Keywords
Longevity regulation, endocrinology & metabolism, insulin sensitivity, growth hormone hormonal signaling, caloric restriction, (neuro)endocrinology of senescence
Longevity regulation, endocrinology & metabolism, insulin sensitivity, growth hormone hormonal signaling, caloric restriction, (neuro)endocrinology of senescence
First we thank the referees for reviewing our article, and also for their cordial, conscientious commentary in doing so. In response to their commentary, we have made the following two changes:
1. We have added the following sentence to the first paragraph of the Discussion: “Ultimately, whether insulin sensitivity is truly one of the mechanisms by which the aging-retarding (and thus, lifespan-extending) effects of the Ghr/bp gene disruption are mediated, and/or account for the differential response of GHR-KO mice to CR, will require direct experimental demonstration.”
2. We have also added a penultimate Discussion paragraph focusing on the analyses of metabolism, particularly noting that:
A. total caloric output is greater in GHR-KO mice, relative to their littermate controls, regardless of diet; yet heat production does not differ by genotype (inc. speculation on why that incongruence exists);
B. although not statistically significant, caloric restriction reduces heat production in GHR-KO mice but raises it in littermate controls;
C. for oxygen consumption, carbon dioxide expulsion, and energy expenditure, gas-exchange-based measures of metabolism are robustly heightened by C.R. in littermates, but this effect is muted/ absent in GHR-KO’s (inc. implications that this has for explaining C.R.’s effects on senescence in littermate controls vs. GHR-KO’s).
To read any peer review reports and author responses for this article, follow the "read" links in the Open Peer Review table.
Improvements in insulin sensitivity or blood glucose homeostatic management are hallmarks of many slow-aging mutant and dietarily restricted animals, supporting the conjectures that these endocrine and metabolic phenomena may be positive regulators of (or simply indicators of interventions that might promote) longevity [Arum et al., 2009; Bartke, 2008; Bonkowski et al., 2006; Lawler et al., 2008; Longo & Finch, 2003; Masoro, 2003; Masoro, 2005; Mattison et al., 2007; Piper & Bartke, 2008]. A recently proffered approach to biomedical ventures endeavoring to delay aging, and thus increase healthspan (the period of life during which an organism is free of substantial morbidity or physiological disability/inability), begins with studying interventions that increase lifespan [Kenyon, 2010; Miller, 2009; Olshansky et al., 2007; Warner & Sierra, 2009]. Therefore, it is of high gerontological interest to study causal associations between longevity and physiological correlates that might result in anti-aging healthspan therapies based on engendering those physiological correlates, or that might serve as useful biomarkers for pharmacological or lifestyle interventions to delay the onset and/or decelerate the rate of senescence.
The growth hormone receptor/binding protein (Ghr/bp) gene-disrupted (knockout) (GHR-KO) mouse is homozygous for a targeted disruption (knock-out, KO) of the growth hormone (GH) receptor (GHR)/binding protein gene, and is thus GH-resistant, resulting in decreased GH hormonal signaling. GHR-KO mice were generated by insertional mutagenesis that disrupted the Ghr/bp gene; this results in decreased hepatic production of insulin-like growth factor 1 (IGF-1), which leads to markedly reduced levels of circulating IGF-1, a reduced growth rate, an approximately 20% reduction in adulthood length, and an approximately 40% reduction in adult body weight [Zhou et al., 1997].
Of particular note, the GHR-KO mouse outlives its littermate control by approximately 40% [Coschigano et al., 2000; Coschigano et al., 2003].
Produced in the β-cells of the pancreatic Islets of Langerhans, the hormone insulin regulates metabolism and energy homeostasis, partly by inducing the tissue uptake of glucose from blood. The GHR-KO mouse exhibits markedly decreased plasma insulin levels, due partly to decreased proliferation of β-cells [Liu et al., 2004]. As blood insulin concentration inversely mediates the systemic insulin sensitivity, insulin sensitivity is greater in the GHR-KO mouse than in its littermate control [Liu et al., 2004]. This is also the case with multiple other long-lived mice [Brown-Borg et al., 1996; Conover & Bale, 2007; Conover et al., 2008; Dominici et al., 2002; Selman et al., 2008].
Results from survivorship studies reveal that aging-retarding (and thus, lifespan-increasing) dietary restriction (DR), including yet not limited to caloric restriction (CR), further increases insulin sensitivity and survivorship for some long-lived mutants, the Ames (Prop1df/df) Dwarf mouse [Bartke et al., 2001] and the growth hormone releasing hormone KO (Ghrh-/-) mouse (data not shown). However it has been reported that CR doesn’t influence insulin sensitivity, and only modestly increases the survivorship of females, in the GHR-KO mouse [Bonkowski et al., 2006].
As an initial hypothesis, if CR fails to exert much effect on one senescence-associated trait (longevity) of the GHR-KO mouse because its level of insulin sensitivity is already as great as permissible for a viable animal, then a GHR-KO mouse on CR should not vary from a GHR-KO mouse on an ad libitum (A.L.) diet in other aging-associated characteristics (namely, metabolism and cognition).
Therefore, we attempted to investigate whether insulin sensitivity is sufficient to explain the severely attenuated response to CR of slow-aging associated phenotypes in GHR-KO mice. Surprisingly, in the course of our experiments we discovered that CR actually increases blood insulin concentration and starkly reduces insulin sensitivity in GHR-KO mice. These results question the assertion that CR has no effect on GHR-KO mouse blood glucose homeostatic management and the relationship, if any, between insulin sensitivity and slowed senescence in GHR-KO mice.
Ethics statement. Animal Protocol #178-02-001 was approved by the Laboratory Animal Care and Use Committee of Southern Illinois University-School of Medicine.
Ghr/bp gene-disrupted (GHR-KO) mice were generated by inserting a neomycin cassette replacing the 3′-end of the fourth exon and the 5′-end of intron 4/5 of the genomic sequence [Zhou et al., 1997]. The founder population of GHR-KO mice was provided by Dr. John J. Kopchick (Ohio University, Athens, OH). GHR-KO and GHR-N (heterozygous littermate controls for GHR-KO mice) mice were generated by mating of GHR-KO males with females heterozygous for the Ghr/bp-disrupted allele (GHR-N). These breeding schemes produce littermate control mice that have the same genetic background and are subject to the same intra-uterine and post-natal environment as the mutants.
Abiding by service provider’s instructions for sample collection and shipping, genotyping was conducted via quantitative polymerase chain reaction (q-PCR)-based technologies (Transnetyx, Inc., Cordova, TN).
The resulting mice had elements of a 129/Ola, a Balb/c, two C57Bl/6J, and two C3H/HeJ stocks; therefore, although lacking the methodological benefits of “reproducible genetic heterogeneity” [Miller et al., 1999], this stock possesses considerable genetic variation, and thus the results are likely applicable to other mouse populations.
The animals were maintained in shoebox-type cages in light- (12 hours light to 12 hours darkness) and temperature- (22 ± 2ºC) controlled rooms with constant access to Lab Diet Formula 5001 (23% protein, 4.5% fat, 6% fiber) (Nestlē Purina, St. Louis, MO) and tap water. Littermate control pups were weaned at the age of 21–23 days, and GHR-KO pups two weeks later or at the time of weaning control pups from the next litter.
All experiments were performed in female mice, as GHR-KO stock male littermate controls are insensitive to the common dosage of insulin (0.75 U.S.P.U./kg B.W.) during insulin tolerance testing [Arum et al., 2009; Bonkowski et al., 2006].
Mice were 4–8 months of age at inception of restriction.
The amount of food allotted each cage of mice designated for caloric restriction was determined based on (weekly calculated) ad libitum food consumption for entire cages of gender-, genotype-, and birth date-matched controls; these values were averaged over the number of cages within each such group. Two hundred grams of the above-described food was placed in each A.L. cage-hopper on a weekly basis. After six days of food consumption, the remaining food was weighed on a Scout Pro Balance (Ohaus Co., Pine Brook, NJ) calibrated to weight standards on a monthly basis; food consumption values were calculated as follows: {(200 g.) – [food remaining after six days (in g.)]}/six (days)/number of subjects in cage.
As a protection against dissimilar food consumption within CR cages, part of the food was broken into pieces small-enough to pass through the hopper-grate (but not crumbs). Our observations confirmed that this method allowed every restricted mouse to feed ad libitum during the initial surge of food consumption. Considering the valid concerns related to differential restriction resulting from a dominant cage-mate consuming more than their fair proportion, we paid particular attention to any individual mouse weight loss and health (e.g. fight wounds indicative of physical conflicts with a cage-mate) throughout our studies. It is also worth noting that our chosen level of restriction (30%) is moderate compared to the 40% level that causes considerable concerns [Liao et al., 2010; Mattson, 2010]. This moderate level does not lead to an extinguishment of food supply after the initial gorge (thus, even subordinate mice have ample, albeit possibly delayed, access to food) and does not result in substantial weight loss for any sub-cohort of animal-subjects within our stocks (Figure 1A and B).
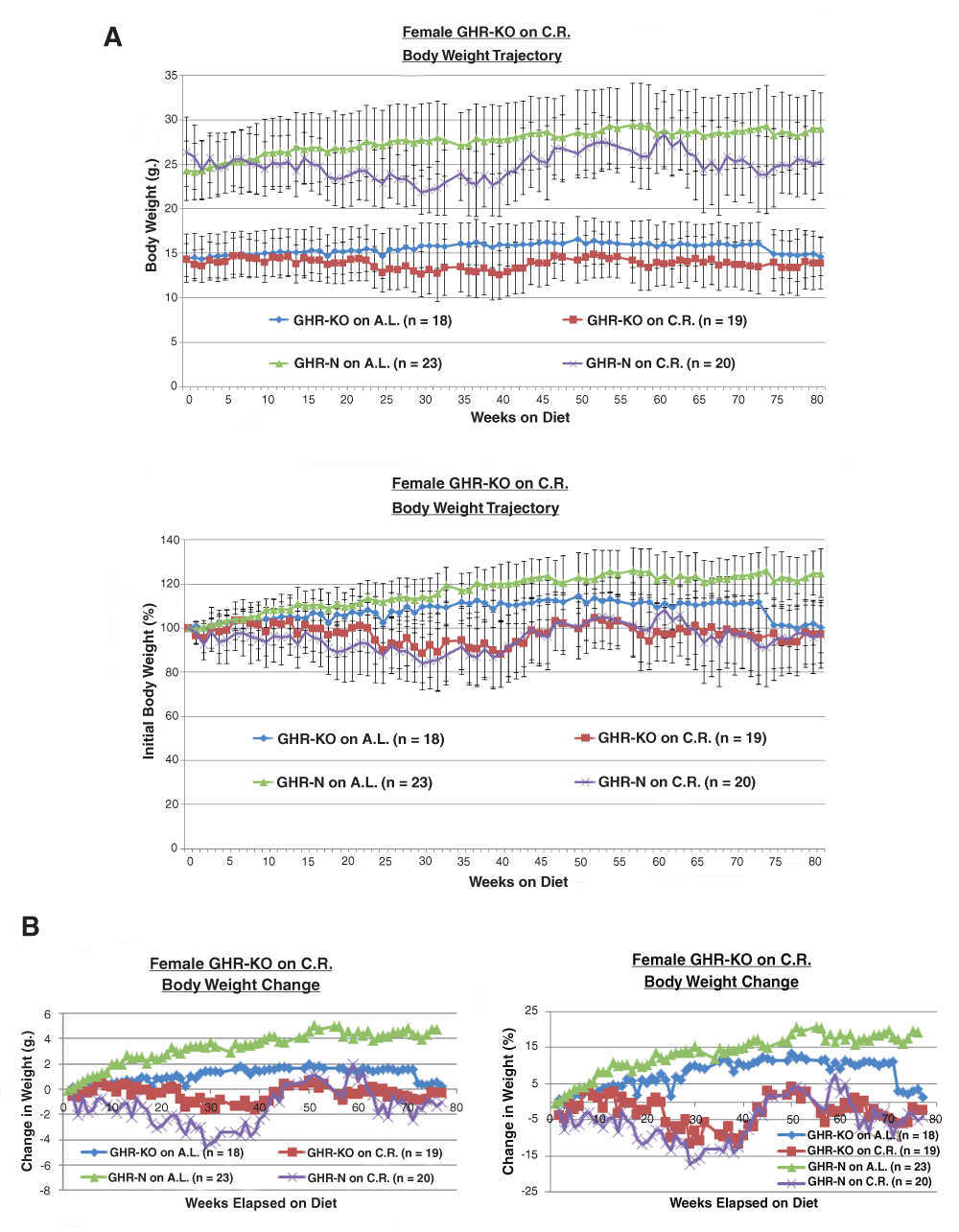
A. 30% caloric restriction represses body weight gain (absolute or normalized-to-initial) in female GHR-KO mice and their littermate controls. B. 30% caloric restriction reins change in body weight (absolute or normalized-to-initial) in GHR-KO females and their littermate controls.
Of relevance to obviating unintended interactions between experimental factors, which might produce confusing or obfuscating variation within the data, mice were housed in genotype-, age-, and diet-specific cages.
Mice were weighed weekly on a Scout Pro Balance (Ohaus Co., Pine Brook, NJ) that was calibrated to weight standards on a monthly basis. All mice were weighed in the late afternoon, approximately 20 hours after the restricted mice had been fed.
Mice were young-adults in all experiments except for the indirect calorimetry trials involving CR, the spontaneous locomotor activity experiments and the behavior (anxiety & memory) experiments, where mice had to be middle-aged in order to address gerontological queries.
Age-staging was based on a combination of 1) quantitative extrapolation from prior stock-specific survivorship data [Bonkowski et al., 2006], 2) presence/appearance of aging-associated wizening (as represented quantitatively by declining body weight), and 3) spontaneous, testing-independent, (and presumably) aging-resultant mortality. Thus, young-adulthood is marked by at least 90% of reproductively competent negative control subjects being alive; middle-age is the period between when approximately 90% of the control subjects are still alive and median survivorship; old-age is the period between median survivorship and when approx. 10% of the subjects are alive; and oldest-old age is designated as the period when ≤ 10% of the controls remain.
All animals underwent home-cage assessments of gross health (Supplemental Table I) and any animal exhibiting questionable health by these criteria, or which was aberrantly hypoglycemic at the inception of a test, was excluded from the testing and/or data analysis. In addition, all animals were given at-least two weeks of recuperation in-between tests.
Glucose tolerance testing [ad libitum (A.L.)-fed or fasted]
For A.L.-fed tests, animals had access to food for at least 16 hours before the test. For fasted tests, animals were fasted for 16 hours, although CR animals were A.L.-fed the day before the 16-hour fast commenced. Thirty minutes prior to beginning the test, each animal was weighed, had a small nick placed at the tip of its tail with a razor, and re-housed without access to food. After 30 minutes to recover from the handling stress of the weighing and tail-nicking, blood glucose concentration was assessed in each animal. Blood was obtained by applying a gentle pressure to the tail-tip, with a blood glucose monitoring system (glucometer and testing strips) (OneTouch Ultra 2, Lifescan, Inc., Milpitas, CA). Without releasing the grasp on the animal, it was manually repositioned to a nearly supine pose, and injected inter-peritoneally with 2 g. D-(+)-glucose (Sigma-Aldrich Co., St. Louis, MO) per kg of body weight. [The powdered glucose was dissolved in 0.9% sodium chloride (Sigma-Aldrich Co., St. Louis, MO)]. Subsequent blood glucose measurements were at 10, 20, 30, 40, 50, 60, 75, 90, and 120 minutes after the injection. Animals were given A.L. access to food immediately after completion of the test.
Insulin tolerance testing
Animals had access to food for at least 16 hours before the test. Animals were prepared for injection as described for glucose tolerance testing (above). Animals were injected inter-peritoneally with 0.75 U.S.P.U. of porcine insulin (Sigma-Aldrich Co., St. Louis, MO) per kg of body weight. (The lyophilized insulin was dissolved in 0.9% sodium chloride). Subsequent blood glucose measurements were at 10, 20, 30, 40, 50, 60, 75, 90, and 120 minutes after the injection. Animals were given A.L. access to food immediately after completion of testing.
Pyruvate conversion testing
Animals were fasted for 16 hours and CR animals were A.L.-fed the day before the fast commenced. Animals were prepared for injection as described for glucose tolerance testing (above). Animals were injected inter-peritoneally with 2 g of sodium pyruvic acid (Sigma-Aldrich Co., St. Louis, MO) per kg of body weight. (The lyophilized sodium pyruvate was dissolved in 0.9% sodium chloride). Subsequent blood glucose measurements were at 15, 30, 45, 60, and 120 minutes after the injection. Animals were given A.L. access to food immediately after completion of testing.
Non-stimulated blood glucose comparisons [ad libitum (A.L.)-fed or fasted]
Un-stimulated blood glucose values were obtained from young-adult mice at the beginnings of A.L.-fed and fasted glucose tolerance tests, drawn from a small nick at the tip of the tail and measured with a blood glucose monitoring system (glucometer and testing strips) (OneTouch Ultra 2, Lifescan, Inc., Milpitas, CA). A.L.-fed blood glucose values were collected after an overnight (~16 hrs.) period of A.L. feeding for all subjects; fasted blood glucose values were gathered after equivalent overnight fasting (~16 hrs.) for all subjects.
A.L.-fed blood glucose values recorded immediately preceding a sacrifice and tissue harvesting from middle-aged mice were consistent with the results obtained as above. As a control against inferences drawn from the possible effects of short-term fasting, CR mice were noted to have stomachs freighted with foodstuff upon the sacrifice that followed the blood glucose assessment.
Indirect calorimetry was conducted as previously described by Westbrook et al., (2009) (Accuscan Instruments, Inc., Columbus, OH). Acclimation day testing, A.L.-fed day testing and fasted day testing were all conducted in one longitudinal stretch. Data were normalized per unit of lean body weight [Butler & Kozak, 2010] as determined by fat depot sub-dissection [Berryman et al., 2010; Muzumdar et al., 2008]. The values at the 17:00 hour were excluded from the statistical analyses, as this time was used for maintenance activities (e.g. removal of food, weighing of remaining food, and weighing of mice) during longitudinal testing paradigm. Parameters assessed are annotated in Supplemental Table II a.
Spontaneous locomotion was assessed using the same equipment and the indirect calorimetry procedure described above (Accuscan Instruments, Inc., Columbus, OH). The values measured at the 17:00 hour were excluded from the statistical analyses, as this time was used for maintenance activities (e.g. removal of food, weighing of remaining food, and weighing of mice) during longitudinal testing paradigm. Parameters assessed are annotated in Supplemental Table II b.
Blood cell counting was accomplished using a VetScan HM2 Hematology System (Abaxis, Union City, CA) and ≥ 25 μL of whole blood [collected in EDTA-coated Microvette 100 μL capillary tubes (Sarstedt AG & Co., Nümbrecht, Germany)] drawn from ad libitum-fed subjects. The following 18 parameters were assessed: concentration of leukocytes/white blood cells (W.B.C.), concentration of lymphocytes (LYM), concentration of monocytes (MON), concentration of granulocytes (GRA), proportion of leukocytes that are lymphocytes (LYM%), proportion of leukocytes that are monocytes (MON%), proportion of leukocytes that are granulocytes (GRA%), concentration of erythrocytes/red blood cells (R.B.C.), concentration of hemoglobin (g/dL) (HGB), hematocrit (%) (HCT), mean (erythrocytic) cell volume (M.C.V.), mean corpuscular hemoglobin (pg.) (M.C.H.), mean corpuscular hemoglobin concentration (g/dL) (M.C.H.C.), red cell (erythrocytic) distribution width (%) (R.D.W.), concentration of thrombocytes/platelet cells (PLT), mean platelet volume (M.P.V.), plateletocrit/platelet hematocrit (%) (PCT), platelet distribution width (%) (P.D.W.).
Plasma insulin was measured with the multiplexed Mouse Endocrine Lincoplex ELISA kit (LINCO Research, St. Charles, MO).
All animals underwent home-cage assessments of gross health, locomotor ability and activity (Supplemental Table I). Animals exhibiting questionable health based on these criteria were excluded from the testing.
During the light-phase of their day, the mice were individually placed in the center of a lid-less, opaque, white, 44 × 44 × 40 cm. (length × width × height) polymer box with the floor divided into 16 11 × 11 cm2. The number of squares entered within the allotted time was noted per mouse per trial; as the experiment is contingent upon the novelty of the aberrant context, each subject was only tested once. The methods used derived from standard methodologies previously used “to analyze general activity and exploratory drive” [Crawley, 2007; Selman et al., 2009].
All animals underwent home-cage assessments of gross health, and locomotor ability & activity (Supplemental Table I). Animals exhibiting questionable health based on these criteria were excluded from the testing.
During the light-phase of their day, mice were individually placed in the center of a lid-less, opaque, white, 44 × 44 × 40 cm (length × width × height) polymer box with the floor divided into 16 11 × 11 cm2. The number of squares entered within one minute was noted per mouse per trial; 24 hours after the initial evaluation (acquisition), the mice were re-tested (retention). Memory index values were calculated per mouse as follows: (Retention activity/Acquisition activity); these reflect the degree to which the subject remembered the context presented 24 hours prior (with enhanced memory putatively resulting in more movement due to less anxiety). Final location scores evaluate the ultimate (after the 60-second testing interval) position of a mouse on the retention day, with a more-ensconced placement being indicative of greater anxiety (and, thus, worse memory of prior context) than a more-exposed positioning. The methods derived from standard methodologies used by other investigators [Crawley, 2007].
Graphs were generated with Excel (Microsoft, Redmond, WA) and IrfanView Image Viewer (Irfan Skiljan, Wiener Neustadt, Austria; http://www.irfanview.com/). The measures of central tendency are arithmetic means, and all depictions of variation (error bars) represent the standard deviations (S.D.) [Glantz, 2002].
Pre-hoc statistical measures
In brief, experimental design approaches were taken to maximize robustness while lessening the potential need to increase sample size; utilizing 1) an a priori specification of a limited number of well-defined hypotheses, 2) refinement of experimental techniques, and 3) grouping of animals so that the effect of unit variability on the treatment was minimized.
Post-hoc statistical analysis
Levene’s tests (to investigate scedasticity) and Kolmogorov-Smirnov tests (to determine deviations from Gaussian distribution) were conducted to guide the choice of statistical algorithms for analysis of differences amongst groups. The combined parameters of effect size and Type 1 error probability were considered when determining phenomena meriting presentation and discussion.
Most data were contrasted with unpaired, homoscedastic Student’s t-test, Analysis of Variance, or Analysis of Variance for Repeated Measures (ANOVA or ANOVA-R.M., resp.), as appropriate; followed by the Tukey’s Honestly Significant Difference (H.S.D.) or the Dunnett’s t-test post-hoc tests for multiple pairwise comparisons, as appropriate.
For repeatedly measured blood glucose regulatory assessments, the p-value for a given pairwise comparison at a given time-point represents the result of testing all of the time-points, up-to-and-including that time-point, within the repeated measures analysis; this permits testing whether both groups have experienced similar excursions in blood glucose (the null hypothesis) relative to their initial values and with consideration of all intermediate values. This mode of analysis poses more discrete and descriptive inquiries than analyzing the area under respective curves or utilizing isolated, independent blood glucose values/percentages at lone time-points. The data that are normalized to initial blood glucose values were used for the precise, time-point-specific p-values reported, yet the inferences of differences amongst groups do not depend on the use of these normalized data. For a particular pairwise comparison within a particular assay, the p-value reported in the text is the most conservative (i.e. highest) sub-0.05 p-value from the series of repeated measures analyses.
In instance of considerable variation in data confounding inferences, the data outside of 1 S.D. might have been equilaterally excluded from the data used for statistical analysis.
Statistical comparisons were conducted with PSPP for Windows (Free Software Foundation, Inc., http://www.gnu.org/software/pspp/get.html).
Probing parameters pursuant to proliferation
A 30% CR resulted in the standard body weight (B.W.) gain attenuation, whether represented in absolute grams or in percentage-of-initial (Figure 1A) or in body weight change in grams or in percentage-of-initial (Figure 1B).
When scrutinizing proliferation on a cellular level, hematocytometric analyses of various blood cell parameters (e.g. erythrocytes, leukocytes, and platelets) in late-middle-aged (~25 months-of-age) revealed no effect of CR on either GHR-N mice or GHR-KO mice (Table 1).
Blood glucose homeostatic regulation experiments
Importantly, there was no effect of the 30% CR diet on B.W. measured immediately preceding the testing for any of the blood glucose homeostasis regulation assays (Figure 2A).
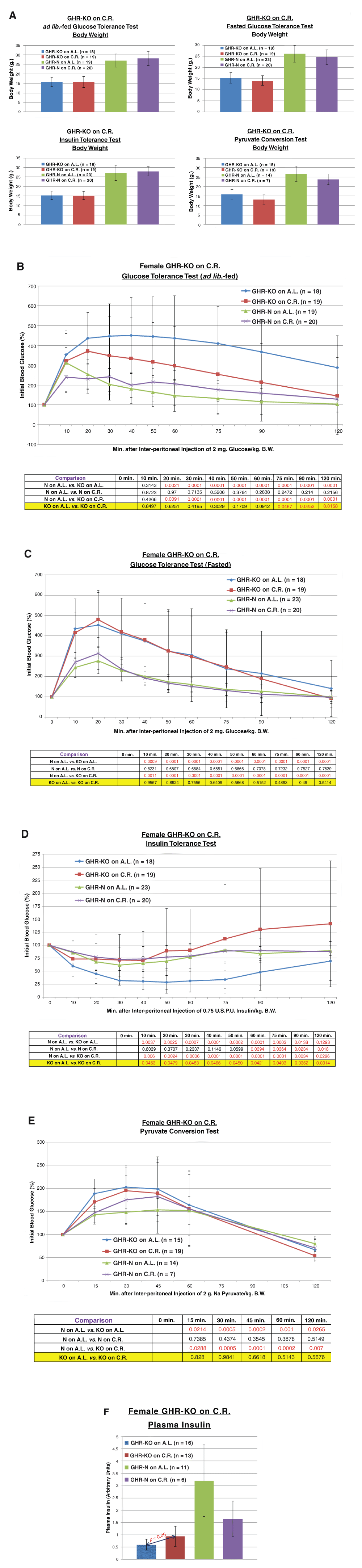
A. 30% caloric restriction does not affect body weight immediately preceding a tolerance or conversion test for GHR-N or GHR-KO females. B. 30% caloric restriction partially corrects the glucose intolerance of female GHR-KO mice under A.L.-fed conditions (including repeated-measures statistical analysis table). C. 30% caloric restriction does not affect the glucose intolerance of GHR-KO females under fasted conditions (including repeated-measures statistical analysis table). D. 30% caloric restriction corrects the enhanced insulin sensitivity of female GHR-KO mice (including repeated-measures statistical analysis table). E. 30% caloric restriction does not significantly alter the heightened de novo hepatic glucose production of GHR-KO females (including repeated-measures statistical analysis table). F. 30% caloric restriction increases plasma insulin in female GHR-KO mice.
In relation to our hypothesis, CR increased A.L.-fed glucose incorporation in fed female GHR-KO mice during glucose tolerance testing [(p = 0.0467), (Figure 2B)], but had no effect in fasted GHR-KO mice (Figure 2C). As for the insulin tolerance tests, CR attenuated the sensitivity of GHR-KO females to 0.75 U.S.P.U./kg B.W. of insulin [(p = 0.0483), (Figure 2D)]. CR did not alter the pyruvate conversion potential in female GHR-KO mice (Figure 2E). Additionally, CR increased the plasma insulin content in GHR-KO mice [(p < 0.05), (Figure 2F)].
Therefore, our data show additive or synergistic effects of CR with the GHR-KO gene disruption on blood glucose homeostasis.
Indirect measures of metabolism
Measurements estimating the general rate of metabolic processes have long been correlated with ultimate survivorship, and have been proffered as sufficient to explain the rate of senescence [Rubner, 1908]. Whether the mechanisms by which CR retards senescence include alterations (particularly, decreases) in metabolism has been an active research hypothesis for some time [Ramsey et al., 2000].
Indirect (gas exchange) calorimetric measurements of metabolism have been reported to be increased [Westbrook et al., 2009], as well as decreased [Carrillo & Flouris, 2011; Mookerjee et al., 2010], in animals with extended longevity. Identifying metabolic phenotypes that transcend one particular genetic background or mode of delaying and/or decelerating aging would be important for proposing or testing mechanisms of extended lifespan and healthspan.
During our analyses of oxygen consumption (VO2), respiratory quotient (R.Q.)/respiratory exchange ratio (R.E.R.), heat production (Calories/hr), and energy expenditure (E.E.) in A.L.-fed and fasted female GHR-KO mice on CR, no genotype- or diet-based differences were detected for food consumption, changes in body weight induced by either acclimation or fasting, or thermogenesis (as crudely measured with an ambient thermometer in each chamber) while the subjects were in the indirect calorimetry chambers from the acclimation day through the A.L.-fed day to the fasted day (Table 2).
CR did not affect these A.L.-fed indirect calorimetry-based measures of metabolism effects of female littermate controls, nor that of female GHR-KO mice (Table 3).
Spontaneous locomotor activity late in life, as it can serve as a measure of the multi-factorial syndrome of frailty [Walston et al., 2006] (i.e. frail mice would presumably be disinclined to move, or cover less area when trying) is often used as a behavioral marker of delayed and/or decelerated senescence [Ingram, 2000; Manini, 2010; Minor et al., 2011; Neff et al., 2013; Wilkinson et al., 2012; Zhang et al., 2014].
Similarly, CR had no germane effect on the spontaneous locomotion of female littermate control mice, or female GHR-KO mice (Table 4).
Cognitive assessments
The retention of cognitive capability (in particular, memory function) into middle-age and beyond in the GHR-KO mice further supports that the effects of the Ghr/bp disruption extend beyond increasing survivorship, to include ameliorating senescence and its resultant functional decrements [Bartke, 2005; Kinney et al., 2001a; Kinney et al., 2001b; Kinney-Forshee et al., 2004; O. Arum & A. Bartke, (unpublished data)]. With noteworthy exceptions, which are partly due to varied methodologies of caloric restriction or cognitive assessment amongst scientists, CR is broadly considered to be beneficial for retarding aging-resultant cognitive decline [Arslan-Ergul et al., 2013; Joseph et al., 2009].
Regarding the cognitive assessments, CR had no effect on the anxiety of either littermate control or GHR-KO females (Figure 3A). The memory index results derived from open-field activity (Figure 3B) did not support the initial hypothesis that the greater insulin sensitivity of GHR-KO mice precludes their full benefits from CR (as the GHR-KO mice on A.L. and the GHR-KO mice on CR, which have differing insulin responsiveness, did not differ in memory performance). Similar inferences were concluded for the open-field activity-based memory tests regarding the final location of the mice (Figure 3C).
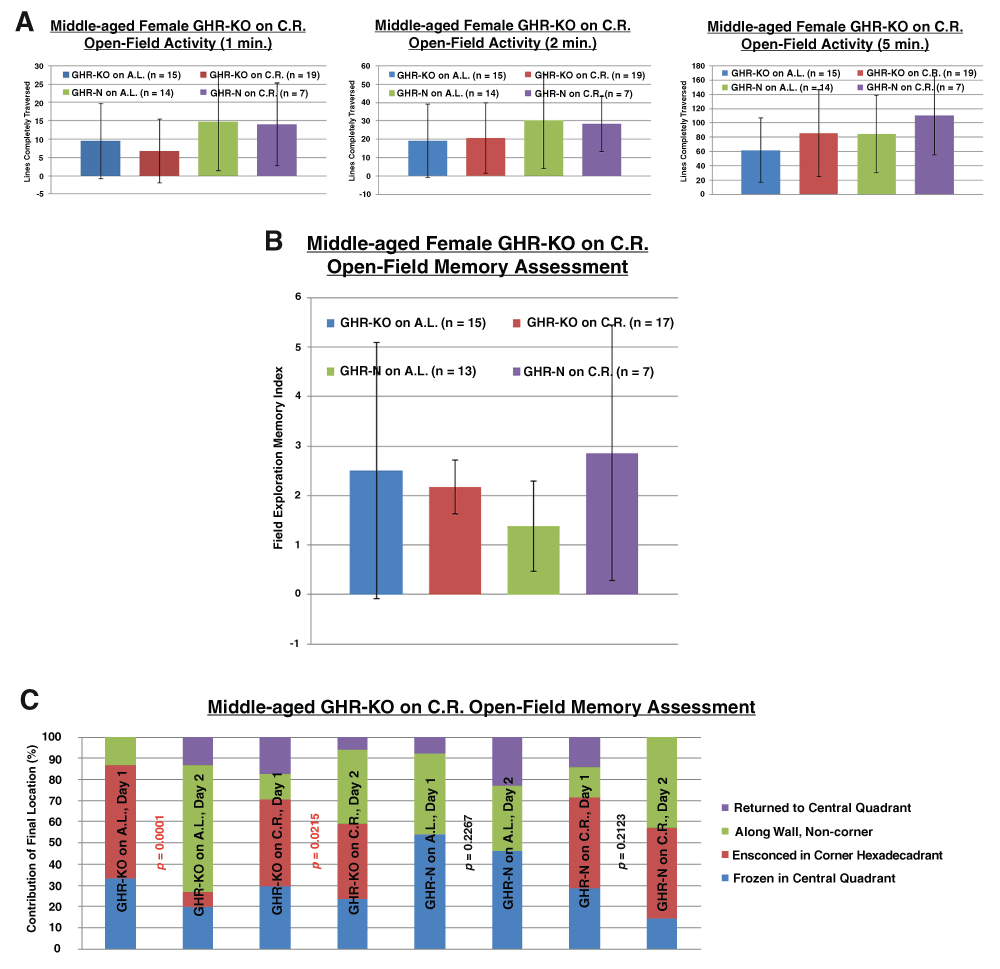
A. Neither Ghr/bp disruption nor 30% caloric restriction alters anxiety-betraying activity in an open field for female mice. B. 30% caloric restriction does not change the (memory index) performance of female GHR-KO mice in the open-field paradigm. C. 30% caloric restriction does not change the (final location) performance of female GHR-KO mice in the open-field paradigm.
The initial aim of this study was to investigate if the very limited response of the GHR-KO mouse to a 30% CR diet in terms of longevity [Bonkowski et al., 2006] is related to the inability of these mutants to respond to a 30% CR diet with regards to insulin sensitivity [Bonkowski et al., 2006]. This was based on the hypothesis that it is the maximization of the response to CR in the insulin sensitivity test that acts as a “ceiling/floor” effect limiting the survivorship response to CR [Bonkowski et al., 2006]. Our insulin sensitivity results in GHR-KO mice on 30% CR differed from those obtained in a previous study, showing that caloric restriction promotes euglycemia in GHR-KO mice (Figure 2C). These differences might have been due to the difference in ages of the animals {12 months in [Bonkowski et al., 2006] vs. 8–13 months in the present report}, or different durations of CR (10 or 12 months vs. 4–6 months, respectively). Those caveats emptor notwithstanding, that blood insulin content is increased by CR in GHR-KO mice (Figure 2E) dovetails with the improved performance in glucose bolus assimilation (Figure 2A), decreased insulin sensitivity (Figure 2C), and (statistically indistinguishable) decreased gluconeogenic capability (Figure 2D) of GHR-KO mice on CR relative to their A.L. counterparts. Moreover, data from macromolecular analysis of insulin signaling in GHR-KO mice on CR, including decreased insulin receptor (INSR) and thymoma viral proto-oncogene 1/protein kinase b (AKT1/PKB) concentrations in the skeletal musculature of GHR-KO’s on CR, and decreased phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) subunits content in the livers of GHR-KO’s on CR, (all relative to GHR-KO’s on AL), also corroborate and portend decreased insulin sensitivity in GHR-KO mice on CR [Bonkowski et al., 2009]. Additionally, it is worth noting that a tight regulation of euglycemia would be more consistent with health and survival than a predilection for hypoglycemia [Tan & Flanagan, 2013]; thus, “improving health”, as CR has been broadly documented as doing, might mean preventing the innate endocrinological/metabolic derangements that are merely coincident with the longevity of the GHR-KO mouse. Finally, to the best of our knowledge, published reports on CR-mediated induction of insulin sensitivity (vis-à-vis increased blood glucose assimilation dynamics) using insulin tolerance tests or hyperinsulinemic-euglycemic clamping assays on healthy mice are either lacking or are not consistently reproduced. This is an important limitation, and caveat emptor, given that mutant mice with abnormal growth and adult body composition have been documented to have insulin tolerance testing results in disagreement with the molecular biology-based assumptions of their insulin sensitivity [Boparai et al., 2010]. Ultimately, whether insulin sensitivity is truly one of the mechanisms by which the aging-retarding (and thus, lifespan-extending) effects of the Ghr/bp gene disruption are mediated, and/or account for the differential response of GHR-KO mice to CR, will require direct experimental demonstration.
We also investigated the effects of CR on the performance of GHR-KO mice in other gerontologically associated measures. We discovered that CR did not alter the metabolism or spontaneous activity of GHR-KO mice, and also revealed that CR has no effect on the anxiety or memory function of GHR-KO mice. This documentation of lacking amenability of GHR-KO mice to effects of CR further underscore a seeming epistasis of the genetic effect of Ghr/bp disruption to the environmental effect of dietary restriction.
Certain aspects of the indirect calorimetry results deserve special treatment. Firstly, energy expenditure (EE) was greater in GHR-KO mice compared to their littermate controls, regardless of diet; yet heat production (HP) did not differ by genotype. We suspect that a technical reason exists for this incongruence. The formula for calculating HP with indirect calorimetric gas-exchange data, formulated based on reconciling direct calorimetric measures with indirect calorimetric gas-exchange measures in normal-sized, lean mice [Arch et al., 2006], is [((4.33 × VO2) + (0.67 × VCO2)) + (Wt.(kg.)) × (60 Min./Hr.)]; and, thus, is not necessarily appropriate for accurately measuring HP via indirect calorimetry in dwarf mice, which have divergent body composition (most-notably obesity) compared to their littermate controls [Berryman et al., 2004; Berryman et al., 2010]. More directly, that the heat production equation factors in body weight results in lighter dwarf mice having lower calculated HP, for given amounts of oxygen inhalation (VO2) and carbon dioxide expiration (VCO2), than their normal-sized littermates. Additionally of note (although not statistically significant), caloric restriction tended to reduce HP in GHR-KO mice but tended to raise it in littermate controls. Finally, (as alluded to in the paragraph above) for VO2, VCO2, and EE, gas-exchange-based measures of metabolism were robustly heightened by CR in littermates, but this effect was muted or absent in GHR-KO’s. Therefore, CR-effected increased metabolism could be a mean by which that diet slows senescence in littermates; moreover, the attenuation of this metabolic effect in GHR-KO mice (whether due to a ceiling effect, in which VO2 and CO2 being innately high in GHR-KO mice on A.L. makes it so that CR is unable to raise them much higher, or other rationales) might partially explain the attenuation of the aging-retarding (including life expectancy-increasing) effect of CR on GHR-KO mice.
In summary, our results question the notion of maximized insulin sensitivity obviating further lifespan increase in GHR-KO mice. Future studies aimed at elucidating concordant physiological, and ultimately (macro)molecular, underpinnings of disparate instances of longevity would benefit from heeding analyses that reduce or eliminate the likelihood of suspected mechanisms.
Dataset 1. Experimental data showing the effect(s) of growth hormone (GH) receptor (GHR)/binding protein (Ghr/bp) gene disruption and/or caloric restriction on the various outcomes, http://dx.doi.org/10.5256/f1000research.5378.d37530 [Arum et al., 2014].
O.A., R.K.K., and A.B. acquired funding for this study; O.A. and A.B. conceived and designed this study; J.G.T. provided accommodations for the blood glucose regulatory dynamics, and technical training for the anxiety and cognitive assessments in this study; O.A., R.K.B., & J.K.S. methodologically executed this study; O.A. statistically analyzed the data from this study; and O.A. & A.B. prepared the manuscript for this study. J.J.K. provided the founder population of the growth hormone receptor/binding protein gene-disrupted mice bred for this study. All authors approved the final content of the manuscript.
This work was supported by National Institute on Aging Grants AG19899, U19 AG023122, and 3R01AG019899-07S1, as well as a Senior Scholar Award in Aging from The Ellison Medical Foundation, and The Glenn Foundation for Medical Research.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Characteristics of interest in home-cage assessments are succinctly detailed.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 27 Apr 15 |
||
|
Version 1 28 Oct 14 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)