and impaired development of sexual characteristics due to decreased production of adrenal androgens. Patients that are genetically male often present complete male pseudohermaphroditism while female patients may be infertile44–46.
It has been reported that CYP17A1 strongly prefers 17α-hydroxypregnenolone over 17α-hydroxyprogesterone as a substrate for 17,20-lyase activity in humans28,47. 17α-Hydroxyprogesterone may, however, be a substrate for CYP17A1 in other species47.
CYP21A2 is a steroid 21-hydroxylase catalyzing the production of deoxycorticosterone and 11-deoxycortisol, which are precursors for the production of corticosterone, aldosterone and cortisol. 21-Hydroxylase deficiency may lead to severe conditions such as congenital adrenal hyperplasia and Addison´s disease48. CYP21A2 is exclusively expressed in the adrenal cortex28. Therefore, glucocorticoids and mineralocorticoids cannot be synthesized in other tissues than the adrenal cortex.
Sex hormone production
Sex hormones are mainly produced in the gonads, breast and prostate. The sex hormones in these tissues are produced either by in situ synthesis from cholesterol or by enzyme catalyzed conversion of DHEA or androstenedione excreted to the circulation from the adrenal cortex.
The sex hormones are divided into two groups; androgens and estrogens. Androgens such as testosterone and 5α-dihydrotestosterone (DHT) are produced via reactions catalyzed by 17β-hydroxysteroid dehydrogenase (17β-HSD) and 5α-reductase. Estrogens, such as 17β-estradiol (estradiol), are produced by aromatization of androgenic precursors, a reaction catalyzed by CYP19A1 (aromatase). Hence, the tissue-selective expression and activity of 5α-reductase and aromatase regulates the production of androgens and estrogens49,51. For some of these steroids, it remains unclear if they act as estrogens or as androgens or if they are inactive metabolites52. To fully understand the estrogenic and/or androgenic signaling exerted by these steroids is of utmost importance in research on eg. breast and prostate carcinogenesis.
It is crucial to regulate the levels of sex hormones in order to achieve normal gonadal development. Abnormally high levels of estrogens and androgens are associated with increased risk for breast cancer and prostate cancer, respectively50,53–55.
Vitamin D and analogs as regulators of steroidogenic enzymes
Adrenal steroidogenesis
A link between vitamin D and the adrenal steroidogenesis has been proposed in a few very early reports56,57. In a paper from 1959, De Toni et al.56 describes several clinical cases involving children with rickets having changes in urinary 17-ketosteroid levels. It is suggested in the paper that the altered steroid production may involve some action of vitamin D. Furthermore, the authors propose that disturbances in steroid metabolism might be due to sensitivity or resistance of organisms to antirachitic vitamins57. More recently, a case has been described where a woman with osteomalacia was reported to have elevated serum levels of aldosterone and simultaneously low levels of 25-hydroxyvitamin D58. After 24 months of treatment with vitamin D, the condition was normalized.
Recently, Lundqvist et al.59 investigated the effects of 1α,25-dihydroxyvitamin D3 on the adrenal steroidogenesis. We studied the effects of 1α,25-dihydroxyvitamin D3 on the gene expression of key steroidogenic enzymes, the enzyme activity and the hormone production. The study was performed in the human adrenocortical carcinoma cell line NCI-H295R. We found that the mRNA levels of three key enzymes in the adrenal steroidogenesis, CYP11A1, CYP17A1 and CYP21A2 were altered by 1α,25-dihydroxyvitamin D3 treatment. CYP11A1 and CYP17A1 mRNA levels were upregulated after vitamin D treatment, while CYP21A2 mRNA level was suppressed by the same treatment. No significant changes were observed in the mRNA levels of CYP11B1, CYP11B2 and 3βHSD. Further, we found that 1α,25-dihydroxyvitamin D3 treatment decreased the production of corticosterone, androstenedione, dehydroepiandrosterone (DHEA) and DHEA-sulfate (DHEA-S), while the production of aldosterone and cortisol was unaltered. Moreover, we measured the enzyme activity of CYP21A2 and CYP17A1 by adding a known amount of substrate to the cell culture and measuring the turnover of substrate to product. In resemblance with the effects on mRNA level, CYP21A2 enzyme activity was suppressed by 1α,25-dihydroxyvitamin D3. CYP17A1 catalyzes two separate reactions, the 17α-hydroxylation and the 17,20-lyase reaction. We found that treatment with vitamin D resulted in increased 17α-hydroxylation activity of CYP17A1, but in decreased 17,20-lyase activity of CYP17A1.
The regulation of the two activities of CYP17A1 uses two principally different mechanisms. The 17α-hydroxylase activity is regulated by alterations of the gene expression of CYP17A1. The 17,20-lyase activity, on the other hand, is regulated via posttranscriptional mechanisms28,31–34. It has been reported that the 17,20-lyase activity is regulated via three mechanisms; the abundance of P450 oxidoreductase (POR)35,36, allosteric action of cytochrome b537 and serine phosphorylation of CYP17A138–41. The discrepancy between the 1α,25-dihydroxyvitamin D3-mediated increase in expression of CYP17A1 mRNA and the suppression of 17,20-lyase activity could be a result of posttranscriptional mechanisms affected by 1α,25-dihydroxyvitamin D3.
In a subsequent report22, we investigated the molecular mechanism for the effect of 1α,25-dihydroxyvitamin D3 on CYP21A2 gene expression. We found that 1α,25-Dihydroxyvitamin D3 altered the promoter activity of CYP21A2 via a mechanism involving VDR and a vitamin D response element in the CYP21A2 promoter. Further, we found that the mechanism included interaction of the comodulators VDR interacting repressor (VDIR) and Williams syndrome transcription factor (WSTF) to the gene promoter.
Chatterjee and collaborators60,61 have reported that ligand-activated VDR upregulates the expression of sulfotransferase 2A1 (SULT2A1), an enzyme that catalyzes the conversion of dehydroepiandrosterone (DHEA) to dehydroepiandrosterone-sulfate (DHEA-S).
Sex hormone production
The production of sex hormones is regulated by multiple enzymes. Vitamin D has been reported to affect the expression and activity of several of these enzymes.
17β-hydroxysteroiddehydrogenases. Wang and Tuohimaa62 have reported that 1α,25-dihydroxyvitamin D3 upregulates the mRNA level for 17β-hydroxysteroid dehydrogenases (17β-HSD) type 2, 4 and 5 in cell lines derived from human prostate. In keratinocytes, Hughes et al.63 have reported that 1α,25-dihydroxyvitamin D3 stimulates the expression of 17β-HSD type 1 and 2.
Aromatase. It has been shown that 1α,25-dihydroxyvitamin D3 alters the aromatase activity in placental cells64,65, prostate cells66 and osteoblasts67,68. Kinuta et al.69 have reported that vitamin D receptor null mutant mice have a decreased aromatase activity in the ovary, testis and epididymis. Recently, it was reported that 1α,25-dihydroxyvitamin D3 alters the gene expression of aromatase in a tissue-selective manner70. In breast cancer cell lines, vitamin D treatment resulted in decreased aromatase gene expression, while the same treatment increased the aromatase gene expression in osteosarcoma cell lines. 1α,25-Dihydroxyvitamin D3 has therefore been proposed to be a tissue-selective aromatase modulator70.
We have investigated the effects of 1α,25-dihydroxyvitamin D3 on the aromatase gene expression and estradiol production in human breast carcinoma MCF-7 cells, human adrenocortical carcinoma NCI-H295R cells and human prostate cancer LNCaP cells71. We found that the hormonally active form of vitamin D altered the estrogen and androgen metabolism in a cell line specific manner. Aromatase gene expression and estradiol production was found to be decreased in breast cancer cells, while the androgen production was markedly increased in the same cell line. 1α,25-dihydroxyvitamin D3 was found to increase the aromatase gene expression and decrease dihydrotestosterone production in adrenocortical cells. In prostate cancer cells, aromatase gene expression was found to be increased after 1α,25-dihydroxyvitamin D3 treatment. Furthermore, we studied the effects of 1α,25-dihydroxyvitamin D3 on three different aromatase promoters, and found that the transcriptional rate of these promoters were affected by 1α,25-dihydroxyvitamin D3 in a cell line-specific manner.
In a subsequent study, we have shown that the substance EB1089 (a vitamin D analog with decreased hypercalcemic effect) is able to inhibit the aromatase gene expression by dissociation of comodulator WSTF from the CYP19A1 promoter in human breast cancer MCF-7 cells23. Furthermore, 1α,25-dihydroxyvitamin D3 and analogs have been reported to alter sex hormone signaling by suppressing the expression of estrogen receptor α72–77. It has also been reported that vitamin D deficiency alters reproductive functions in both male and female rats, indicating that vitamin D may affect sex hormone signaling78,79.
Concluding remarks
In conclusion, 1α,25-dihydroxyvitamin D3 has been shown to regulate the gene expression and enzyme activity of a number of steroidogenic enzymes, and the corresponding hormone production. The physiological role of these vitamin D effects remains to be elucidated in many cases, especially for the adrenal steroidogenic enzymes. More research has been conducted on the physiological role and potential medical use of the effects of vitamin D on estrogen production and action in breast cancer. Cell culture based experiments and laboratory animal experiments has clearly shown that 1α,25-dihydroxyvitamin D3 is able to decrase the production and action of estrogens in breast cancer models, and also the growth of breast cancer xenotumors70,72–75,80. Based on these findings, vitamin D or analogs has been proposed to be of potential use as an anti-cancer agent76,81–84 and a large number of clinical trials (e.g. ClinicalTrials.gov identifiers NCT00656019, NCT01472445, NCT01965522, NCT01948128, NCT01787409, and NCT01097278) are currently being conducted using vitamin D or analogs in different settings to investigate the potential use in the prevention or treatment of breast cancer.
Competing interests
No competing interests were disclosed.
Grant information
The author is grateful for financial support from the Swedish Academy of Pharmaceutical Sciences and the Swedish Society for Medical Research.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Faculty Opinions recommendedReferences
- 1.
Plum LA, DeLuca HF:
Vitamin D, disease and therapeutic opportunities.
Nat Rev Drug Discov.
2010; 9(12): 941–55. PubMed Abstract
| Publisher Full Text
- 2.
Dusso AS, Brown AJ, Slatopolsky E:
Vitamin D.
Am J Physiol Renal Physiol.
2005; 289(1): F8–28. PubMed Abstract
| Publisher Full Text
- 3.
Norman AW:
From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health.
Am J Clin Nutr.
2008; 88(2): 491S–499S. PubMed Abstract
- 4.
Holick MF:
Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease.
Am J Clin Nutr.
2004; 80(6 Suppl): 1678S–88S. PubMed Abstract
- 5.
Jones G, Strugnell SA, DeLuca HF:
Current understanding of the molecular actions of vitamin D.
Physiol Rev.
1998; 78(4): 1193–1231. PubMed Abstract
- 6.
DeLuca HF:
Overview of general physiologic features and functions of vitamin D.
Am J Clin Nutr.
2004; 80(6 Suppl): 1689S–96S. PubMed Abstract
- 7.
Holick MF:
Vitamin D: A millenium perspective.
J Cell Biochem.
2003; 88(2): 296–307. PubMed Abstract
| Publisher Full Text
- 8.
Prosser DE, Jones G:
Enzymes involved in the activation and inactivation of vitamin D.
Trends Biochem Sci.
2004; 29(12): 664–673. PubMed Abstract
| Publisher Full Text
- 9.
Karlgren M, Miura S, Ingelman-Sundberg M:
Novel extrahepatic cytochrome P450s.
Toxicol Appl Pharmacol.
2005; 207(2 Suppl): 57–61. PubMed Abstract
| Publisher Full Text
- 10.
Deeb KK, Trump DL, Johnson CS:
Vitamin D signalling pathways in cancer: potential for anticancer therapeutics.
Nat Rev Cancer.
2007; 7(9): 684–700. PubMed Abstract
| Publisher Full Text
- 11.
Zbytek B, Janjetovic Z, Tuckey RC, et al.:
20-Hydroxyvitamin D3, a product of vitamin D3 hydroxylation by cytochrome P450scc, stimulates keratinocyte differentiation.
J Invest Dermatol.
2008; 128(9): 2271–80. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 12.
Tuckey RC, Janjetovic Z, Li W, et al.:
Metabolism of 1alpha-hydroxyvitamin D3 by cytochrome P450scc to biologically active 1alpha,20-dihydroxyvitamin D3.
J Steroid Biochem Mol Biol.
2008; 112(4–5): 213–9. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 13.
Slominski A, Semak I, Wortsman J, et al.:
An alternative pathway of vitamin D2 metabolism. Cytochrome P450scc (CYP11A1)-mediated conversion to 20-hydroxyvitamin D2 and 17,20-dihydroxyvitamin D2.
FEBS J.
2006; 273(13): 2891–2901. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 14.
Slominski A, Semak I, Zjawiony J, et al.:
Enzymatic metabolism of ergosterol by cytochrome P450scc to biologically active 17alpha,24-dihydroxyergosterol.
Chem Biol.
2005; 12(8): 931–939. PubMed Abstract
| Publisher Full Text
- 15.
Slominski A, Semak I, Zjawiony J, et al.:
The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism.
FEBS J.
2005; 272(16): 4080–4090. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 16.
Matilainen M, Malinen M, Saavalainen K, et al.:
Regulation of multiple insulin-like growth factor binding protein genes by 1alpha,25-dihydroxyvitamin D3.
Nucleic Acids Res.
2005; 33(17): 5521–5532. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 17.
Pike JW, Meyer MB:
The vitamin D receptor: new paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin D(3).
Endocrinol Metab Clin North Am.
2010; 39(2): 255–269. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 18.
Fujiki R, Kim MS, Sasaki Y, et al.:
Ligand-induced transrepression by VDR through association of WSTF with acetylated histones.
EMBO J.
2005; 24(22): 3881–3894. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 19.
Murayama A, Kim MS, Yanagisawa J, et al.:
Transrepression by a liganded nuclear receptor via a bHLH activator through co-regulator switching.
EMBO J.
2004; 23(7): 1598–1608. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 20.
Kim MS, Fujiki R, Kitagawa H, et al.:
1alpha,25(OH)2D3-induced DNA methylation suppresses the human CYP27B1 gene.
Mol Cell Endocrinol.
2007; 265–266: 168–173. PubMed Abstract
| Publisher Full Text
- 21.
Kim MS, Fujiki R, Murayama A, et al.:
1alpha,25(OH)2D3-induced transrepression by vitamin D receptor through E-box-type elements in the human parathyroid hormone gene promoter.
Mol Endocrinol.
2007; 21(2): 334–342. PubMed Abstract
| Publisher Full Text
- 22.
Lundqvist J, Wikvall K, Norlin M:
Vitamin D-mediated regulation of CYP21A2 transcription - a novel mechanism for vitamin D action.
Biochim Biophys Acta - General Subjects.
2012; 1820(10): 1553–1559. PubMed Abstract
| Publisher Full Text
- 23.
Lundqvist J, Hansen SK, Lykkesfeldt AE, et al.:
Vitamin D analog EB1089 inhibits aromatase expression by dissociation of comodulator WSTF from the CYP19A1 promoter-a new regulatory pathway for aromatase.
Biochim Biophys Acta.
2013; 1833(1): 40–47. PubMed Abstract
| Publisher Full Text
- 24.
Tuoresmäki P, Väisänen S, Neme A, et al.:
Patterns of Genome-Wide VDR Locations.
PLoS One.
2014; 9(4): e96105. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 25.
Meyer MB, Pike JW:
Corepressors (NCoR and SMRT) as well as coactivators are recruited to positively regulated 1α,25-dihydroxyvitamin D3-responsive genes.
J Steroid Biochem Mol Biol.
2013; 136: 120–124. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 26.
Pike JW, Meyer MB:
Fundamentals of vitamin D hormone-regulated gene expression.
J Steroid Biochem Mol Biol.
In press. 2013. PubMed Abstract
| Publisher Full Text
- 27.
Norman AW, Okamura WH, Bishop JE, et al.:
Update on biological actions of 1alpha,25(OH)2-vitamin D3 (rapid effects) and 24R,25(OH)2-vitamin D3.
Mol Cell Endocrinol.
2002; 197(1–2): 1–13. PubMed Abstract
| Publisher Full Text
- 28.
Miller WL:
Steroidogenic Enzymes.
Endocr Dev.
2008; 13: 1–18. PubMed Abstract
| Publisher Full Text
- 29.
Miller WL:
Androgen synthesis in adrenarche.
Rev Endocr Metab Disord.
2009; 10(1): 3–17. PubMed Abstract
| Publisher Full Text
- 30.
Rainey WE, Nakamura Y:
Regulation of the adrenal androgen biosynthesis.
J Steroid Biochem Mol Biol.
2008; 108(3–5): 281–286. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 31.
Nguyen AD, Conley AJ:
Adrenal androgens in humans and nonhuman primates: production, zonation and regulation.
Endocr Dev.
2008; 13: 33–54. PubMed Abstract
| Publisher Full Text
- 32.
Miller WL, Huang N, Agrawal V, et al.:
Genetic variation in human P450 oxidoreductase.
Mol Cell Endocrinol.
2009; 300(1–2): 180–184. PubMed Abstract
| Publisher Full Text
- 33.
Miller WL, Auchus RJ, Geller DH:
The regulation of 17,20 lyase activity.
Steroids.
1997; 62(1): 133–142. PubMed Abstract
| Publisher Full Text
- 34.
Hall PF:
Cytochrome P-450 C21scc: one enzyme with two actions: hydroxylase and lyase.
J Steroid Biochem Mol Biol.
1991; 40(4–6): 527–532. PubMed Abstract
| Publisher Full Text
- 35.
Lin D, Black SM, Nagahama Y, et al.:
Steroid 17 alpha-hydroxylase and 17,20-lyase activities of P450c17: contributions of serine106 and P450 reductase.
Endocrinology.
1993; 132(6): 2498–2506. PubMed Abstract
| Publisher Full Text
- 36.
Yanagibashi K, Hall PF:
Role of electron transport in the regulation of the lyase activity of C21 side-chain cleavage P-450 from porcine adrenal and testicular microsomes.
J Biol Chem.
1986; 261(18): 8429–8433. PubMed Abstract
- 37.
Auchus RJ, Lee TC, Miller WL:
Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer.
J Biol Chem.
1998; 273(6): 3158–3165. PubMed Abstract
| Publisher Full Text
- 38.
Pandey AV, Mellon SH, Miller WL:
Protein phosphatase 2A and phosphoprotein SET regulate androgen production by P450c17.
J Biol Chem.
2003; 278(5): 2837–2844. PubMed Abstract
| Publisher Full Text
- 39.
Pandey AV, Miller WL:
Regulation of 17,20 lyase activity by cytochrome b5 and by serine phosphorylation of P450c17.
J Biol Chem.
2005; 280(14): 13265–13271. PubMed Abstract
| Publisher Full Text
- 40.
Zhang LH, Rodriguez H, Ohno S, et al.:
Serine phosphorylation of human P450c17 increases 17,20-lyase activity: implications for adrenarche and the polycystic ovary syndrome.
Proc Natl Acad Sci U S A.
1995; 92(23): 10619–10623. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 41.
Wang YH, Tee MK, Miller WL:
Human Cytochrome P450c17: single step purification and phosphorylation of serine 258 by protein kinase a.
Endocrinology.
2010; 151(4): 1677–1684. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 42.
Zachmann M, Vollmin JA, Hamilton W, et al.:
Steroid 17,20-desmolase deficiency: a new cause fo male pseudohermaphroditism.
Clin Endocrinol (Oxf).
1972; 1(4): 369–385. PubMed Abstract
| Publisher Full Text
- 43.
Kok RC, Timmerman MA, Wolffenbuttel KP, et al.:
Isolated 17,20-lyase deficiency due to the cytochrome b5 mutation W27X.
J Clin Endocrinol Metab.
2010; 95(3): 994–999. PubMed Abstract
| Publisher Full Text
- 44.
New MI:
Inborn errors of adrenal steroidogenesis.
Mol Cell Endocrinol.
2003; 211(1–2): 75–83. PubMed Abstract
| Publisher Full Text
- 45.
Miller WL:
Disorders of androgen synthesis--from cholesterol to dehydroepiandrosterone.
Med Princ Pract.
2005; 14(Suppl 1): 58–68. PubMed Abstract
| Publisher Full Text
- 46.
Flück CE, Pandey AV:
Clinical and biochemical consequences of p450 oxidoreductase deficiency.
Endocr Dev.
2011; 20: 63–79. PubMed Abstract
- 47.
Gilep AA, Sushko TA, Usanov SA:
At the crossroads of steroid hormone biosynthesis: the role, substrate specificity and evolutionary development of CYP17.
Biochim Biophys Acta (BBA) - Proteins & Proteomics.
2011; 1814(1): 200–209. PubMed Abstract
| Publisher Full Text
- 48.
Krone N, Arlt W:
Genetics of congenital adrenal hyperplasia.
Best Pract Res Clin Endocrinol Metab.
2009; 23(2): 181–192. PubMed Abstract
| Publisher Full Text
- 49.
Ellem SJ, Risbridger GP:
Aromatase and regulating the estrogen:androgen ratio in the prostate gland.
J Steroid Biochem Mol Biol.
2010; 118(4–5): 246–251. PubMed Abstract
| Publisher Full Text
- 50.
Risbridger GP, Davis ID, Birrell SN, et al.:
Breast and prostate cancer: more similar than different.
Nat Rev Cancer.
2010; 10(3): 205–212. PubMed Abstract
| Publisher Full Text
- 51.
Simpson ER, Clyne C, Rubin G, et al.:
Aromatase--a brief overview.
Annu Rev Physiol.
2002; 64: 93–127. PubMed Abstract
| Publisher Full Text
- 52.
Lardy H, Marwah A, Marwah P:
C(19)-5-ene steroids in nature.
Vitam Horm.
2005; 71: 263–299. PubMed Abstract
| Publisher Full Text
- 53.
Ali S, Coombes RC:
Endocrine-responsive breast cancer and strategies for combating resistance.
Nat Rev Cancer.
2002; 2(2): 101–112. PubMed Abstract
| Publisher Full Text
- 54.
Scher HI, Sawyers CL:
Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis.
J Clin Oncol.
2005; 23(32): 8253–8261. PubMed Abstract
| Publisher Full Text
- 55.
Balk SP, Knudsen KE:
AR, the cell cycle, and prostate cancer.
Nucl Recept Signal.
2008; 6: e001. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 56.
De Toni E Jr, Nordio S:
The relationship between calcium-phosphorus metabolism, the 'Krebs cycle' and steroid metabolism.
Arch Dis Child.
1959; 34: 371–382. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 57.
Natanson AO, Chuvaev AV:
[The effect of a rachitogenic regimen on the function of the rat adrenal cortex].
Vopr Pitan.
1970; 29(3): 46–47. PubMed Abstract
- 58.
Taylor HC, Elbadawy EH:
Renal tubular acidosis type 2 with Fanconi's syndrome, osteomalacia, osteoporosis, and secondary hyperaldosteronism in an adult consequent to vitamin D and calcium deficiency: effect of vitamin D and calcium citrate therapy.
Endocr Pract.
2006; 12(5): 559–567. PubMed Abstract
| Publisher Full Text
- 59.
Lundqvist J, Norlin M, Wikvall K:
1alpha,25-Dihydroxyvitamin D3 affects hormone production and expression of steroidogenic enzymes in human adrenocortical NCI-H295R cells.
Biochim Biophys Acta (BBA) - Molecular and Cell Biology of Lipids.
2010; 1801(9): 1056–1062. PubMed Abstract
| Publisher Full Text
- 60.
Chatterjee B, Echchgadda I, Song C:
Vitamin D receptor regulation of the steroid/bile acid sulfotransferase SULT2A1.
Methods Enzymol.
2005; 400: 165–191. PubMed Abstract
| Publisher Full Text
- 61.
Echchgadda I, Song CS, Roy AK, et al.:
Dehydroepiandrosterone sulfotransferase is a target for transcriptional induction by the vitamin D receptor.
Mol Pharmacol.
2004; 65(3): 720–729. PubMed Abstract
| Publisher Full Text
- 62.
Wang JH, Tuohimaa P:
Regulation of 17beta-hydroxysteroid dehydrogenase type 2, type 4 and type 5 by calcitriol, LXR agonist and 5alpha-dihydrotestosterone in human prostate cancer cells.
J Steroid Biochem Mol Biol.
2007; 107(1–2): 100–105. PubMed Abstract
| Publisher Full Text
- 63.
Hughes SV, Robinson E, Bland R, et al.:
1,25-dihydroxyvitamin D3 regulates estrogen metabolism in cultured keratinocytes.
Endocrinology.
1997; 138(9): 3711–3718. PubMed Abstract
| Publisher Full Text
- 64.
Barrera D, Avila E, Hernández G, et al.:
Estradiol and progesterone synthesis in human placenta is stimulated by calcitriol.
J Steroid Biochem Mol Biol.
2007; 103(3–5): 529–532. PubMed Abstract
| Publisher Full Text
- 65.
Sun T, Zhao Y, Mangelsdorf DJ, et al.:
Characterization of a region upstream of exon I.1 of the human CYP19 (aromatase) gene that mediates regulation by retinoids in human choriocarcinoma cells.
Endocrinology.
1998; 139(4): 1684–1691. PubMed Abstract
| Publisher Full Text
- 66.
Lou YR, Murtola T, Tuohimaa P:
Regulation of aromatase and 5alpha-reductase by 25-hydroxyvitamin D(3), 1alpha,25-dihydroxyvitamin D(3), dexamethasone and progesterone in prostate cancer cells.
J Steroid Biochem Mol Biol.
2005; 94(1–3): 151–157. PubMed Abstract
| Publisher Full Text
- 67.
Takayanagi R, Goto K, Suzuki S, et al.:
Dehydroepiandrosterone (DHEA) as a possible source for estrogen formation in bone cells: correlation between bone mineral density and serum DHEA-sulfate concentration in postmenopausal women, and the presence of aromatase to be enhanced by 1,25-dihydroxyvitamin D3 in human osteoblasts.
Mech Ageing Dev.
2002; 123(8): 1107–1114. PubMed Abstract
| Publisher Full Text
- 68.
Tanaka S, Haji M, Takayanagi R, et al.:
1,25-Dihydroxyvitamin D3 enhances the enzymatic activity and expression of the messenger ribonucleic acid for aromatase cytochrome P450 synergistically with dexamethasone depending on the vitamin D receptor level in cultured human osteoblasts.
Endocrinology.
1996; 137(5): 1860–1869. PubMed Abstract
| Publisher Full Text
- 69.
Kinuta K, Tanaka H, Moriwake T, et al.:
Vitamin D is an important factor in estrogen biosynthesis of both female and male gonads.
Endocrinology.
2000; 141(4): 1317–1324. PubMed Abstract
| Publisher Full Text
- 70.
Krishnan AV, Swami S, Peng L, et al.:
Tissue-selective regulation of aromatase expression by calcitriol: implications for breast cancer therapy.
Endocrinology.
2010; 151(1): 32–42. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 71.
Lundqvist J, Norlin M, Wikvall K:
1α,25-Dihydroxyvitamin D3 exerts tissue-specific effects on estrogen and androgen metabolism.
Biochim Biophys Acta (BBA) - Molecular and Cell Biology of Lipids.
2011; 1811(4): 263–270. PubMed Abstract
| Publisher Full Text
- 72.
Swami S, Krishnan AV, Feldman D:
1alpha,25-Dihydroxyvitamin D3 down-regulates estrogen receptor abundance and suppresses estrogen actions in MCF-7 human breast cancer cells.
Clin Cancer Res.
2000; 6(8): 3371–3379. PubMed Abstract
- 73.
Simboli-Campbell M, Narvaez CJ, VanWeelden K, et al.:
Comparative effects of 1,25(OH)2D3 and EB1089 on cell cycle kinetics and apoptosis in MCF-7 breast cancer cells.
Breast Cancer Res Treat.
1997; 42(1): 31–41. PubMed Abstract
| Publisher Full Text
- 74.
James SY, Mackay AG, Binderup L, et al.:
Effects of a new synthetic vitamin D analogue, EB1089, on the oestrogen-responsive growth of human breast cancer cells.
J Endocrinol.
1994; 141(3): 555–563. PubMed Abstract
| Publisher Full Text
- 75.
Stoica A, Saceda M, Fakhro A, et al.:
Regulation of estrogen receptor-alpha; gene expression by 1, 25-dihydroxyvitamin D in MCF-7 cells.
J Cell Biochem.
1999; 75(4): 640–651. PubMed Abstract
- 76.
Larsen SS, Heiberg I, Lykkesfeldt AE:
Anti-oestrogen resistant human breast cancer cell lines are more sensitive towards treatment with the vitamin D analogue EB1089 than parent MCF-7 cells.
Br J Cancer.
2001; 84(5): 686–690. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 77.
Swami S, Krishnan AV, Peng L, et al.:
Transrepression of the estrogen receptor promoter by calcitriol in human breast cancer cells via two negative vitamin D response elements.
Endocr Relat Cancer.
2013; 20(4): 565–577. PubMed Abstract
| Publisher Full Text
- 78.
Halloran BP, DeLuca HF:
Effect of vitamin D deficiency on fertility and reproductive capacity in the female rat.
J Nutr.
1980; 110(8): 1573–1580. PubMed Abstract
- 79.
Kwiecinski GG, Petrie GI, DeLuca HF:
Vitamin D is necessary for reproductive functions of the male rat.
J Nutr.
1989; 119(5): 741–744. PubMed Abstract
- 80.
Krishnan AV, Swami S, Feldman D:
Equivalent anticancer activities of dietary vitamin D and calcitriol in an animal model of breast cancer: importance of mammary CYP27B1 for treatment and prevention.
J Steroid Biochem Mol Biol.
2013; 136: 289–295. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 81.
Christensen GL, Jepsen JS, Fog CK, et al.:
Sequential versus combined treatment of human breast cancer cells with antiestrogens and the vitamin D analogue EB1089 and evaluation of predictive markers for vitamin D treatment.
Breast Cancer Res Treat.
2004; 85(1): 53–63. PubMed Abstract
| Publisher Full Text
- 82.
Krishnan AV, Swami S, Feldman D:
Vitamin D and breast cancer: inhibition of estrogen synthesis and signaling.
J Steroid Biochem Mol Biol.
2010; 121(1–2): 343–348. PubMed Abstract
| Publisher Full Text
- 83.
Welsh J:
Targets of vitamin D receptor signaling in the mammary gland.
J Bone Miner Res.
2007; 22(Suppl 2): V86–V90. PubMed Abstract
| Publisher Full Text
- 84.
Ingraham BA, Bragdon B, Nohe A:
Molecular basis of the potential of vitamin D to prevent cancer.
Curr Med Res Opin.
2008; 24(1): 139–149. PubMed Abstract
| Publisher Full Text



Comments on this article Comments (0)