Introduction
Neural circuits generated during embryonic development are remodeled by environmental inputs during periods of heightened plasticity in postnatal development1. These critical periods are limited to specific windows of time that are different for each sensory system. In the visual system, the primary visual cortex is subjected to a critical period for ocular dominance plasticity during which connections from a weak or absent eye can be permanently overtaken by the strong eye2. The integrated action between inhibitory and excitatory circuits determines critical period onset, with a major role being played by the maturation of fast-spiking parvalbumin (FSPV) interneurons3. We have shown that Otx2 homeoprotein helps determine critical period timing by signaling FSPV cells in mice4. Conditional knock-down in heterozygous floxed mice just prior to normal critical period timing is sufficient to delay onset, while cortical infusion of recombinant Otx2 protein accelerates both onset and closure4.
Remarkably, Otx2 protein in the cortex is non-cell autonomous. The Otx2 locus is silent, as shown by PCR, in situ hybridization and Otx2+/GFP mice, while Otx2 protein is detectable by immunohistochemistry and immunoblot4,5. We have shown that cortical infusion of recombinant Otx2 protein results in specific uptake by FSPV cells, while injection in the retina results in its transport along the visual pathway and into these same cells4. Blocking extracellular Otx2 through infusion of antibodies or specific peptides reduces uptake of endogenous Otx2 in FSPV cells4,6. Furthermore, we recently showed that the choroid plexus expresses Otx2 and secretes it into the cerebrospinal fluid5. Conditional knockdown of Otx2 expression in the choroid plexus results in reduced cortical levels of Otx2 protein. Needless to say, all of these approaches that alter Otx2 protein levels in the visual cortex have resulted in changes in cortical plasticity timing7.
An outstanding question is whether cortical Otx1 homeoprotein also plays a role in the critical period. Indeed, Otx1 is expressed in the cerebral cortex during development and continues to be expressed by layer V neurons in the adult8. It is thus possible that it is secreted by layer V cells and transferred into above granular and supragranular layers where Otx2 protein is detected. Unfortunately, most antibodies (commercial and academic) for Otx1 and Otx2 are pan-Otx thereby ruling out immunohistochemical approaches for conclusive evidence. Since genetic manipulation of the Otx2 locus has resulted in reduced protein in layer IV visual cortex7, we sought confirmation whether Otx1 is also present in these layers in the adult by using Otx1 knockout mice and proteomic approaches.
Methods
Animals
All experiments were conducted in accordance with European Union Council Directives (86/609/EEC) and conform to Directive 2010/63/EU of the European Parliament. This study falls under project #00704.02 authorized by the French Ministry of Research. Adult male C57Bl/6J mice (Janvier) aged 2 to 3 months were used throughout this study and euthanized by cervical dislocation. Otx1 knockout mice, in which Otx1 is replaced with lacZ gene, have been previously described14. Adult (~3 months) male Otx1LacZ/LacZ mice were fixed by intracardiac perfusion.
Quantitative RT-PCR
Total RNA from frozen tissue samples were extracted by using RNeasy Mini kits (Qiagen) and were reverse transcribed (~400 ng) with Superscript II and oligo-dT primers (Invitrogen). For real-time PCR, samples were analyzed with a LightCycler Instrument (Roche) and SYBR green (Sigma) detection with the following primers:
Otx2-forward ATTCACGTTTCATGACCAACAG;
Otx2-reverse ATTGACTCCGTATGAGCGGTAT;
Otx1-forward GAACCTTCCTTCTCCGAAATCT;
Otx1-reverse GATCTTCACATCGGACAAATCA;
GAPDH-forward TGACGTGCCGCCTGGAGAAAC;
GAPDH-reverse CCGGCATCGAAGGTGGAAGAG.
The experiment was performed in duplicate. For calculating fold expression, gene-to-GAPDH ratios were determined by using the 2-∆∆Ct method referenced to Otx1 expression in the lateral geniculate nucleus (LGN).
Immunoprecipitation and immunoblots
Dissected tissue was lysed in 100 mM Tris-HCl, pH 7.4, 10 mM EDTA, 150 mM NaCl, 1% Triton X-100, and protease inhibitor (Roche) then triturated (22G and 26G needles). Lysates were centrifuged at 16,000 × g for 10 min at 4°C and supernatants were incubated either directly with antibodies or with antibody-coupled Dynabeads (Life Technologies) at 4°C for 16 h. For samples with uncoupled antibodies, Protein A Dynabeads (Life Technologies) were added and incubated at 4°C for 1 h. Proteins were eluted with 2X SDS sample buffer after five washes with lysis buffer. Antibodies were rabbit polycolonal IgG (Abcam ab27478) and anti-Otx2 (rabbit polyclonal, Abcam ab21990) and were coupled to Dynabeads according to manufacturer instructions (Life Technologies). For immunoblots, samples were separated on NuPAGE 4%–12% Bis-Tris precast gels (Invitrogen) and transferred onto PVDF membrane. Membranes were incubated overnight at 4°C with anti-Otx2 (rabbit polyclonal, 1/1,000, Abcam ab21990) and then with HRP-coupled anti-rabbit IgG (1/2,000, GE Healthcare NA934) 1 h at RT.
Immunohistochemistry
For immunostaining, 50 µm floating sections were incubated with anti-Otx2 (rat polyclonal, 1/200, in-house) and biotinylated-WFA (1/100, Sigma L1516) in TBS, 1% Triton-X, 0.2% Tween-20, 10% fetal calf serum, overnight at 4°C. Sections were extensively washed at RT, incubated 2 h at RT with anti-rat Alexa Fluor-488 (Molecular Probes A21208, 1/2,000) and streptavidin Alexa Fluor-546 (Molecular Probes S11225, 1/2,000), washed again and finally mounted in Fluoromount medium (SouthernBiotech). Images were acquired with an Eclipse 90i microscope (Nikon).
Mass spectrometry analysis
After immunoprecipitation, proteins were separated on SDS–PAGE gels (Invitrogen) and stained with colloidal blue staining (LabSafe GEL BlueTM GBiosciences). Gel slices were excised and proteins were reduced with 10 mM DTT prior to alkylation with 55 mM iodoacetamide. After washing and shrinking the gel pieces with 100% MeCN, in-gel digestion was performed using trypsin (Promega) overnight in 25 mM NH4HCO3 at 30°C.
Peptides were extracted and analyzed by nano-LC-MS/MS using an Ultimate 3000 system (Dionex S.A.) coupled to an Orbitrap Fusion mass spectrometer (Q-OT-qIT, Thermo Fisher Scientific). Samples were loaded on a C18 precolumn (300 µm inner diameter × 5 mm; Dionex) at 20 µl/min in 5% MeCN, 0.1% TFA. After a desalting for 3 min, the precolumn was switched on the C18 column (75 μm i.d. × 50 cm, packed with C18 PepMap™, 3 μm, 100 Å; LC Packings) equilibrated in solvent A (2% MeCN, 0.1% HCO2H). Bound peptides were eluted using a 150 min linear gradient (from 5 to 30% (v/v)) of solvent B (80% MeCN, 0.085% HCO2H) at a 150 nl/min flow rate and an oven temperature of 40°C. We acquired Survey MS scans in the Orbitrap on the 400–1500 m/z range with the resolution set to a value of 240,000 and a 4 × 105 ion count target. Each scan was recalibrated in real time by co-injecting an internal standard from ambient air into the C-trap. Tandem MS was performed by isolation at 1.6 Th with the quadrupole, HCD fragmentation with normalized collision energy of 35, and rapid scan MS analysis in the ion trap. The MS2 ion count target was set to 104 and the max injection time was 200 ms. Only those precursors with charge state 2–7 were sampled for MS2. The dynamic exclusion duration was set to 60 s with a 10 ppm tolerance around the selected precursor and its isotopes. The instrument was run in top speed mode with 3 s cycles.
Data were acquired using the Xcalibur software (v 3.0.63) and the resulting spectra were interrogated by the MascotTM Software through Proteome Discoverer (v 1.4.0.288, Thermo Scientific) with the SwissProt Mus musculus database (20140402, 16,671 sequences). We set carbamidomethyle cysteine, oxidation of methionine and N-terminal acetylation as variable modifications. We set specificity of trypsin digestion and allowed 2 missed cleavage sites and we set the mass tolerances in MS and MS/MS to 2 ppm and 0.5 Da, respectively. The resulting Mascot files were further processed by using myProMS (v 3.0)15 and the estimated false discovery rate (FDR) by automatically filtering the Mascot score of all peptide identifications was less than 0.5%.
Results
Dataset 1.Quantitative PCR and mass spectrometry data of Otx2 homeoprotein in the mouse visual cortex.
qPCR data: This excel file (“brain qpcr otx data”) contains two worksheets. One worksheet has the Ct values for GAPDH, Otx2 and Otx1 obtained from extracts of different brain structures (LGN, Visual Cortex, Superior Colliculus and Cerebellum). Also included are the delta-Ct calculations. The second sheet contains the normalisation calculations and corresponding graph of expression values.
Mass spectroscoy data: This Mascot .dat file (“F022130_part.dat”) contains the experiment description and the data pertaining to the 2 peptides that match Otx2 protein.Otx1 locus but not Otx2 locus is active in the mouse visual cortex
We compared the expression of the Otx1 and Otx2 loci of various brain regions by performing quantitative PCR (Figure 1A). While both mRNA were detected in thalamic and cerebellar structures, only Otx1 mRNA was found in the visual cortex. This result confirms the previously reported absence of GFP expression in the visual cortex of Otx2+/GFP knockout mice and lack of signal in the visual cortex of wild type mice after Otx2 in situ hybridization, even though Otx2 antibodies label FSPV cells4. These cells are enwrapped by a dense extracellular matrix called perineuronal nets (PNNs) when localized to layer IV of the cortex (Figure 1B). Otx1 locus is active in layer V8. In immunohistochemical analysis of Otx1 knockout mouse, almost all Otx signal is lost in layer V while signal from Otx2 protein continues in PNN-labeled cells (Figure 1B). However, we cannot preclude that some Otx1 is also present in layer IV cells in wild type mice.
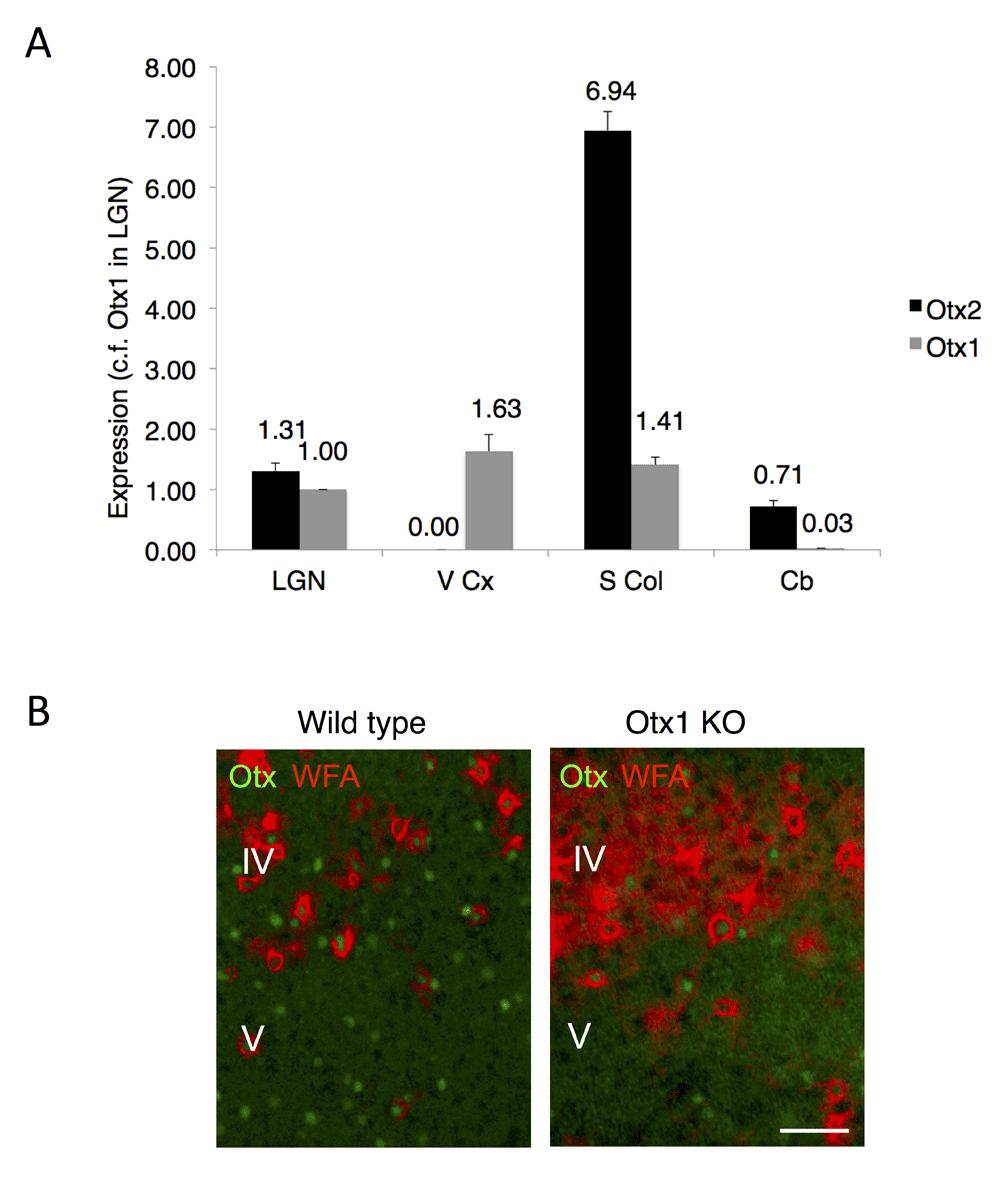
Figure 1. Expression of Otx1 and Otx2 in adult mouse brain.
(A) Analysis of Otx1 and Otx2 expression by quantitative RT-PCR on extracts from lateral geniculate nucleus (LGN), visual cortex (V Cx), superior colliculus (S Col) and cerebellum (Cb). The fold-difference in expression is calculated relative to Otx1 in LGN. The Otx2 locus is silent in visual cortex. (B) Non-cell autonomous Otx2 is found in visual cortex. Immunostaining in wild type mice reveals Otx1/2 cells in layers IV and V of visual cortex, including cells with perineuronal nets (stained by WFA lectin) enriched in layer IV. Staining for Otx2 persists in Otx1 null mice (Otx1 KO). Scale bar, 50 µm.
Otx2 protein but not Otx1 protein is found in granular and supragranular layers of visual cortex
In order to analyze Otx homeoprotein distribution in the visual cortex, we turned to immunoprecipitation (IP) experiments. We first performed IP on whole visual cortex extracts, which showed both Otx1 and Otx2 protein (Figure 2A). This result is confirmed by IP of choroid plexus, which strongly expresses Otx2 but only very weakly expresses Otx1. To analyze granular and supragranular content, we dissected and extracted the superior layers of posterior adult mouse cortex (Figure 2B) and performed IP by using cross-linked magnetic beads. Immunoblot analysis detected only Otx2 but not Otx1 protein (Figure 2C). This result was confirmed by mass spectrometry analysis on these extracts, which identified 2 Otx2 peptides (6.6% coverage with 100% specificity, Figure 2D). While this number of peptides is low, it was expected given that Otx2 is predicted to be poorly ionizable. Furthermore, identification has an error of 1 protein in 20,000 (19,999 are true) with a FDR of less than 0.5% for 2 peptides in our database of 16,671 sequences, thus the chance of misinterpretation is very low. These peptides are 100% specific for Otx2 and they have the same ions as the reference spectrum from a sample of purified Otx2 protein. While the second peptide is also specific for Otx1 and Crx homeoproteins, the first peptide is unique for Otx2 (Figure 2D). These results confirm that the homeoprotein localized in granular and supragranular FSPV cells is indeed Otx2 and not Otx1.
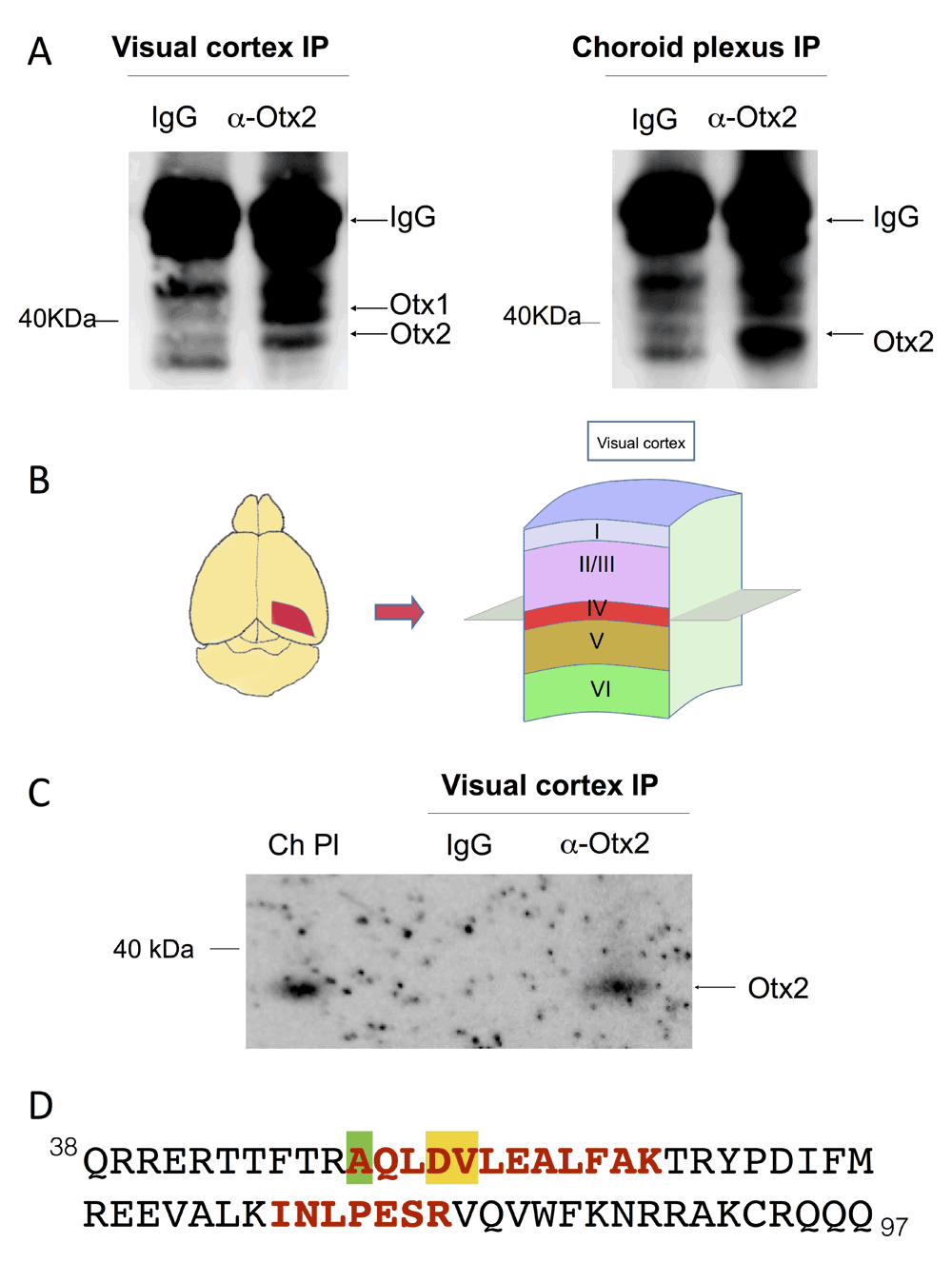
Figure 2. Otx2 protein in the granular and supragranular layers of adult mouse visual cortex.
(A) Immunoblots for Otx1/2 of immunoprecipitation (IP) experiments on extracts from visual cortex and choroid plexus. (B) Diagram of finely dissected region for extracts containing granular (IV) and supragranular (I-III) layers of visual cortex. (C) Immunoblot for Otx1/2 of samples from IP using cross-linked magnetic beads and finely dissected extracts. Choroid plexus (Ch Pl) extract was used to control for Otx2 migration velocity. (D) The peptides (red, bold) matching Otx2 protein identified by high-resolution mass spectrometry. Only the homeodomain sequence of Otx2 (amino acids 38–97) is shown. The amino acid differing in Otx1 is highlighted in green, while amino acids that differ in Crx are highlighted in green and in yellow.
Discussion
The non-cell autonomous activity of homeoprotein transcription factors is now well established. There are clear phenotypes with recently developed in vivo single-chain secreted antibodies that neutralize extracellular homeoproteins yet leave intact cell autonomous activities9–11. Non-autonomy can also be demonstrated by comparing mRNA and protein expression. Indeed, the absence of mRNA in presence of the protein argues in favor of non-cell autonomy. However, when the receiving territory is a short distance from the producing territory, one could invoke the possibility of cell migration or mRNA instability to bring into question the reality of homeoprotein transfer.
In the visual system, Otx2 protein is found in the visual cortex far from two potential sources of Otx2 (where the Otx2 locus is active), namely the eye and the choroid plexus5,12. Indeed, the Otx2 locus is not active in the adult cerebral cortex as verified by using the Otx2+/GFP mouse, quantitative RT-PCR and in situ hybridization4. In addition, conditional Otx2 ablation in the choroid plexus reduces its content in FSPV cells, further supporting non-cell autonomy5.
However, since most Otx1 and Otx2 antibodies are pan-Otx antibodies, it was still conceivable that some of the protein seen in FSPV cells by immunohistochemistry could correspond to Otx1 expressed in layer V of the cerebral cortex and transferred into PV cells. The present study shows that the staining in layer IV is maintained in the Otx1 knockout mouse and that IP experiments of layers I-IV give immunoblot bands with expected Otx2 size and can be used to identified Otx2 by mass spectrometry. These results confirm that FSPV cells in granular and supragranular layers of the cerebral cortex only contain non-cell autonomous Otx2 and do not contain Otx1.
It may seem surprising that Otx1 expressed in layer V is not secreted and internalized by FSPV cells. Indeed the protein presents a homeodomain nearly identical to that of Otx2 and thus contains the two sequences necessary for internalization and secretion (for review see7). However, previous studies have demonstrated that homeoproteins are transported from the basolateral to the apical side of polarized cells and thus into the axon7,13. Given their polarity and orientation, the pyramidal cells of layer V that express Otx1 are thus very unlikely to release it at the level of FSPV cells. In contrast, the choroid plexus epithelial cells present their apical surface toward the ventricles allowing Otx2 secretion into the cerebral spinal fluid. In conclusion, this study demonstrates that Otx2 is the only non-cell autonomous Otx family protein in the granular and supragranular FSPV cells.
Data availability
F1000Research: Dataset 1. Quantitative PCR and mass spectrometry data of Otx2 homeoprotein in the mouse visual cortex, 10.5256/f1000research.4869.d3338416
Author contributions
NK and AAD carried out experimental work
DA and AS made Otx1 knockout mice
DF carried out the mass spectroscopy experimental work
LD supervised mass spectroscopy and proteomic data analysis
AP and AAD conceived the ideas of the study, designed protocols, and drafted the manuscript
All authors read, critically revised, and approved the final manuscript
Competing interests
No competing interests were disclosed.
Grant information
This work was supported by the Région Ile-de-France, the FRM, GRL Program n°2009-00424, ANR grant BRAINEVER n° 11-BLAN-069467, and ERC Advanced Grant HOMEOSIGN n°339379.
Faculty Opinions recommendedReferences
- 1.
Hensch TK:
Critical period plasticity in local cortical circuits.
Nat Rev Neurosci.
2005; 6(11): 877–88. PubMed Abstract
| Publisher Full Text
- 2.
Morishita H, Hensch TK:
Critical period revisited: impact on vision.
Curr Opin Neurobiol.
2008; 18(1): 101–7. PubMed Abstract
| Publisher Full Text
- 3.
Hensch TK, Fagiolini M:
Excitatory-inhibitory balance and critical period plasticity in developing visual cortex.
Prog Brain Res.
2005; 147: 115–24. PubMed Abstract
| Publisher Full Text
- 4.
Sugiyama S, Di Nardo AA, Aizawa S, et al.:
Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity.
Cell.
2008; 134(3): 508–20. PubMed Abstract
| Publisher Full Text
- 5.
Spatazza J, Lee HHC, Di Nardo AA, et al.:
Choroid-plexus-derived Otx2 homeoprotein constrains adult cortical plasticity.
Cell Rep.
2013; 3(6): 1815–23. PubMed Abstract
| Publisher Full Text
- 6.
Beurdeley M, Spatazza J, Lee HHC, et al.:
Otx2 binding to perineuronal nets persistently regulates plasticity in the mature visual cortex.
J Neurosci.
2012; 32(27): 9429–37. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 7.
Spatazza J, Di Lullo E, Joliot A, et al.:
Homeoprotein signaling in development, health, and disease: a shaking of dogmas offers challenges and promises from bench to bed.
Pharmacol Rev.
2013; 65(1): 90–104. PubMed Abstract
| Publisher Full Text
- 8.
Frantz GD, Weimann JM, Levin ME, et al.:
Otx1 and Otx2 define layers and regions in developing cerebral cortex and cerebellum.
J Neurosci.
1994; 14(10): 5725–40. PubMed Abstract
- 9.
Lesaffre B, Joliot A, Prochiantz A, et al.:
Direct non-cell autonomous Pax6 activity regulates eye development in the zebrafish.
Neural Dev.
2007; 2: 2. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 10.
Wizenmann A, Brunet I, Lam JSY, et al.:
Extracellular Engrailed participates in the topographic guidance of retinal axons in vivo.
Neuron.
2009; 64(3): 355–66. PubMed Abstract
| Publisher Full Text
- 11.
Layalle S, Volovitch M, Mugat B, et al.:
Engrailed homeoprotein acts as a signaling molecule in the developing fly.
Development.
2011; 138(11): 2315–23. PubMed Abstract
| Publisher Full Text
- 12.
Sugiyama S, Prochiantz A, Hensch TK:
From brain formation to plasticity: insights on Otx2 homeoprotein.
Dev Growth Differ.
2009; 51(3): 369–77. PubMed Abstract
| Publisher Full Text
- 13.
Dupont E, Prochiantz A, Joliot A:
Identification of a signal peptide for unconventional secretion.
J Biol Chem.
2007; 282(12): 8994–9000. PubMed Abstract
| Publisher Full Text
- 14.
Acampora D, Mazan S, Avantaggiato V, et al.:
Epilepsy and brain abnormalities in mice lacking the Otx1 gene.
Nat Genet.
1996; 14(2): 218–22. PubMed Abstract
| Publisher Full Text
- 15.
Poullet P, Carpentier S, Barillot E:
myProMS, a web server for management and validation of mass spectrometry-based proteomic data.
Proteomics.
2007; 7(15): 2553–6. PubMed Abstract
| Publisher Full Text
- 16.
Kim N, Acampora D, Dingli F, et al.:
Quantitative PCR and mass spectrometry data of Otx2 homeoprotein in the mouse visual cortex.
F1000Research.
2014. Data Source


We are well aware that the rat antibody recognizes both Otx1 and Otx2 (as it was produced in our laboratory ... Continue reading We thank Pierre Godement for his insightful reading of our article.
We are well aware that the rat antibody recognizes both Otx1 and Otx2 (as it was produced in our laboratory and kindly provided to Pierre Godement through Marion Wassef). Indeed, the Otx1 knockout mouse contains a truncated form of Otx1. In a previous study to which Pierre Godement makes reference, protein staining with an Otx1-specific antibody is extremely faint and non-nuclear throughout all cortical layers of the homozygous Otx1 knockout mouse (Weimann et al., 1999). While it is possible that our antibody also recognizes a truncated form of Otx1, Figure 1B clearly shows that staining in layer V (a bona fide site of Otx1 expression) is decreased in the knockout as compared to wild type. Furthermore, we continue to find nuclear staining in other cortical layers, contrary to what was found with the Otx1 antibody. This link contains the raw microscope images and includes an image of the heterozygous Otx1 mouse visual cortex, which shows similar staining to wild type. (Ratio of Otx cells in infragranular versus supragranular layers in these images is 1.43, 1.46, and 1.11 for WT, het, and homozygote mice, respectively.)
We have not stained for PV (since it was not necessary in the context of this paper) but have used the WFA lectin (that recognizes the PNNs surrounding PV-cells). Contrary to what Pierre Godement writes, we did not say in Sugiyama et al. (2008) that the Otx2 antibody stains "all parvalbumin positive" cells. Figure 1J and page 3 of the article clearly state that 71.4 +/- 0.5% of Otx2-positive cells are PV-positive and that 78.9 +/- 1.7% of the PV-positive cells are Otx2-positive.
One must indeed be cautious with pan-Otx antibodies; genetic evidence for non-cell autonomous cortical Otx2 is paramount. In Sugiyama et al. (2008), in addition to using 6 different Otx2 antibodies, we recombined Otx2 by crossing Otx2 floxed mouse with a CamKII-Cre mouse and lost Otx2 staining in PV-cells. Because CamKII is not expressed in PV-cells, this demonstrated the non-cell autonomous accumulation of Otx2. In addition, we have identified the choroid plexus as a source of Otx2. Recombining the Otx2 locus specifically in this structure reduced the amount of Otx2 in cortical PV-cells and reopened plasticity in the adult (Spatazza et al., 2013).
It is correct that immunoprecipitation (IP) gels show Otx2 with an apparent MW just under 40 kD. This is where Otx2 runs under our conditions (NuPAGE 4%-12%, MES buffer). This link contains the full western blots corresponding to Figures 2A and 2C. Along with the IPs from visual cortex and choroid plexus, the full blot corresponding to Figure 2A includes an extract from retina (a primary structure strongly expressing Otx2). The velocity for Otx2 is identical for all 3 structures. MW markers and samples from an IP with another pan-Otx antibody (Millipore AB9566) are also included.
No in situ studies have shown Otx1 mRNA expression above layer V in the cortex, but the presence of Otx1 protein due to transfer between the layers is a possibility. Our mass spectroscopy analysis of supgranular layer IPs found two peptides corresponding to Otx1 and Otx2, but also one peptide unique for Otx2 (Dataset 1 and Figure 2D). In Figure 2C (supragranular layers), we only find one band migrating with the Otx2 velocity and recognized by Otx antibodies (IP and western blot). It is based on this result that we conclude there is no Otx1 in these layers. The questions of Otx2 amount, location and provenance are not within the scope of this article.
Finally, Pierre Godement raises two additional points. First is that he has made plasticity-related observations concerning axon guidance based on the regulation of extracellular matrix protein expression by Otx2 (Nguyen Ba-Charvet et al., 1998). We agree with this potential impact on cortical plasticity and indeed cited this work in Sugiyama et al. (2008).
Second, Pierre Godement proposes that it is Otx1 that regulates the termination of the critical period and not Otx2. We do not exclude that Otx1 and many other proteins participate in critical period regulation but we maintain that Otx2 is a primary factor in the regulation of postnatal and adult cortical plasticity. Whether Otx1 is involved still awaits demonstration.
In conclusion, we sincerely thank Pierre Godement for giving us the opportunity to discuss some points that may not have been explained with enough clarity.
We are well aware that the rat antibody recognizes both Otx1 and Otx2 (as it was produced in our laboratory and kindly provided to Pierre Godement through Marion Wassef). Indeed, the Otx1 knockout mouse contains a truncated form of Otx1. In a previous study to which Pierre Godement makes reference, protein staining with an Otx1-specific antibody is extremely faint and non-nuclear throughout all cortical layers of the homozygous Otx1 knockout mouse (Weimann et al., 1999). While it is possible that our antibody also recognizes a truncated form of Otx1, Figure 1B clearly shows that staining in layer V (a bona fide site of Otx1 expression) is decreased in the knockout as compared to wild type. Furthermore, we continue to find nuclear staining in other cortical layers, contrary to what was found with the Otx1 antibody. This link contains the raw microscope images and includes an image of the heterozygous Otx1 mouse visual cortex, which shows similar staining to wild type. (Ratio of Otx cells in infragranular versus supragranular layers in these images is 1.43, 1.46, and 1.11 for WT, het, and homozygote mice, respectively.)
We have not stained for PV (since it was not necessary in the context of this paper) but have used the WFA lectin (that recognizes the PNNs surrounding PV-cells). Contrary to what Pierre Godement writes, we did not say in Sugiyama et al. (2008) that the Otx2 antibody stains "all parvalbumin positive" cells. Figure 1J and page 3 of the article clearly state that 71.4 +/- 0.5% of Otx2-positive cells are PV-positive and that 78.9 +/- 1.7% of the PV-positive cells are Otx2-positive.
One must indeed be cautious with pan-Otx antibodies; genetic evidence for non-cell autonomous cortical Otx2 is paramount. In Sugiyama et al. (2008), in addition to using 6 different Otx2 antibodies, we recombined Otx2 by crossing Otx2 floxed mouse with a CamKII-Cre mouse and lost Otx2 staining in PV-cells. Because CamKII is not expressed in PV-cells, this demonstrated the non-cell autonomous accumulation of Otx2. In addition, we have identified the choroid plexus as a source of Otx2. Recombining the Otx2 locus specifically in this structure reduced the amount of Otx2 in cortical PV-cells and reopened plasticity in the adult (Spatazza et al., 2013).
It is correct that immunoprecipitation (IP) gels show Otx2 with an apparent MW just under 40 kD. This is where Otx2 runs under our conditions (NuPAGE 4%-12%, MES buffer). This link contains the full western blots corresponding to Figures 2A and 2C. Along with the IPs from visual cortex and choroid plexus, the full blot corresponding to Figure 2A includes an extract from retina (a primary structure strongly expressing Otx2). The velocity for Otx2 is identical for all 3 structures. MW markers and samples from an IP with another pan-Otx antibody (Millipore AB9566) are also included.
No in situ studies have shown Otx1 mRNA expression above layer V in the cortex, but the presence of Otx1 protein due to transfer between the layers is a possibility. Our mass spectroscopy analysis of supgranular layer IPs found two peptides corresponding to Otx1 and Otx2, but also one peptide unique for Otx2 (Dataset 1 and Figure 2D). In Figure 2C (supragranular layers), we only find one band migrating with the Otx2 velocity and recognized by Otx antibodies (IP and western blot). It is based on this result that we conclude there is no Otx1 in these layers. The questions of Otx2 amount, location and provenance are not within the scope of this article.
Finally, Pierre Godement raises two additional points. First is that he has made plasticity-related observations concerning axon guidance based on the regulation of extracellular matrix protein expression by Otx2 (Nguyen Ba-Charvet et al., 1998). We agree with this potential impact on cortical plasticity and indeed cited this work in Sugiyama et al. (2008).
Second, Pierre Godement proposes that it is Otx1 that regulates the termination of the critical period and not Otx2. We do not exclude that Otx1 and many other proteins participate in critical period regulation but we maintain that Otx2 is a primary factor in the regulation of postnatal and adult cortical plasticity. Whether Otx1 is involved still awaits demonstration.
In conclusion, we sincerely thank Pierre Godement for giving us the opportunity to discuss some points that may not have been explained with enough clarity.