Introduction
The degradation of proteins, in particular of those that are damaged or are present in excess, is an important vital function of biological systems and is implicated in several cellular processes such as cell cycle control, proliferation, differentiation, apoptosis and protein quality control1. Impairments in protein degradation lead to the formation of protein aggregates2,3, promote the aging process4,5 and convey the development of neurodegenerative diseases like Alzheimer’s or Parkinson’s disease6. There are two major pathways involved in protein degradation: autophagy and degradation by the ubiquitin proteasome system (UPS)7. Autophagy is effective in nutrient recycling and protein degradation. During autophagy proteins or whole organelles are engulfed by a double membrane forming autophagosomes that deliver their cargo to the lysosome in animals and the vacuole in plants and fungi8. The UPS consists of a large number of different ubiquitin ligases that act jointly with the proteasome, a multi-protein complex with proteolytic activities. The ubiquitin ligases identify and mark proteins that need to be removed, by formation of a chain of ubiquitin on the target protein9. A ubiquitin chain linked at K-48 is recognized by the 26S proteasome10. The 26S proteasome consists of two subcomplexes, the catalytic 20S core particle and the 19S regulatory particle. The 19S regulatory particle conveys the identification, deubiquitination, unfolding and transport of the substrate into the proteolytic chamber. The core particle is responsible for the degradation of the target proteins. It is composed of four stacked rings, which enclose the proteolytic chamber. The inner rings consist of 7 β-subunits, including the proteolytic active PRE3 (β1), PUP1 (β2) and PRE2 (β5). The three catalytic subunits are the first substrates of the proteasome. Each contains a prosequence that is removed during assembly of the proteasome by an autocatalytic mechanism11,12. The assembled β-subunits are framed by rings of seven α-subunits, blocking the entrance to the proteolytic chamber, if no regulatory particle is bound (reviewed in:13).
Previous studies revealed that aging reduces the expression of genes coding for proteasome subunits and the activity of the proteasome in several model systems14–16. Also, several studies indicate a health and lifespan prolonging effects of high proteasome activity. For example, the proteasome activity is elevated in human fibroblast cell cultures derived from centenarians14 and in the liver of the naked mole rat17,18, a long-living rodent. Moreover, the overexpression of genes coding for proteasome subunit β1 or β5 in human fibroblasts was reported to lead to an increase in overall proteasome abundance and activity, resulting in an increased capacity to cope with stress19. Another component influencing proteasome activity is the proteasome assembly protein UMP1. Saccharomyces cerevisiae overexpressing ScUmp1 shows increased lifespan and viability in response to oxidative stress20. In S. cerevisiae, high levels of proteasome subunit ScRPN4 were reported to increase UPS capacity, enhance resistance to proteotoxic stress and increase replicative lifespan21. Overall, it appears that the proteasome is a relevant target for aging research. The data suggest that keeping protease activity high during aging can lead to an increase in the healthy lifespan of biological systems.
We use the filamentous ascomycete Podospora anserina as a model organism to investigate the mechanisms of aging including the role of different quality control pathways (for recent reviews see:22–24). In this study we investigated the impact of protein degradation by the UPS and autophagy. Although we could not demonstrate a role of the UPS, we established that the degradation of GFP-CL1 protein, that was expected to be a target of the proteasome, occurred via autophagy.
Materials and methods
P. anserina strains and cultivation
P. anserina was grown on plates with M2 medium (0.25 g/l KH2PO4 (Merck Cat# 5099.1000), 0.3 g/l K2HPO4 (Roth Cat# P749.1), 0.25 g/l MgSO4 × 7 H2O (Merck Cat# 1.05886.0500), 0.5 g/l urea (Merck Cat# 1.08487.0500) and 10 g/l yellow dextrin (Roth Cat# 6777.1), supplemented with 2.5 mg/l biotin (Serva Cat# 15060), 50 mg/l thiamine (Serva Cat# 36020), 5 mg/l citric acid × 1 H2O (Sigma-Aldrich Cat# C-0759), 5 mg/l ZnSO4 × 7 H2O (Merck Cat# Z-0625), 1 mg/l Fe(NH4)2(SO4)2 × 6 H2O (Merck Cat# 1.03861.0250), 2.5 mg/l CuSO4 × 5 H2O (Merck Cat# 2790.1000), 25 mg/l MnSO4 × 1 H2O (Serva Cat# 28405), 50 mg/l Na2MoO4 × 2 H2O (Serva Cat# 30207) and 50 mg/l H3BO3 (Merck Cat# 100165.5000) after sterilization of the basal medium) or in shaking Erlenmeyer flasks with CM-Medium (70 mM NH4Cl (Merck Cat# 1.01145.5000), 7.3 mM KH2PO4, (Merck Cat#1.04873.100), 6.7 mM KCl (Merck Cat# 1.04936.1000), 2 mM MgSO4 (Merck Cat# 1.05886.0500), 1% glucose (Sigma Cat# G-5400), 0.2% tryptone (Otto Nordwald Cat# 211701), 0.2% yeast extract (DIFCO Cat# 0127-07), 5 mM FeCl2 × 7 H2O (Merck Cat# 13478-10-9), 3.5 mM ZnSO4, (Merck Cat# 108883), 6.2 mM MnCl2, (Merck Cat# 5934.0100), pH 6.5) under constant light at 27°C. For germination, spores were incubated for two days in the dark on standard cornmeal agar supplemented with 60 mM ammonium acetate (Merck Cat# 1116.1000)25. Pieces of the mycelium derived from germinated spores were transferred on M2 medium to obtain cultures of specific age.
Quantitative Real-time PCR (qRT-PCR)
After germination of spores, pieces of the mycelium were directly used or grown at 27° and constant light on M2 medium for 13 – 16 days (middle-aged) or 21 – 24 days (senescent), depending on the lifespan of the specific individual, to obtain cultures of specific age. A piece of the growth front was subsequently spread on a fresh M2 plate covered with cellophane (BioRad Cat# 1650963) and grown for 3 days. RNA was extracted with RNA-Plant kit (Machery-Nagel Cat# 740.949.250) and cDNA synthesis was performed using iScript kit (BioRad Cat# 170-8891). After dilution of cDNA to a concentration of 10 ng/µl, 20 ng was used per qRT-PCR reaction (IQ SybrGreen SuperMix, BioRad cat# 170-8882). The primers summarized in Table 1 were used to perform the qRT-PCR with three technical replicates per sample. A specific culture was compared to the mean CP of the juvenile cultures. Relative expression was normalized to PaPorin with the following formula26.
E = PCR-Efficiency; CP = crossing point
Table 1. Primers used for qRT-PCR.
| PaNo. | Gene | Primer | Sequence |
|---|
| Pa_5_4560 | PaPre3 | Pre3 for | ATGGAATTCGGTACATCGGG |
| | Pre3 rev | GAGGATAACGCCGTCTTTGA |
| Pa_1_12250 | PaPre2 | rTPre2for | TTGTTCACAGAGCAGGAGCAG |
| | rTPre2rev | CGATCTTGATGGGGCAGT |
| Pa_1_24350 | PaUmp1 | ump1for | TCAACCGAACTCCGATACTC |
| | ump1rev2 | TGGCCTCCCACTGTTTTAAG |
Western blot analysis
To obtain total protein extracts, fungi of specific age were spread on a cellophane foil covered M2 surface for 3 days. Proteins were extracted as described in24. Briefly, the mycelia were harvested, ground under liquid nitrogen, mixed with extraction buffer and centrifuged at 14,000 g at 4°C for 10 min. The supernatant was recovered and used for the experiments. The protein extracts were fractionated by 2-phase SDS-PAGE (14% separating gels) according to standard protocol27. Proteins were subsequently transferred to PVDF membranes (Millipore Cat# IPFL00010). Blocking, antibody incubation and washing was performed according to western blot analysis handbook (LIC-OR Bioscience, Bad Homburg, Germany). The following primary antibodies were used: anti-PaPRE2 (rabbit, 1:500 dilution, raised against the specific peptide [H]-WKTKLEKGEFSNVT-[OH]; Sigma), anti-PaPRE3 (1:2500 dilution, raised against the specific synthetic peptide [H]-LYLPDTDYKVRHEN-[OH]; Sigma), anti-PaPUP1 (rabbit, 1:5000 dilution, raised against the specific peptide Ac-CLKRNYIKPNERT-amid, NEP), anti-HSP60 (mouse, 1:4000 dilution, Biomol, Cat# SPA-807), Anti-GFP (mouse, 1:10000 dilution, Sigma-Aldrich Cat# G6795 RRID:AB_563117). Secondary antibodies conjugated with IRDye 680 (1:15000 dilution, goat anti-mouse 680RD: LI-COR Biosciences Cat# 926-68070 RRID:AB_10956588) or IRDye CW 800 (1:15000 dilution, goat anti-rabbit 800: LIC-OR Biosciences, Cat# 926-3221) were used. After western transfer, the polyacrylamid gels were stained 1 h with coomassie blue as additional loading control.
Cloning procedures and generation of P. anserina mutants
The vector pExMthph28 was used as backbone for the generation of PaPre2, PaPre3 and PaUmp1 overexpression plasmids. For the assembly of pPaPre2Ex1, pExMthph was cut with BamHI and XbaI. The PaPre2 gene and terminator were amplified with the primers Pre2ExpFor (AAGGATCCATGGACACCCTCGTTGCG; restriction sites are underlined) and Pre2ExpXbaRev (AAAGATCTTGGCCCTCCTTACTAGAC), cut with BamHI and XbaI and ligated with the backbone. For the generation of pPaPre3Ex1, the PaPre3 gene and terminator were amplified with the primers PaPre3FwdBam (TTGGATCCATGGAATTCGGTACATCGGG) and PaPre3RevPst (TTCTGCAGCCCACAACCAGAACCTTTCAC) cut with BamHI and PstI and ligated with the similarly restricted vector pExMthph. pPaUmp1Ex1 was generated by amplification of PaUmp1, including the terminator, with the primers PaUmp1FwdBamHI (TTGGATCCATGGTAAGTTGCAGCCAACC) and PaUmp1RevPst1 (TTCTGCAGGCTCCCGTGAGGGCAGGAC), restriction of the product with BamHI and PstI, and ligation into the similarly cut vector pExMthph. The generation of a Gfp-Cl1 overexpression plasmid was performed by 3 fragment ligation. The Gpd promotor from Aspergillus nidulans, the eGfp gene and the first part of the Cl1-sequence were amplified by PCR with the plasmid pSM5 (based on pSM229) as template and with primers eGfp-Pgpd-cl1for (CCTCGAGGTCGACGGTATCGATAAGCTTGATATCGAATT) containing a HindIII restriction site and eGfp-Pgpd-cl1rev (GTGGCTAGC GCTGCTGAACCAGTTCTTGCAGGC CTTGTACAGCTCGTCCAT), containing half of the Cl1 sequence (italic) optimized for codon usage of P. anserina and a Eco47III restriction site. The second half of the Cl1-sequence was amplified with the TrpC terminator in a similar manner with the primers Ttrpc-cl1for (CTTCAGCGAG CTCAGCCACTTCGTCATCCACCTCTA ATCCACTTAACGTTACTGA) containing an Eco53kI restriction sites (underlined) and Ttrpc-cl1rev (CCACCGCGGTGGCGGCCGCTCTAGAAAGAAGGATTACCTC) containing a XbaI restriction site. The PCR products were ligated into the purified backbone of pSM5, previously cut with HindIII and XbaI. The plasmids were used to transform P. anserina wild-type spheroplasts according to24. pSM5 was used to generate a strain expressing Gfp without degron sequence. Briefly, mycelium of wild type “s” was blended and the cell wall digested with an enzyme solution. After filtration and concentration of sphaeroblasts by centrifugation, the sphaeroblasts were mixed with 10 µg plasmid DNA. Subsequently, polyethylene glycole (Serva Cat# 33136) was added to the sphaeroblasts. Transformants were selected for hygromycin B (Calbiochem Cat# 400051) resistance and the number of integrations was verified by Southern blot analysis.
Fluorescence microscopy
A piece of 2 day old mycelium was grown on a glass slide with a piece of PASM-medium27 covered with a coverslip and incubated at 27°C for 2 days. Heat stressed samples were incubated at 27°C for 24 h followed by an incubation at 37°C for 24 h. The cover slip with hyphae on it was visualized using a fluorescence microscope (DM LB, Leica, Wetzlar, Germany) with the appropriate excitation and emission filter to detect the GFP signal and a digital camera system (DC500, Leica, Wetzlar, Germany).
Results
Regulation of proteasome components during aging and oxidative stress
In order to address the role of the UPS on aging of P. anserina, we investigated the expression of the genes coding for proteolytic subunits PaPre2 (β5) and PaPre3 (β1) and of the proteasome assembly factor PaUmp1. First, we determined the abundance of transcript by using total RNA of juvenile, middle-aged and senescent cultures (Figure 1A). No significant changes in mRNA levels were observed in cultures of different age although mRNA abundance of all three genes was slightly reduced in senescent cultures. Next we analyzed protein levels of proteasome subunits in cultures of different age grown in standard growth medium and in medium to which paraquat was added as an inducer of oxidative stress. An increased abundance of mitochondrial HSP60 verified an increase in oxidative stress in senescent and in paraquat treated cultures (Figure 1B). However, no changes in the abundance of subunits PaPRE2, PaPRE3 and PaPUP1 (β3) of the proteasome were observed in the corresponding P. anserina cultures. We thus were unable to demonstrate a role of the ubiquitin proteasome system in counteracting adverse effects on cellular proteins in aged cultures and in cultures challenged with exogenous oxidative stress.
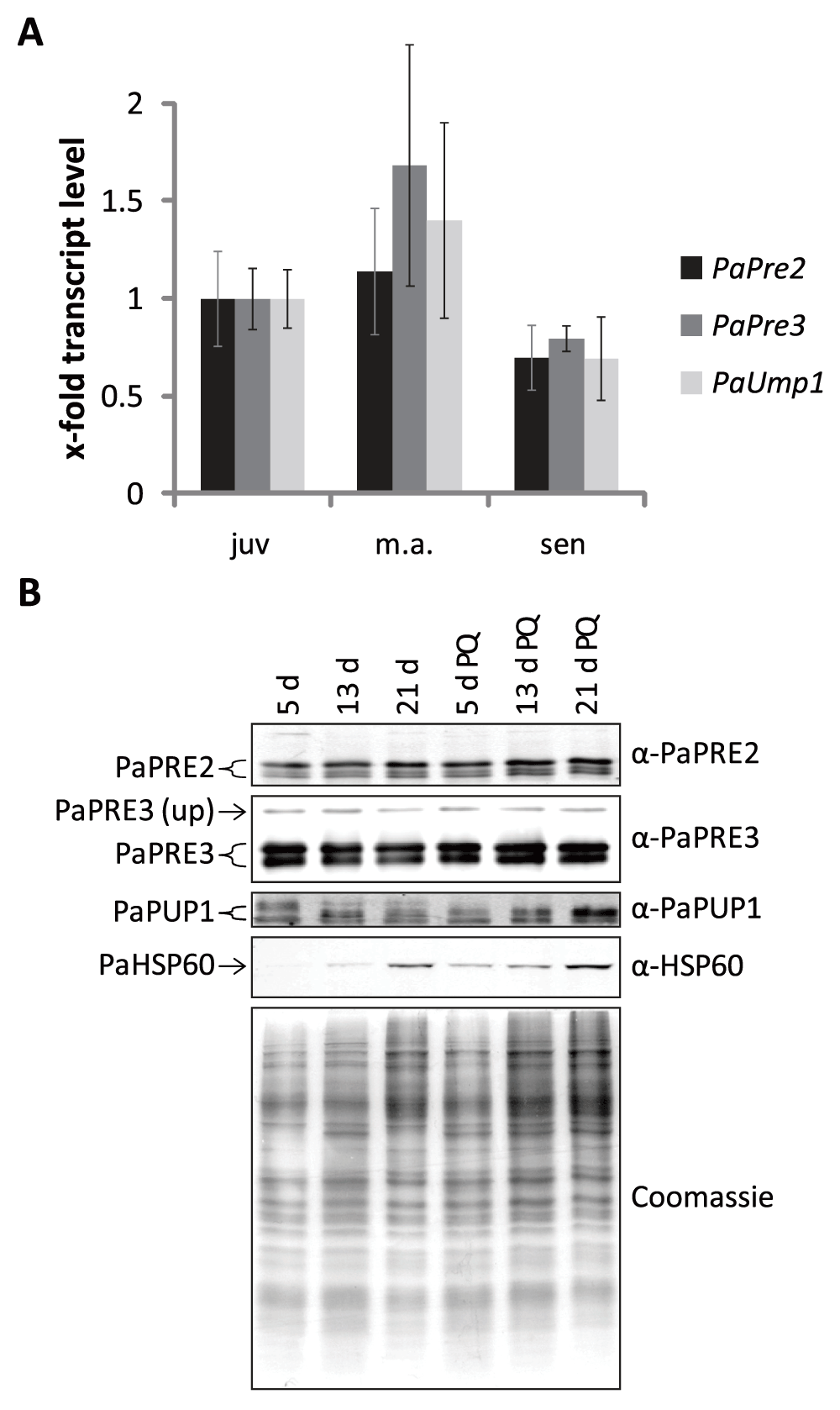
Figure 1. Gene expression and protein abundance of proteasome subunits during aging and PQ-stress.
(A) The expression of PaPre2, PaPre3 and PaUmp1 transcripts in juvenile, middle-aged and senescent samples is depicted relative to the juvenile wild type as mean ± SEM (5 – 7 biological replicates). (B) Western blot analysis of 50 µg total protein extracts of 5 d, 13 d and 21 d old wild type cultures grown on medium with and without the addition of 5 µM paraquat. Used antibodies are indicated on the right. The polyacrylamid gel stained with coomassie after blotting is used as loading control.
Overexpression of PaPre2 or PaPre3 does not increase the total amount of proteasome
High activity of the proteasome has been linked to increased health and lifespan30–33. In human cell cultures, the overexpression of the subunits β5 and β1 was found to increase the overall abundance of the proteasome, as well as its activity and resistance to oxidative stress34. To investigate whether or not such an effect is also observed in P. anserina, strains overexpressing the homolog subunits PaPre2 (β5) and PaPre3 (β1) were generated. First, plasmids conveying PaPre2 and PaPre3 overexpression were constructed and transformed into P. anserina spheroplasts (Table 2). Subsequently, overexpression of the genes was verified by qRT-PCR. PaPre3 expression was increased by factor 140 to 380 in the respective overexpression strain compared to wild type (Figure 2A). The PaPre2 overexpression strain exhibited a 94 times higher PaPre2 expression than the wild type (Figure 2B). In the next step, we evaluated protein levels in the overexpression strains by western blot immunodetection. The analysis of three independent PaPre3 overexpressors revealed two strains with unchanged protein abundance and one strain (PaPre3_OEx2) in which increased PaPRE3 signals occurred (Figure 2C). However, the detected signals are larger or smaller than expected for processed PaPRE3 and probably represent unprocessed PaPRE3 and a degradation product. A strain overexpressing PaPre2 showed no increase in PaPRE2 abundance compared to the wild type (Figure 2D). Thus, despite the strong increase in mRNA abundance, no substantial change in protein levels of the two investigated proteasome subunits was observed in the generated strains.
Table 2. Number of ectopic integrations.
| Strain | Number of
integrations |
|---|
| Gfp | 2 |
| Gfp-Cl1-1 | 1 |
| Gfp-Cl1-2 | 1 |
| PaPre2_OEx | 1 |
| PaPre3_OEx1 | 1 |
| PaPre3_OEx2 | 3 |
| PaPre3_OEx3 | 2 |
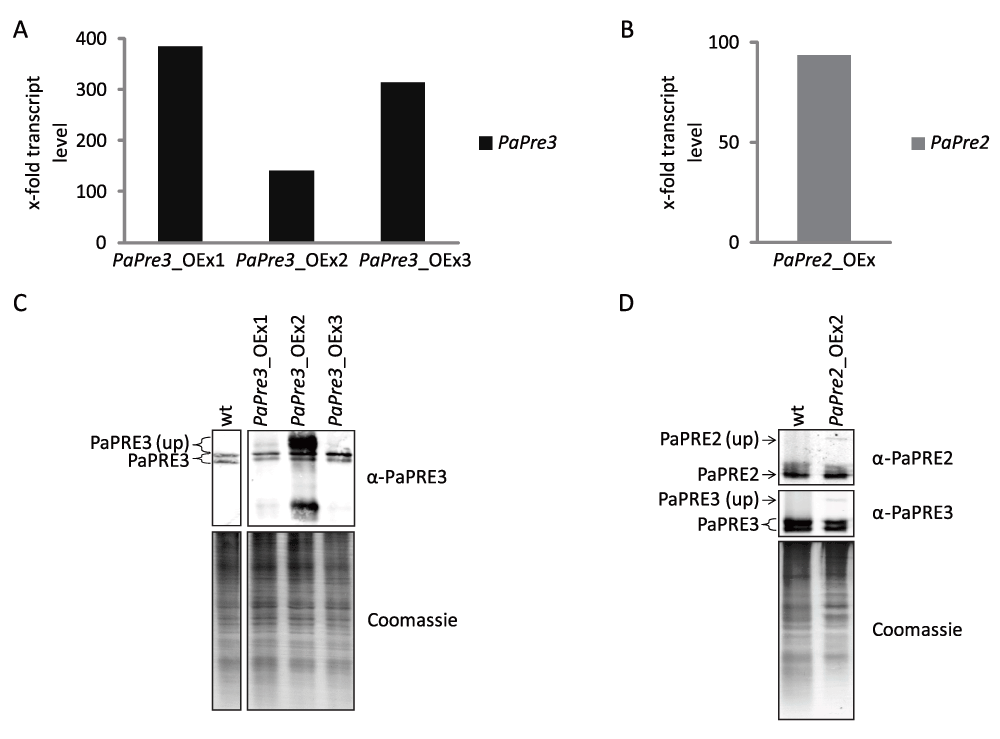
Figure 2. Overexpression of catalytic 20S subunits PaPre2 or PaPre3 does not alter the level of processed protein.
The expression of PaPre3 (A) and PaPre2 (B) in the respective overexpression strain was examined by qRT-PCR. (C, D) Total protein extracts of PaPre3 (50 µg) and PaPre2 (60 µg) overexpression strains were probed with α-PaPRE3 and α-PaPRE2 for the amount of processed proteasome subunits. The polyacrylamid gel stained with coomassie after blotting is displayed as loading control.
The proteasome activity reporter GFP-CL1 is degraded by autophagy
The activity of the UPS is not exclusively defined by the abundance of proteasome subunits but influenced by various factors like ubiquitin ligases, deubiquitin ligases, ATP-level, the regulatory particle, oxidative stress and post-translational modifications. In order to evaluate the efficiency of the UPS during aging of P. anserina, we generated two Gfp-cl1 strains with similar properties and a Gfp strain. Successful transformation of wild type was verified by Southern blot analysis (Table 2). The introduced genes are under the control of the constitutive Gpd promoter of Aspergillus nidulans. Gfp-cl1 codes for a protein containing the CL1 degron sequence fused to GFP. The CL1 sequence is a part of the Saccharomyces cerevisiae genome. It was first described in a ScURA3-CL1 fusion protein, which is unstable in wild type, but stable in strains lacking the ubiquitin ligases ScUbc6 and ScUbc7. Due to these characteristics the Cl1 degron has been used to monitor proteasome activity in various species including fly35, mouse36, rat37,38 and human cell cultures39–44.
In our work, we investigated the degradation of the CL1 degron fused to GFP. Fluorescence microscopy revealed diffuse fluorescence in whole cells of strains expressing Gfp-cl1 and Gfp, respectively, indicating a cytoplasmic localization (Figure 3A). Significantly, after applying heat stress, the Gfp-cl1 strain revealed a vacuolar localization of the GFP signal in some parts of the mycelium (Figure 3A). Western-blot analysis revealed two distinct GFP signals in Gfp-cl1-strains (Figure 3B). One signal corresponds to a protein with a size of 28.8 kDa expected for GFP-CL1 fusion protein while the other has the size of free GFP (26.9 KDa). This result was surprising, because proteasomal degradation should result in total decomposition of GFP-CL1 and provided a first clue for the degradation of the CL1 degron sequence by autophagy since the GFP part remains and is not, or only slowly, degraded by vacuolar proteases17,18. To verify the degradation of the CL1 degron by autophagy, we generated a P. anserina strain lacking PaAtg145 and expressing Gfp-cl1 by crossing of single mutants and selection of the double mutant. PaATG1 is necessary for autophagy and ΔPaAtg1-strains are not able to transport proteins to the vacuole for degradation45. Western blot analysis revealed that the double mutant contains only GFP-CL1 and no free GFP (Figure 3C), demonstrating that GFP-CL1 is at least partially degraded via autophagy in the wild type of P. anserina. This conclusion is supported by the accumulation of green fluorescence in the vacuoles of Gfp-cl1 overexpressing strains after the induction of autophagy by heat stress (Figure 3A).
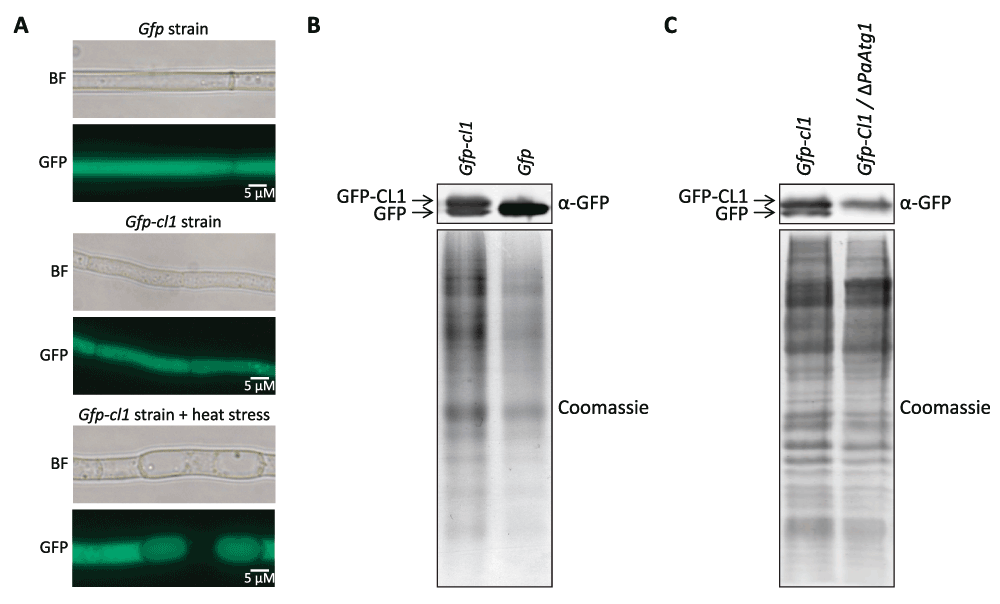
Figure 3. GFP-Cl1 is degraded by autophagy.
(A) Fluorescence microscopy analysis of Gfp and Gfp-cl1-1 mutant. Fungi were grown for 2d at 27°. Heat stressed samples were incubated at 37°C for the last 24 h. (B) Western-blot analysis of 60 µg total protein extracts from Gfp-Cl1-1 and Gfp strains. Proteins were detected by western-blot-analysis with α-GFP antibody. The corresponding polyacrylamid gel was stained with coomassie after blotting as loading control. (C) Western-blot analysis of 45 µg total protein extracts from Gfp-cl1-2 strains and from a Gfp-cl1-2/ΔPaAtg1 double mutant. Proteins were detected by western-blot-analysis with α-GFP antibody. The polyacrylamid gel was stained with coomassie after blotting.
Discussion
In the current study, we investigated the role of the proteasome in aging of P. anserina. Contrary to the mammalian model systems, we did not detect significant reduction of transcript or protein levels of proteasome subunits during aging46. Moreover, attempts to modulate the abundance of selected proteasomal subunits failed, although transcript abundance was strongly increased in the generated overexpression strains. It appears that in P. anserina the biosynthesis of the investigated proteasome subunits is under a strong post-transcriptional control.
One aim of our study was the development of an assay to study proteasomal activity. In other systems such assays are based on the microscopic monitoring of fluorescence changes resulting from the degradation of a reporter protein, termed degron, which is fused to GFP. The degron becomes rapidly ubiquitinated and subsequently the whole fusion protein is delivered to the proteasome were it is degraded. A widely used degron is CL1 derived from S. cerevisiae and consisting of 15 hydrophobic amino acids. Although this sequence was successfully used to detect proteasome activity in a wide range of organisms including yeast47, fly35, mouse36, rat37,38 and human cell cultures39–44, our experiments did not reveal a clear degradation of the whole GFP-CL1 fusion protein as it would be expected for degradation by the proteasome. Beside other reasons, it may be that CL1 is not recognized by the P. anserina ubiquitination system and thus does not constitute a functional degron. On the other hand, the sequence may be recognized by both the UPS and the autophagy machinery. Under the investigated conditions autophagy may be by far more efficient than ubiquitination and proteasomal degradation. An overlap of UPS and autophagy substrates has been shown previously48–51. The degradation of this reporter by autophagy may indeed be a severe problem for the establishment of a reporter gene based proteasome activity assay in filamentous fungi because they seem to be characterized by high level of basal autophagy. In Aspergillus oryzea, mitochondria, peroxisomes and nuclei of basal hyphae are degraded during normal growth in an autophagy dependent manner to use the nutrients to support growth52. Previous work in P. anserina also detected a high basal autophagy level under non-starved standard growth conditions45. On the other hand, basal autophagy in yeast and mammalian cell cultures appears to be low53,54. Another complication of the system may be that, as previously shown in Caenorhabditis elegans and neuronal rat cells, CL1 fused to GFP can form toxic aggregates if the expression level exceeds the capacity of the degradation system55. In our experiments this latter problem appears not to be valid since the fluorescence signal is distributed throughout the cell although we detected small condensed signals in some cells, which could indicate the formation of protein aggregates. Since such aggregates were only very small spots compared to those demonstrated in the mentioned studies with C. elegans and rat cells, the formation of toxic GFP-CL1 aggregates appears to be negligible under the chosen expression conditions.
Data availability
figshare: Raw data of qRT-PCR and western blot analyses of proteasome subunits and GFP-CL1 degradation in Podospora anserine. DOI: 10.6084/m9.figshare.117791056
Author contributions
MW performed experiments. HDO initiated and supervised this study. HDO and MW wrote the manuscript.
Competing interests
No competing interests were disclosed.
Grant information
In part, this work was supported by a grant of the European Commission (Acronym: Proteomage, FP6-51830) to HDO.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgements
We thank Dr. J. Servos (Frankfurt) for providing plasmids used in this study and Dr. A. Hamann for discussion of data.
Faculty Opinions recommendedReferences
- 1.
Naujokat C, Hoffmann S:
Role and function of the 26S proteasome in proliferation and apoptosis.
Lab Invest.
2002; 82(8): 965–980. PubMed Abstract
| Publisher Full Text
- 2.
Cummings CJ, Reinstein E, Sun Y, et al.:
Mutation of the E6-AP ubiquitin ligase reduces nuclear inclusion frequency while accelerating polyglutamine-induced pathology in SCA1 mice.
Neuron.
1999; 24(4): 879–892. PubMed Abstract
| Publisher Full Text
- 3.
He L, Lu XY, Jolly AF, et al.:
Spongiform degeneration in mahoganoid mutant mice.
Science.
2003; 299(5607): 710–712. PubMed Abstract
| Publisher Full Text
- 4.
Morimoto RI, Cuervo AM:
Proteostasis and the aging proteome in health and disease.
J Gerontol A Biol Sci Med Sci.
2014; 69(Suppl 1): S33–S38. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 5.
Lindner AB, Demarez A:
Protein aggregation as a paradigm of aging.
Biochim Biophys Acta.
2009; 1790(10): 980–996. PubMed Abstract
| Publisher Full Text
- 6.
Powers ET, Morimoto RI, Dillin A, et al.:
Biological and chemical approaches to diseases of proteostasis deficiency.
Annu Rev Biochem.
2009; 78: 959–991. PubMed Abstract
| Publisher Full Text
- 7.
Lilienbaum A:
Relationship between the proteasomal system and autophagy.
Int J Biochem Mol Biol.
2013; 4(1): 1–26. PubMed Abstract
| Free Full Text
- 8.
Kroemer G, Mariño G, Levine B:
Autophagy and the integrated stress response.
Mol Cell.
2010; 40(2): 280–293. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 9.
Hershko A, Ciechanover A:
The ubiquitin system.
Annu Rev Biochem.
1998; 67: 425–479. PubMed Abstract
| Publisher Full Text
- 10.
Pickart CM, Fushman D:
Polyubiquitin chains: polymeric protein signals.
Curr Opin Chem Biol.
2004; 8(6): 610–616. PubMed Abstract
| Publisher Full Text
- 11.
Seemuller E, Lupas A, Baumeister W:
Autocatalytic processing of the 20S proteasome.
Nature.
1996; 382(6590): 468–471. PubMed Abstract
| Publisher Full Text
- 12.
Chen P, Hochstrasser M:
Autocatalytic subunit processing couples active site formation in the 20S proteasome to completion of assembly.
Cell.
1996; 86(6): 961–972. PubMed Abstract
| Publisher Full Text
- 13.
Jung T, Catalgol B, Grune T:
The proteasomal system.
Mol Aspects Med.
2009; 30(4): 191–296. PubMed Abstract
| Publisher Full Text
- 14.
Chondrogianni N, Petropoulos I, Franceschi C, et al.:
Fibroblast cultures from healthy centenarians have an active proteasome.
Exp Gerontol.
2000; 35(6–7): 721–728. PubMed Abstract
| Publisher Full Text
- 15.
Carrard G, Bulteau AL, Petropoulos I, et al.:
Impairment of proteasome structure and function in aging.
Int J Biochem Cell Biol.
2002; 34(11): 1461–1474. PubMed Abstract
| Publisher Full Text
- 16.
Chondrogianni N, Gonos ES:
Proteasome dysfunction in mammalian aging: steps and factors involved.
Exp Gerontol.
2005; 40(12): 931–938. PubMed Abstract
| Publisher Full Text
- 17.
Pérez VI, Buffenstein R, Masamsetti V, et al.:
Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat.
Proc Natl Acad Sci U S A.
2009; 106(9): 3059–3064. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 18.
Rodriguez KA, Osmulski PA, Pierce A, et al.:
A cytosolic protein factor from the naked mole-rat activates proteasomes of other species and protects these from inhibition.
Biochim Biophys Acta.
2014; 1842(11): 2060–2072. PubMed Abstract
| Publisher Full Text
- 19.
Chondrogianni N, Stratford FL, Trougakos IP, et al.:
Central role of the proteasome in senescence and survival of human fibroblasts: induction of a senescence-like phenotype upon its inhibition and resistance to stress upon its activation.
J Biol Chem.
2003; 278(30): 28026–28037. PubMed Abstract
| Publisher Full Text
- 20.
Chen Q, Thorpe J, Dohmen JR, et al.:
Ump1 extends yeast lifespan and enhances viability during oxidative stress: central role for the proteasome?
Free Radic Biol Med.
2006; 40(1): 120–126. PubMed Abstract
| Publisher Full Text
- 21.
Kruegel U, Robison B, Dange T, et al.:
Elevated proteasome capacity extends replicative lifespan in Saccharomyces cerevisiae.
PLoS Genet.
2011; 7(9): e1002253. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 22.
Fischer F, Hamann A, Osiewacz HD:
Mitochondrial quality control: an integrated network of pathways.
Trends Biochem Sci.
2012; 37(7): 284–292. PubMed Abstract
| Publisher Full Text
- 23.
Osiewacz HD, Brust D, Hamann A, et al.:
Mitochondrial pathways governing stress resistance, life, and death in the fungal aging model Podospora anserina.
Ann NY Acad Sci.
2010; 1197: 54–66. PubMed Abstract
| Publisher Full Text
- 24.
Osiewacz HD, Hamann A, Zintel S:
Assessing organismal aging in the filamentous fungus Podospora anserina.
Methods Mol Biol.
2013; 965: 439–462. PubMed Abstract
| Publisher Full Text
- 25.
Esser K:
Podospora anserina. In: Handbook of Genetics, King,R.C., ed, Plenum Press, New York. 1974; pp. 531–551.
- 26.
Pfaffl MW:
A new mathematical model for relative quantification in real-time RT-PCR.
Nucleic Acids Res.
2001; 29(9): e45. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 27.
Brust D, Hamann A, Osiewacz HD:
Deletion of PaAif2 and PaAmid2, two genes encoding mitochondrial AIF-like oxidoreductases of Podospora anserina, leads to increased stress tolerance and lifespan extension.
Curr Genet.
2010; 56(3): 225–235. PubMed Abstract
| Publisher Full Text
- 28.
Averbeck NB, Borghouts C, Hamann A, et al.:
Molecular control of copper homeostasis in filamentous fungi: increased expression of a metallothionein gene during aging of Podospora anserina.
Mol Gen Genet.
2001; 264(5): 604–612. PubMed Abstract
| Publisher Full Text
- 29.
Pöggeler S, Masloff S, Hoff B, et al.:
Versatile EGFP reporter plasmids for cellular localization of recombinant gene products in filamentous fungi.
Curr Genet.
2003; 43(1): 54–61. PubMed Abstract
| Publisher Full Text
- 30.
Tonoki A, Kuranaga E, Tomioka T, et al.:
Genetic evidence linking age-dependent attenuation of the 26S proteasome with the aging process.
Mol Cell Biol.
2009; 29(4): 1095–1106. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 31.
Vilchez D, Boyer L, Morantte I, et al.:
Increased proteasome activity in human embryonic stem cells is regulated by PSMD11.
Nature.
2012; 489(7415): 304–308. PubMed Abstract
| Publisher Full Text
- 32.
Katsiki M, Chondrogianni N, Chinou I, et al.:
The olive constituent oleuropein exhibits proteasome stimulatory properties in vitro and confers life span extension of human embryonic fibroblasts.
Rejuvenation Res.
2007; 10(2): 157–172. PubMed Abstract
| Publisher Full Text
- 33.
Vilchez D, Morantte I, Liu Z, et al.:
RPN-6 determines C. elegans longevity under proteotoxic stress conditions.
Nature.
2012; 489(7415): 263–268. PubMed Abstract
| Publisher Full Text
- 34.
Chondrogianni N, Tzavelas C, Pemberton AJ, et al.:
Overexpression of proteasome beta5 assembled subunit increases the amount of proteasome and confers ameliorated response to oxidative stress and higher survival rates.
J Biol Chem.
2005; 280(12): 11840–11850. PubMed Abstract
| Publisher Full Text
- 35.
Pandey UB, Nie Z, Batlevi Y, et al.:
HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS.
Nature.
2007; 447(7146): 859–863. PubMed Abstract
| Publisher Full Text
- 36.
Liu Y, Hettinger CL, Zhang D, et al.:
The proteasome function reporter GFPu accumulates in young brains of the APPswe/PS1dE9 Alzheimer’s disease mouse model.
Cell Mol Neurobiol.
2014; 34(3): 315–322. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 37.
Tian Z, Wang C, Hu C, et al.:
Autophagic-lysosomal inhibition compromises ubiquitin-proteasome system performance in a p62 dependent manner in cardiomyocytes.
PLoS One.
2014; 9(6): e100715. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 38.
Ranek MJ, Terpstra EJ, Li J, et al.:
Protein kinase g positively regulates proteasome-mediated degradation of misfolded proteins.
Circulation.
2013; 128(4): 365–376. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 39.
Bence NF, Sampat RM, Kopito RR:
Impairment of the ubiquitin-proteasome system by protein aggregation.
Science.
2001; 292(5521): 1552–1555. PubMed Abstract
| Publisher Full Text
- 40.
Menendez-Benito V, Verhoef LG, Masucci MG, et al.:
Endoplasmic reticulum stress compromises the ubiquitin-proteasome system.
Hum Mol Genet.
2005; 14(19): 2787–2799. PubMed Abstract
| Publisher Full Text
- 41.
Bett JS, Cook C, Petrucelli L, et al.:
The ubiquitin-proteasome reporter GFPu does not accumulate in neurons of the R6/2 transgenic mouse model of Huntington’s disease.
PLoS One.
2009; 4(4): e5128. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 42.
Nonaka T, Hasegawa M:
A cellular model to monitor proteasome dysfunction by alpha-synuclein.
Biochemistry.
2009; 48(33): 8014–8022. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 43.
Tydlacka S, Wang CE, Wang X, et al.:
Differential activities of the ubiquitin-proteasome system in neurons versus glia may account for the preferential accumulation of misfolded proteins in neurons.
J Neurosci.
2008; 28(49): 13285–13295. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 44.
Hope AD, de Silva R, Fischer DF, et al.:
Alzheimer’s associated variant ubiquitin causes inhibition of the 26S proteasome and chaperone expression.
J Neurochem.
2003; 86(2): 394–404. PubMed Abstract
| Publisher Full Text
- 45.
Knuppertz L, Hamann A, Pampaloni F, et al.:
Identification of autophagy as a longevity-assurance mechanism in the aging model Podospora anserina.
Autophagy.
2014; 10(5): 822–834. PubMed Abstract
| Publisher Full Text
- 46.
Chondrogianni N, Sakellari M, Lefaki M, et al.:
Proteasome activation delays aging in vitro and in vivo.
Free Radic Biol Med.
2014; 71: 303–320. PubMed Abstract
| Publisher Full Text
- 47.
Gilon T, Chomsky O, Kulka RG:
Degradation signals for ubiquitin system proteolysis in Saccharomyces cerevisiae.
EMBO J.
1998; 17(10): 2759–2766. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 48.
De Domenico I, Vaughn MB, Li L, et al.:
Ferroportin-mediated mobilization of ferritin iron precedes ferritin degradation by the proteasome.
EMBO J.
2006; 25(22): 5396–5404. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 49.
Webb JL, Ravikumar B, Atkins J, et al.:
Alpha-Synuclein is degraded by both autophagy and the proteasome.
J Biol Chem.
2003; 278(27): 25009–25013. PubMed Abstract
| Publisher Full Text
- 50.
Kabuta T, Suzuki Y, Wada K:
Degradation of amyotrophic lateral sclerosis-linked mutant Cu,Zn-superoxide dismutase proteins by macroautophagy and the proteasome.
J Biol Chem.
2006; 281(41): 30524–30533. PubMed Abstract
| Publisher Full Text
- 51.
Ravikumar B, Duden R, Rubinsztein DC:
Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy.
Hum Mol Genet.
2002; 11(9): 1107–1117. PubMed Abstract
| Publisher Full Text
- 52.
Shoji JY, Kikuma T, Arioka M, et al.:
Macroautophagy-mediated degradation of whole nuclei in the filamentous fungus Aspergillus oryzae.
PLoS One.
2010; 5(12): e15650. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 53.
Abeliovich H, Klionsky DJ:
Autophagy in yeast: mechanistic insights and physiological function.
Microbiol Mol Biol Rev.
2001; 65(3): 463–479. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 54.
Mizushima N, Yoshimori T, Levine B:
Methods in mammalian autophagy research.
Cell.
2010; 140(3): 313–326. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 55.
Link CD, Fonte V, Hiester B, et al.:
Conversion of green fluorescent protein into a toxic, aggregation-prone protein by C-terminal addition of a short peptide.
J Biol Chem.
2006; 281(3): 1808–1816. PubMed Abstract
| Publisher Full Text
- 56.
Wiemer M, Osiewacz HD:
Raw data of qRT-PCR and western blot analyses of proteasome subunits and GFP-CL1 degradation in Podospora anserine.
figshare.
2014. Data Source



Comments on this article Comments (0)