Keywords
Microalgae, Cation Exchange Capacity, Organic Matter, Herbicides, Corn, Crops, Fertilizer, Green, GOgreen, pH, restoration, species diversity, microalgae, soil algae, soil amendments, soil biodiversity.
This article is included in the Agriculture, Food and Nutrition gateway.
Microalgae, Cation Exchange Capacity, Organic Matter, Herbicides, Corn, Crops, Fertilizer, Green, GOgreen, pH, restoration, species diversity, microalgae, soil algae, soil amendments, soil biodiversity.
The size and activity of soil microbial communities is an indicator of soil health, quality and fertility necessary for sustainable agriculture (Doran & Parkin, 1994; Kennedy & Papendick, 1995; Sparling, 1997; Warkentin, 1995). These communities have been classified into eubacteria, cyanobacteria, actinomycetes, archaebacteria, fungi, microalgae, protozoa, viruses, and some nematodes (Paul & Clark, 1989; Roper & Gupta, 1995; Sims, 1990). Out of these groups, microalgae perform several important functions for agro-ecosystems and can also function as a bio-indicator for soil quality.
Four different types of algae are recognized in soil: green (chlorophyta), blue-green (cyanobacteria), yellow-green (xanthophyta), and diatoms (bacillariophyta) (Paul & Clark, 1989). Soil algae are photoautotrophs. These species do not depend on the organic matter (carbon content) of the soil and play a role as primary colonizers. They produce large amounts of secreted polysaccharides that promote soil aggregation at the surface and they are capable of nitrogen fixation (Goyal, 1997; Zenova et al., 1995). Soil habitats are the most important non-aqueous ecosystems for microalgae (Zenova et al., 1995) where these organisms contribute to soil formation and stability (Metting, 1981). In addition, microalgae contribute to energy and matter flux (Kuzyakhmetov, 1998). Green and blue-green microalgae populations in upper topsoil can perform valuable services for soil ecosystems (Metting, 1981; Starks et al., 1981) and agriculture (Ruble & Davis, 1988). One of the major benefits of microalgae is the generation of organic matter from inorganic substances (Alexander, 1977). In addition to providing a food source for other microorganisms, nematodes, and invertebrates, microalgae produce biologically active compounds such as enzymes and ions that can affect other components of soil communities, including plants (Metting, 1981; Zenova et al., 1995).
It has been demonstrated that Gram-negative photosynthetic bacteria play a key role in ecological and plant community succession (Metting, 1981). Primary succession begins in new habitats and it is not influenced by pre-existing communities. Secondary succession follows the disruption of pre-existing communities through external agents such as harvesting, drought or fire. There are few reports on the role that microalgae play in ecological succession. The use of microalgae as fertilizer has recently been suggested (Gaydon et al., 2012). The first objective of this study was to determine the effects of the application of a commercial proprietary suspension of microalgae (Chlorella sp. 1×10e3 cells per mL, Nannochloris sp. 1×10e3 cells per mL, Scenedesmus sp. 1×10e3 cells per mL) formulated to provide nutrients to indigenous soil microorganisms and facilitate microbial density and diversity, as well as to increase soil carbon. The formulation (GOgreen® (Global Organics Group, LLC, Goodyear, AZ) was applied through a center pivot irrigation system in a crop of cultivated corn (Zea mays) on soil algae species diversity after herbicide application and after harvest.
Autogenic and allogenic successions within a particular soil ecosystem are of significant importance to soil communities. In contrast to allogenic succession, caused by abiotic factors such as temperature, light or moisture, autogenic succession is observed when changes in the soil are caused by naturally occurring organisms and plants (Martin & Hine, 2008). These changes include accumulation of organic matter as well as changes in soil nutrient composition or soil pH. Percent organic matter (%OM) is a direct measurement of the amount of organic material (animal and plant residues) in the soil (Bot & Benites, 2005). Organic matter acts as a reservoir for essential nutrients such as nitrogen to be used by the plant or by microbial communities and it is closely related to the Cation Exchange Capacity or CEC of the soil. CEC is directly related to the total amount of cations that a particular soil can hold and in turn it is directly related to the ability of the soil to hold plant nutrients. As OM contributes to cation exchange, the larger the OM content of the soil, the larger the CEC (CUCE, 2007). The secondary objective of this study was to determine the effects of GOgreen® on %OM and CEC in experimental plots with different pH and to compare them with those obtained without GOgreen® treatment.
Experimental plots of approximately 0.5 acres each were planted in four rows. In order to study the effects of consecutive planting and harvesting on algae populations in the soil, one plot (plot 3) was planted in May 2010 and harvested in October 2010. Treatment for plot 3 is shown in Table 1.
Plot size: 4 rows per variety. Planting population: 35,500. NPK: Nitrogen, Phosphorus and Potassium content.
Plots were designated by numbers based on the application rate of GOgreen®. Plot 1 (10 oz/acre), plot 2 (8 oz/acre), plot 3 (10 oz/acre) and plot 4 (20 oz/acre). A control plot (plot 5 no GOgreen®) was also included. Corn (34,000 plants per acre) was planted in May 2011 and harvested in November 2011 allowing each plot to be studied for 6 months. Treatment for all plots is described in detail in Table 2.
Plot size: 4 rows per variety. All plots were divided into high (8.3) and low pH (6.8). Planting population: 34,000. (N/A = not applicable; *second treatment in consecutive years).
Soil samples representative from each of the experimental and control plots were taken at a depth of 6 inches immediately after herbicide application (as described in Table 1) following established protocols at the Irrigation Research Foundation (IRF) in Yuma, CO, USA. These samples were labeled pre-treatment. Analysis included organic matter (OM) content, cation exchange capacity (CEC) and pH. Analysis was performed by Midwest Laboratories, Omaha, NE. Details of protocols are available at https://www.midwestlabs.com/wp-content/uploads/2012/09/139_soil_analysis_methods.pdf.
Each plot received all treatments listed in Table 1 and Table 2 except for the controls. Controls were not treated with GOgreen®. Prior to harvest, soil samples were taken from each of the plots (and control). These samples were labeled post-GOgreen®. Samples were also taken from each of the plots and controls two months after harvesting. These samples were labeled post-GOgreen® two months. Each plot crossed a region of high soil pH (8.3) and neutral soil pH (6.8) and soil samples were taken individually from these regions. Treatments are described in Table 2. After being in use in 2010, plot 3 was again planted in May 2011 and harvested in November 2011 allowing for a year of consecutive corn harvesting and data recording on this plot.
Description of herbicide treatments was relevant to our study because herbicides have been documented to have a detrimental effect on soil algae populations (Kuzyakhmetov, 1998; Zancan et al., 2006). GOgreen® was delivered at the manufacture’s recommended application rate through center pivot irrigation before the V7 stage of corn plant growth. Soil samples were taken at V5 stage of plant growth as well as 4, 6, 7, and 8 weeks after emergence. A final sample was taken at harvest (black layer).
Two-tailed paired difference t-test was used to compare groups pre and post treatment to determine if GOgreen® had an effect on the tested soil parameters. Results of Statistical analysis are shown in Table 4. Statistical analysis was performed using GraphPad Prism software (GraphPad, San Diego, CA, USA).
Media and solutions were prepared using reagents from Sigma-Aldrich (St. Louis, MO) unless otherwise specified. HEPES buffer (50 mM, pH 7.8) was used to prepare Vitamin B12, Biotin and Thiamine solutions. In order to culture algae for identification purposes, 1 gram of soil from each plot, including high and low pH regions as separate samples, was added to 10 mL of selective media containing BG-11, Bolds 3N and DM and plated using approximately 200 μl in Agar plates with the same composition. Plates were prepared using a spread plate method. The media was selective for photosynthetic organisms.
3N Modified Bold’s Basal Media (Bold, 1949) was used for enrichment of green, red, and brown algae. Soil water was prepared using an adaptation of E.G. Pringsheim's biphasic soil-water medium (Pringsheim, 1946). Diatom Medium (DM) was used to culture and identify diatoms (Beakes et al., 1988), Proteose medium (PM) was used for yellow-green algae. PM was made by adding proteose peptone to Bristol Medium at a final concentration of 1 g/L. Bristol medium was prepared according to Bold, (1949). To culture cyanobacteria, BG-11 was used as previously described (Stanier et al., 1971). Cultures were grown at 16:8 under full spectrum grow lights for 4–6 weeks at 22°C. Algae layers (1 mL) were collected using a Pasteur pipette and plates of selective medium were inoculated. Colonies were counted and identified through light microscopy using a Labomed LX500 microscope (Labomed, Hicksville, NY) six days dates post-inoculation.
Results from soil analysis pre-treatment and after GOgreen® treatment at harvest and 2 months post-harvest are shown in Table 3. Plot 2 values are shown prior to plot 1 values as the dose applied in plot 2 was 8 oz./acre vs. 10 oz./acre applied in plot 1. This order facilitated comparison between a one time application of GOgreen® (plot 1) vs. consecutive GOgreen® application (plot 3). Percentages of OM were very low for all plots including the control plot. This is typically observed in soils in the Southwest (USA) (Hargrove & Luxmore, 1988). Differences in OM and CEC pre- and post-GOgreen® treatment for each individual plot are shown in Figure 1A and B respectively.
All units in parts per million (ppm) unless otherwise noted. * indicates pre- GOgreen® treatment the previous year. Abbreviations: OM (%) = Percent Organic Matter, CEC= Cation Exchange Capacity. L = pH < 8.0, H = pH > 8.0 post = after GOgreen® treatment. Post-2M = 2 months after harvest.
Given soil heterogeneity plots were grouped in pre and post treatment groups. Paired Difference t-test analysis was chosen given the small number of samples to analyze. Stedv (Standard Deviation of the Population), df = degrees of freedom.
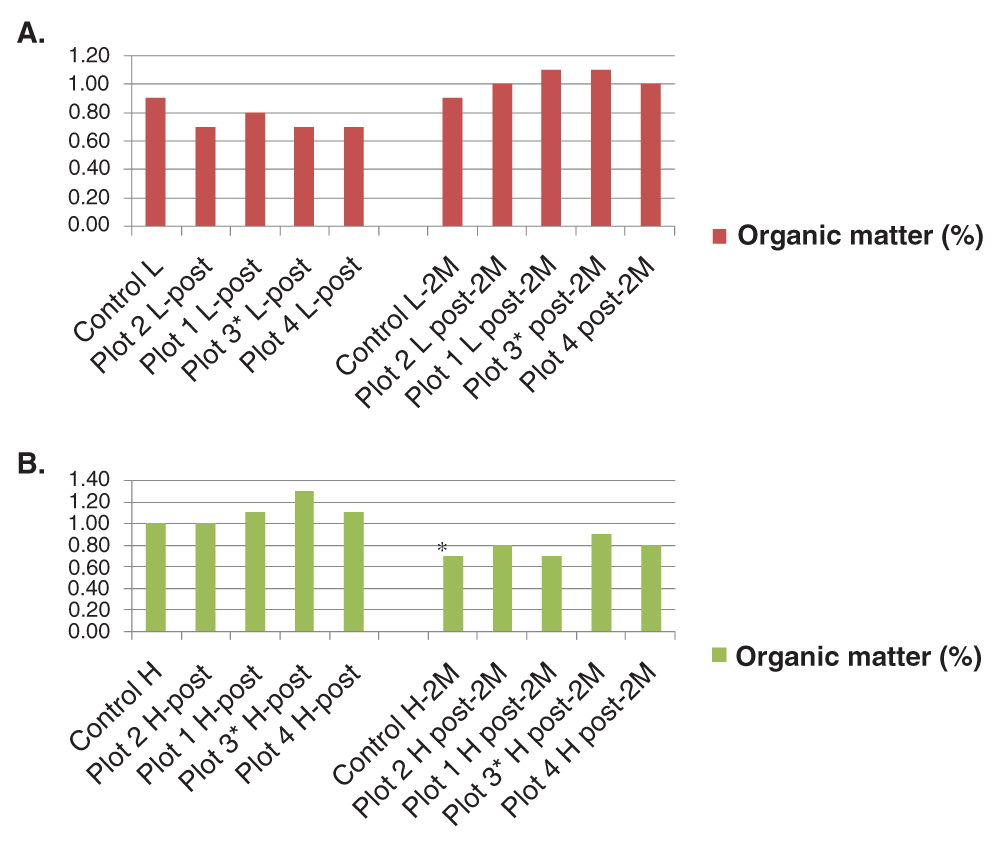
A. In soil with pH < 8.0 (red) a lower OM content when compared to the control was observed. These numbers increased 2 months after harvest as shown to the right. B. Soils with pH > 8.0 (green) showed the opposite trend. These changes indicate that treatment with GOgreen® result in changes in OM content in the soil that vary according to pH.
Our results indicate that GOgreen® has a significant effect in the OM content of soil at both pH values tested. Although lower OM values were observed prior to harvest in soil treated with GOgreen® with pH < 8.0 when compared with the controls (Figure 1A (left)), an increase in OM was observed two months after harvest (Figure 1A (right)). In soil with pH > 8.0, GOgreen® OM content values were higher prior to harvest when compared to those obtained at pH < 8.0. In the first group, treatment with GOgreen® at the recommended manufacturer dose (Table 2) appeared to have a positive effect on CEC. Although GOgreen® initially increased the OM content of the soil prior to harvest (Figure 1B (left)) this effect was not sustained two months after harvest (Figure 1B (right)) in this particular soil and/or crop type. It is worth noting that although the control at pH < 8.0 did not show any changes in OM over time, the control at pH > 8.0 showed a significant decrease in OM (shown as a * in Figure 1B) indicating that other factors independent of GOgreen® application might have played a role in OM content measurements at high pH values.
In terms of CEC, significant differences were observed in CEC two months after harvest when compared to samples collected prior to harvest (Figure 2A and B). It was observed that prior to harvest GOgreen® treatment at the recommended 10 oz./acre increased the CEC of the soil in the treated plots at pH < 8.0. The highest CEC obtained prior to harvest was observed at 20 oz./acre in the soil samples with pH < 8.0. Two months after harvest, the CEC values declined for all plots in this group including the control and the values were uniform. The average CEC for all plots at harvest was 10.6 meq/100g for the controls at this pH value and the average for all plots was 10.68 meq/100g indicating an increase of 0.08 units reflected in a greater ability of the soil to bind and retain ions and nutrients (Table 3). There were no increases observed in CEC in the plots treated with GOgreen® at pH > 8.0 prior to harvest. Although the CEC values for the plots in the pH > 8.0 group were significantly higher (by about 7 meq1/100 g) when compared to the plots in the pH < 8.0 group, two months after harvest, CEC values were also lower than those observed prior to harvest consistent with the CEC values observed for the pH < 8.0 group.
The effect of GOgreen® was studied in plots before and after application. Total algae counts were grouped as percentages of blue-green and green algae as well as diatoms. The results are shown in Figure 3 (and Dataset 1).
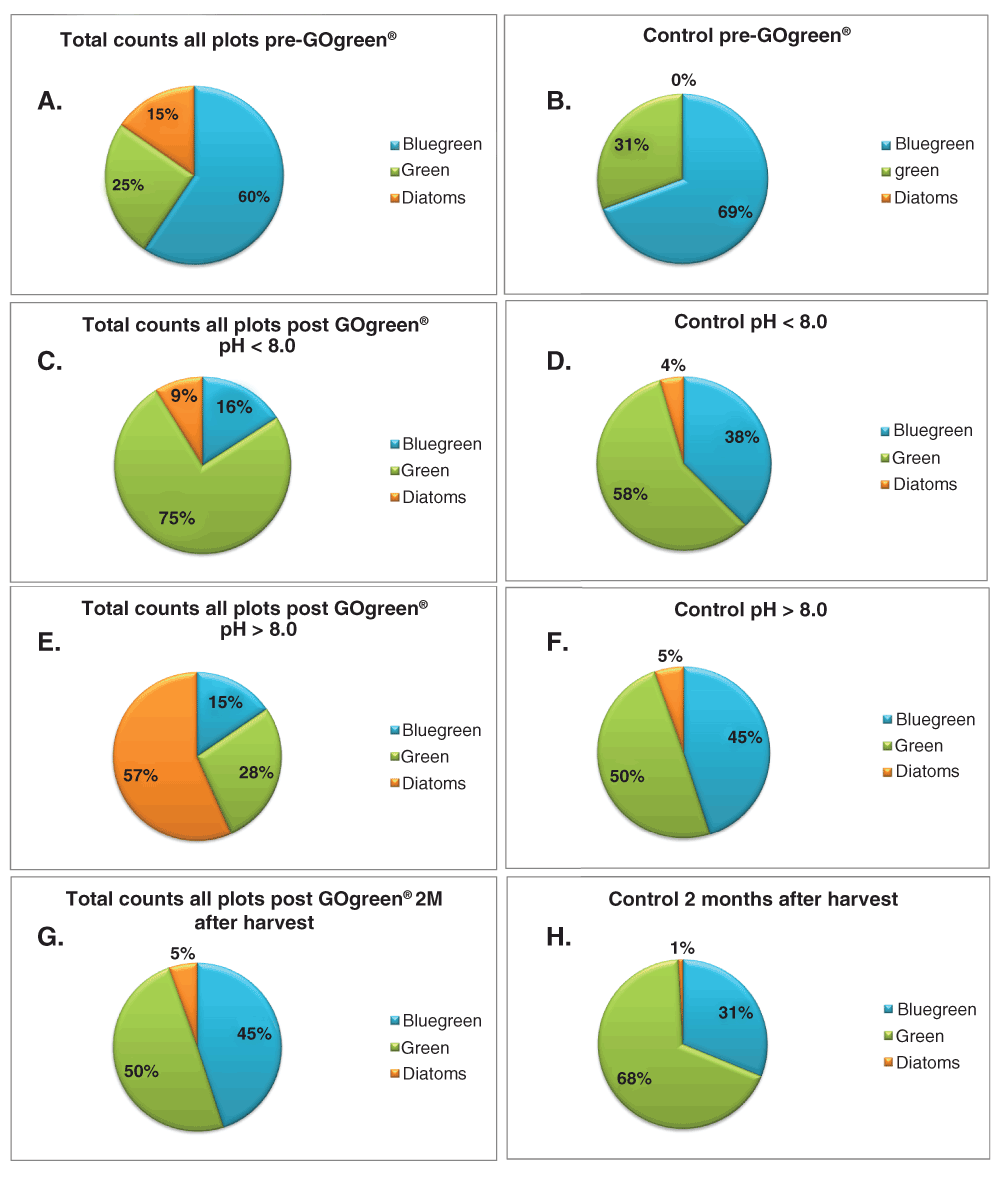
Effects of pH in total counts of groups of species. A. Total counts all plots pre-treatment. B. Control pre-treatment. C. Total counts all plots pH < 8.0. D. Control pH < 8.0. E. Total counts all plots pH > 8.0. F. Control pH > 8.0. G. Total counts all plots 2 months after harvest. H. Control 2 months after harvest. Please see Dataset 1 for the raw data.
Our results indicate that prior to GOgreen® treatment, the total microalgae population in the soil of the test plots was composed of 60% blue-green algae, 25% green algae and 15% diatoms (Figure 3A). The ratio of green to blue-algae was 0.42. In the control plot, the ratio of green to blue-green algae pre-treatment was approximately 0.45 (Figure 3B) indicating uniformity in the distribution of microalgae by plots before treatment.
In soils with pH < 8.0, green algae were more abundant, which also correlated with the control at this pH (Figures 3C and D). The amount of diatoms was higher in the treated plots than in the controls. In soils with pH > 8.0, green algae counts were lower than those of blue algae (Figures 3E and F).
The results obtained two months after harvest indicate the percentages of blue-green algae and green algae were similar after GOgreen® treatment (Figure 3G). In contrast, the controls plots showed a 50% reduction in the blue-green algae counts when compared to the number of green algae (Figure 3H). Although diatoms also appeared in the controls two months after harvest, their counts were significantly higher in the treated plots as shown in Figure 3G.
The comparison between the pH < 8.0 and the pH > 8.0 controls indicates that there were small differences between their values (1.53 vs. 1.11 respectively). However, the difference between green and blue-green algae in the treated plots was significant (4.69 vs 1.87) indicating differences in microalgae diversity based on pH. In both cases, the ratio in terms of percentages was higher than the ratio of the controls, indicating that treatment does have an impact on microalgae populations. Over time, all treated plots showed higher counts of green algae than blue-algae. When compared to untreated plots, those numbers showed a significant increase as the controls pre-treatment indicated higher numbers of blue-green algae in the soil. Furthermore, diatom concentrations increased in all treated plots. The diatom counts were much higher in soil with pH > 8.0.
The results of this study indicate differences between soil pH in the plots with pH < 8.0 treated with GOgreen® two months after harvest (Figure 4A). Although the control plot did not show a substantial change in pH (initial pH was 6.9 and pH after harvest was 7.0), the treated plots did show substantial changes over time. A trend based on rates of application was not obvious.
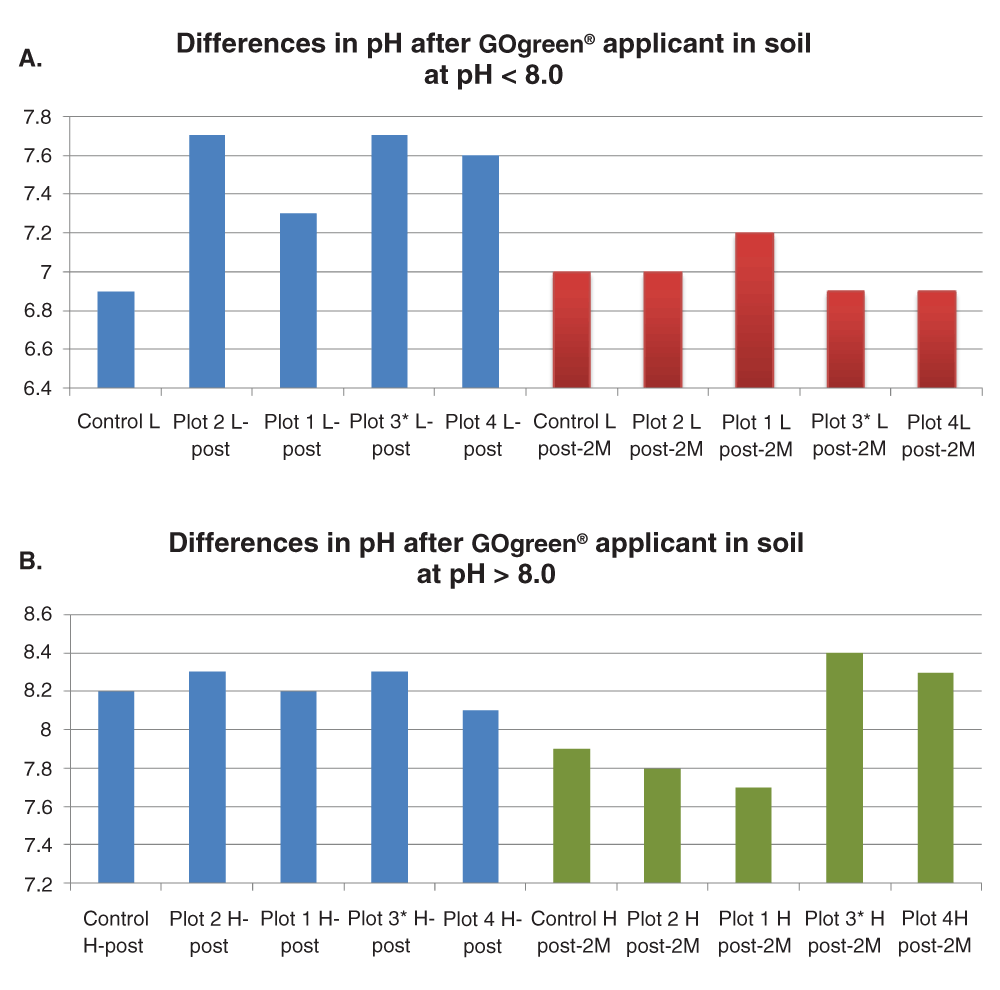
A. Comparison of pH values observed at harvest and two months post-harvest in soils with pH < 8.0. Blue: pH values for plots at harvest. Red: pH values for plots 2 months after harvest. B. Comparison of pH values observed at harvest and two months post-harvest in soils with pH < 8.0. Blue: pH values at harvest. Green: values 2 months after harvest.
It is clear from Figure 4A that in plots with soil pH > 7.0 at the end of harvest, the pH of the soil reached values closer to neutral two months later. In soils with pH > 8.0 the trend was not as noticeable. However, pH decreased to values closer to 7.0 in plots 1 and 2, as well as the controls.
An observation worth noting is the difference in pH in plots 3 and plots 4 at pH > 8.0 when compared to the other plots as seen in Figure 4B. At this pH, treatment with GOgreen® the previous year (plot 3) and with twice the recommended application rate (plot 4) resulted in a pH increase of 0.2 units two months after harvest when compared to the other plots. This result contradicts the observations in which pH decreased in the soil two months after harvest in plots 1 and 2.
A conventional approach was implemented to evaluate microalgae diversity in soil samples collected at a depth of 6 inches after herbicide and fertilizer treatment. Soil was treated with GOgreen® in an effort to restore and improve microalgae diversity and soil properties. This method included isolation, culture and species identification based on morphological criteria. This method has been successfully used by other investigators (Kostikov et al., 2001).
The results presented in this manuscript demonstrate that treatment with GOgreen® has significant and measurable effects on soil OM content, CEC, pH and microalgae species diversity suggesting positive effects of this formulation on soil conditions, making them more suitable to sustain crops. Although studies in soil are affected by a variety of biotic and abiotic factors, the effects of GOgreen® are evident in terms of increases in microalgae species diversity. Abiotic factors considered in this study included light intensity, temperature and humidity. Control of external biotic and abiotic factors in the soil is extremely difficult and therefore the best approximation that could be done in a study of this nature is to compare soils that have been treated under the same conditions with simultaneous sampling.
Temperature changes in the environment during the time of the study were considered since it has been reported that blue-green algae are photo inhibited by high light intensities at low temperatures. Temperature can be considered as the most important limiting factor in outdoor cultivation during the winter (Malakar & Kalita, 2012). Blue-green algae growth is enhanced by increasing light density up to the point of light saturation, at which point photosynthetic activity reaches its maximum (Abu et al., 2007; Pandey & Tiwari, 2010). At high light densities, photosynthetic capacity decreases and blue-green algae growth is inhibited. As soil samples were taken from all plots during the cold months of November and February, it is possible to hypothesize that the low counts of blue-green algae found in the treatment plots and the controls after GOgreen® treatment are directly related to changes in ambient temperature and light intensity between soil sampling times. This could be a possible explanation for the differences encountered in the parameters measured between pre and post GOgreen® applications. However, as all plots (treated and not treated) were exposed to the same environmental conditions, it is highly unlikely that the differences found in the treated plots versus the control plot were due to cold temperatures or intense light. The difference must be related to different soil characteristics as a result of the treatment.
Despite abundant studies on soil algae (Johansen, 1993; Metting, 1981; Lukešová, 1993; Lukešová, 2001; Lukešová & Hoffmann, 1996; Neustupa, 2001; Starks et al., 1981; Sukala & Davis, 1994; Tsujimura et al., 2000), it is still difficult to correlate species diversity and their influence on ecosystem functions. Therefore, this manuscript does not attempt to correlate species diversity with metabolic, ion or gas exchanges. Instead, the focus lies on algae species diversity after use of an organic formulation such as GOgreen® and to correlate these findings with indicators of soil quality such as OM and CEC.
The primary objective of these studies was to determine the effects of GOgreen® on algae species diversity after herbicide application. The results of this study support the findings that Zancan and Zuzyamkhmetov encountered in corn fields subjected to lengthy periods of intense fertilization. Their results indicate a reduction in species diversity and a suppression of blue-green algae development in fields treated with fertilizers and herbicides (Kuzyakhmetov, 1998; Zancan et al., 2006). The control shown in Figure 3B, indicates low species diversity (diatoms are absent) encountered in the soil prior to GOgreen® treatment but after fertilizer and herbicide treatment. The data shown in this manuscript confirms the profound effect of agricultural practices including herbicide and pesticide application on the structure of soil algal communities.
Herbicides have also previously been correlated to changes in microalgae populations in aquatic ecosystems (Bérard & Benninghoff, 2001; Bérard et al., 1999) and decreased density of algal assemblages in plots. Lenacil and Pyrazon for example, have been shown to diminish species diversity and decrease microalgae counts in soil (Zurek, 1981). Herbicides and pesticides influence the range of genera and the number of algal cells, blue-green in particular, present at any given time in vitro and in vivo (McCann & Cullimore,1979; Megharaj et al., 1999; Metting & Rayburn, 1979; Mostafa & Helling, 2002).
One of the main groups considered the diatoms a valuable tool to assess biological conditions in wetlands (Doherty et al. 2000; Stevenson, 2001). The response of diatoms to changes in surrounding land and water column characteristics has been documented previously and many diatom taxa have been identified from a range of sites throughout the world (Stevenson, 2001). Diatoms appear to have a consistent tolerance of a wide range of environmental parameters, such as light, moisture, pH, salinity, oxygen and inorganic and organic nutrients (van Dam et al., 1994). Responses to pH (Pan & Stevenson, 1996) and heavy metal loading (Charles et al., 1996) have also been used to predict environmental pollution. The US Environmental Protection Agency (USEPA) (2002) reported that diatoms are one of the most commonly used microorganisms in observations from aquatic ecosystems for assessing biological, physical, and chemical conditions. Through this study, it can be concluded that GOgreen® increased diatom numbers and species diversity in the treated plots compared to the controls indicating that GOgreen® has a restorative effect on soil quality after herbicide treatment in heavily farmed soil. Additionally, these results indicate that the observations from aquatic ecosystems can be extrapolated to terrestrial environments.
In terms of specific differences in species diversity related to green and blue-green algae, a 17% increase was observed in the total counts of green microalgae in the GOgreen® treated plots at pH < 8.0 when compared to the control, while a 22% decrease was observed in blue-green algae in the same plots. Contrary to these results, a 22% decrease in green microalgae was observed in the GOgreen® treated plots at pH > 8.0 when compared to the controls, while a 30% decrease was observed in blue-green algae counts when compared to the control at this high pH value. These results indicate that the effects of GOgreen® application are different and highly dependent on soil pH.
The pH of all plots tested varied between 6.9 to 8.4, therefore many of the microalgal classes were represented. Blue-green algae are unable to survive in acidic conditions (Brock, 1973), but green algae are able to survive in soils with pH < 7.0 (Lukešová & Hoffmann, 1995). Neutral conditions support the growth of algal communities representing all major taxonomic groups (Lukešová, 2001; Metting, 1981). The percentage of blue-green algae observed in the control plot at pH < 8.0 was 38% (Figure 3D) versus 45% in the control plot at pH > 8.0 (Figure 3F), which supports the findings by the above mentioned investigators. In contrast, the values for green algae in the two controls were similar (58% and 50%). These results indicate increased microalgal species diversity in the GOgreen® treated plots versus the controls within the pH range tested.
An increase in diatom numbers and diversity was observed at all pH values when treatment plots were compared to controls not treated with GOgreen®. The results of treatment with GOgreen® were different between soil with pH < 8.0 and soil with pH > 8.0. At all pH values, an increase in diatom concentration was observed in the GOgreen® treated plots when compared to the controls, but this effect was higher in soils with pH > 8.0, resulting in a 57% of total diversity composed of diatoms (Figure 3E) versus 9% of diatoms at pH < 8.0 (Figure 3C). The main species found in the study were: Pennate diatom, Caposira, Raphid diatom, Geminella, Navicula diatom, Centric diatom, Tessillaria, Pinnularia.
The second objective of these studies was to determine the effect of GOgreen® on OM and CEC. Both OM and CEC depend on soil pH. Optimum pH for corn ranges between 5.5–7.0 (Havlin et al., 1999). Our results suggest that soils starting at pH < 8.0 are most likely to fall within the optimal pH range for corn after GOgreen® treatment.
Soil OM serves multiple functions including nutrient storage and soil aggregation. Soils with high CECs are able to bind more monovalent and divalent cations through available sites in clay and OM particles. A soil with a high CEC also has an increased buffering capacity indicating that this soil is able to resist fluctuations in pH.
Soils with a high clay content and/or OM will typically have higher CEC and buffering capacity than silty or sandy soils, as organic materials provide additional binding sites for cations (CUCE, 2007). In this study, high OM correlated with high CEC values of the plots before GOgreen® application. At harvest, CEC was higher in soil with pH > 8.0, which is consistent with the presence of negative charges in the absence of acidic values that in turn have the ability to bind cations. Two months after harvest, values for CEC were lower at both pH ranges as it is expected after harvest due to soil depletion. These changes also correlated with pH values two months after harvest as the pH lowered for both types of soil. The most interesting observation was the fact that the CEC was around 8.0 at both pH values two months after harvest indicating a stabilization of CEC in the soil regardless of pH. Although this value is lower than those encountered at harvest, a low CEC value indicates that fewer cations such as K+, Ca2+, Mg2+, to name a few, will be bound to soil particles and more available for nutrient uptake by the plant. Nitrogen will also be more available and less lime will be necessary to correct pH fluctuations. In soils with pH around 8.0 such as those found in the Western U.S., large amounts of naturally-occurring lime are typically responsible for the increased pH. This “free lime” buffers pH in the alkaline range making it very difficult to change soil pH. Calcium carbonate (CaCO3) is commonly found in these soils. For these particular soils, larger quantities of amendments are needed to lower the pH converting pH modification in alternatives that are not cost effective. Addition of organic matter is typically used to lower pH but not all sources of organic matter are effective or safe for human consumption. GOgreen® lowers the pH in soils with a pH higher than 7.0 emerging as an economical alternative that is safe for humans and the environment.
In soil, the presence of living organisms has proven critical to OM formation. Soils with OM values around 1.0% are typically found in the desert (CUCE, 2007). Soil OM – the product of on-site biological decomposition – affects the chemical and physical properties of the soil and its overall health. Its composition and breakdown rate affect the soil structure and porosity, the water infiltration rate and moisture holding capacity of soils, the diversity and biological activity of soil organisms, and plant nutrient availability (Bot & Benites, 2005). Where the rate of OM addition is less than the rate of its decomposition, soil OM declines. Conversely, where the rate of addition is higher than the rate of decomposition, soil OM increases. The observations presented in this study regarding the differences in OM at the pH values tested indicate that the rate of OM addition after GOgreen® application is higher than the rate of decomposition in soils with pH < 8.0. The opposite is true for soils with pH > 8.0. However, further studies are needed to test this hypothesis. As changes in OM are lower than 1–2% per year of the total OM in the soil, the effects of GOgreen® algae application will only become significant after several years. For this purpose, this study has been extended in order to monitor the plots described during subsequent years of continuous harvest usage.
As land available for cultivation becomes scarce due to increasing populations, the need for improved soil quality is now recognized at a global scale (Elliott et al., 1996). Soil communities co-exist in constant interactions with each other (Gerson, 1974; Thirup et al., 2000; Yeates et al., 1993). These interactions are crucial to regulate soil activity and may be disturbed by soil pollution, herbicides, pesticides, fertilizers and management practices (Pankhurst, 1997; Paoletti et al., 1988).
Methods to determine soil quality are varied but scholars have identified microorganisms as potential bio indicators of soil quality (Pipe & Cullimore, 1980; Roper & Opel-Keller, 1997). It has been proposed that approaches to study ecological and eco-toxicological impacts of management and agricultural practices on soil quality should be monitored with the use of multimicrobial bio indicators (Bérard et al., 2005). Soil microalgae depend on soil physical and chemical characteristics and therefore, these organisms have been used for decades as bio indicators to estimate the eco-toxicological impact of agricultural management practices and herbicide application (Fujita & Nakahara, 1999; McCann & Cullimore, 1979; Pipe, 1992). Biodiversity is therefore an indicator of soil quality and a crucial component to evaluate the success of a particular remediation strategy. The use of biodiversity as an indicator is limited by the incomplete knowledge regarding microorganisms present in a particular ecosystem (Paoletti, 1999).
Microalgae, and diatoms in particular, respond to different ecological gradients and are therefore useful tools for bio monitoring studies in aquatic and terrestrial ecosystems. It has been demonstrated that diatom diversity tends to be higher in biodynamic systems than in conventional systems. Redundancy analysis (RDA) has suggested that diatom community structure differs significantly between organic and conventional systems (Heger et al., 2012).
Microalgae concentrate in the top few inches of the soil because they need moisture and light to perform photosynthesis. They are located in the first biological layer directly affected by environmental changes, both natural and manmade. Consequently, microalgae composition varies in the soil over several weeks depending on treatment and their numbers are highly influenced by environmental and seasonal factors specific to each region (Hoffmann, 1989; Metting, 1981; Whitton, 2000). These results corroborate previous findings and highlight the importance of CEC, OM and pH variations in increased microalgae species diversity through GOgreen® application.
F1000Research: Dataset 1. Microalgae counts raw data, 10.5256/f1000research.4016.d36846 (Hastings, 2014).
KH, ML, LB and GD conceived the study. KH and LS designed the experiments. KH, LS, GD, JD, and DZ carried out the research. LB and ML contributed to the design of experiments and provided expertise in rates of application, costs and budgets. MCM prepared the first draft of the manuscript. KH, JD, DZ, ML, MCM and GD contributed to the experimental design and preparation of the manuscript. All authors were involved in the revision of the draft manuscript and have agreed to the final content.
The authors would like to thank Irrigation Research Facility (IRF) Yuma, CO and Midwest Labs Omaha, NE for their help with plot and crop treatments and collection.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 14 Nov 14 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)