Keywords
levosimendan, cardiogenic shock, hemodynamic variables, mortality
levosimendan, cardiogenic shock, hemodynamic variables, mortality
Cardiogenic shock (CS) is a clinical condition of acute inadequate tissue perfusion caused by the heart’s inability to pump an adequate amount of blood1,2. Pharmacological treatment of CS is based on positive inotropic agents3,4. Due to the failure of classic inotrope agents, a sensitizer agent, levosimendan, has been used as rescue therapy in such situations. This drug has both positive inotropic and myocardial relaxing properties, which increase the affinity of myofilaments within the myocardial cell to calcium without raising intracellular concentrations of calcium or AMPc5–8. Levosimendan can improve the hemodynamic status of patients with CS without increasing the consumption of myocardial oxygen and without increasing the risk of arrhythmia9,10; however, few studies have been conducted on the use of levosimendan to treat CS.
The purpose of our study was to assess the effectiveness of levosimendan to treat CS by studying its hemodynamic effects on 26 patients with CS and who were refractory to catecholamines.
A retrospective study was conducted at the ICU in the Military Hospital of Tunis for two periods: between January 2004 and December 2009, and from January 2011 until December 2013. All patients had CS and were aged >18 years. Patients who died within 48 h were excluded from the study. The diagnosis of CS was made if hypotension was <90 mmHg for more than 30 min and there were signs of low perfusion in the absence of a hypovolemia or cardiac arrhythmia. These signs were associated with a cardiac index of <2.2 L/min/m2, a pulmonary capillary wedge pressure of >18 mmHg, and high doses of catecholamines had been ineffective to restore mean arterial pressure to 65 mmHg (i.e., dobutamine at >15 µg/kg/min and noradrenaline at >0.6 µg/kg/min).
When catecholamines failed to improve the hemodynamic condition and there were persistent signs of low peripheral perfusion, levosimendan was introduced (Orion Pharma, Espoo, Finlande). This treatment was administered in two steps: a loading dose of 12 µg/kg/min was infused for 30 min; and then continuous infusion was given for 24 h at a dose of 0.1 µg/kg/min. The pulmonary artery was catheterized using a 7.5-Fr Swan Ganz catheter with continuous cardiac output and mixed venous saturation measurements (Edwards Life sciences, North Carolina, USA). This catheter allowed us to collect hemodynamic parameters from each patient at just before administration of levosimendan (i.e., T0), at 30 and 90 min, and at 2, 4, 8, 12, 24, and 48 h.
Doses of catecholamine and plasma-lactate concentrations were recorded on days 0, 1, 2, and 3. Values of the left-ventricular ejection fraction were collected on days 0, 1, 2, 7, and 15. The primary endpoints were the evolution of hemodynamic parameters (the cardiac index, pulmonary pressure, and SvO2). Data were recorded and analyzed using Microsoft Excel (2007) software and Epi Info 6.0.4 (http://www.cdc.gov). Continuous variables were expressed as their means ± standard deviations and were compared between groups using Student’s two-tailed t-test. Non-parametric tests were also used where necessary (Mann–Whitney U test). Fisher’s exact (or the chi-squared) test was used to compare categorical variables, as appropriate. A p-value of <0.05 was considered statistically significant.
The local ethics committee approved the use of the patients’ data for this study.
Twenty-six patients who were hospitalized for CS met our criterion for inclusion in this study. The status of CS was mainly secondary to myocardial infarction (in 42% of cases) and to cardiomyopathy of the peripartum (in 31% of cases). The demographic data are shown in Table 1.
The hemodynamic data at inclusion showed a mean arterial pressure of 63 ± 7 mmHg, a cardiac index of 1.96 ± 0.29 l/min/m2, and a pulmonary capillary wedge pressure of 28 ± 4 mmHg.
Levosimendan resulted in significantly improved mean arterial pressure to 76 ± 7 mmHg at 48 h (p=0.001 compared to T0) and cardiac index to 3.19 ± 0.68 L/min/m2 (p<0.0001 compared to T0) (Figure 1) without any significant modification to either heart rate (101 ± 28 to 86 ± 13, p=0.26) or the emergence of rhythm disturbance.
Levosimendan decreased pulmonary wedge pressure to 17 ± 3 mmHg at 48h (p=0.001 compared to T0). Pulmonary arterial systolic pressure was significantly reduced to 38 ± 5 mmHg after 48 h, compared to 49 ± 6 mmHg at inclusion (p=0.016 compared to T0). Pulmonary arterial diastolic pressure was significantly reduced to 20 ± 4 mmHg at 24 h, compared to 29 ± 4 mmHg at inclusion (p<0.001 compared to T0). Mean pulmonary arterial pressure was significantly reduced to 26 ± 5 mmHg after 24 h compared to 35 ± 5 mmHg at inclusion (p=0.001 compared to T0).
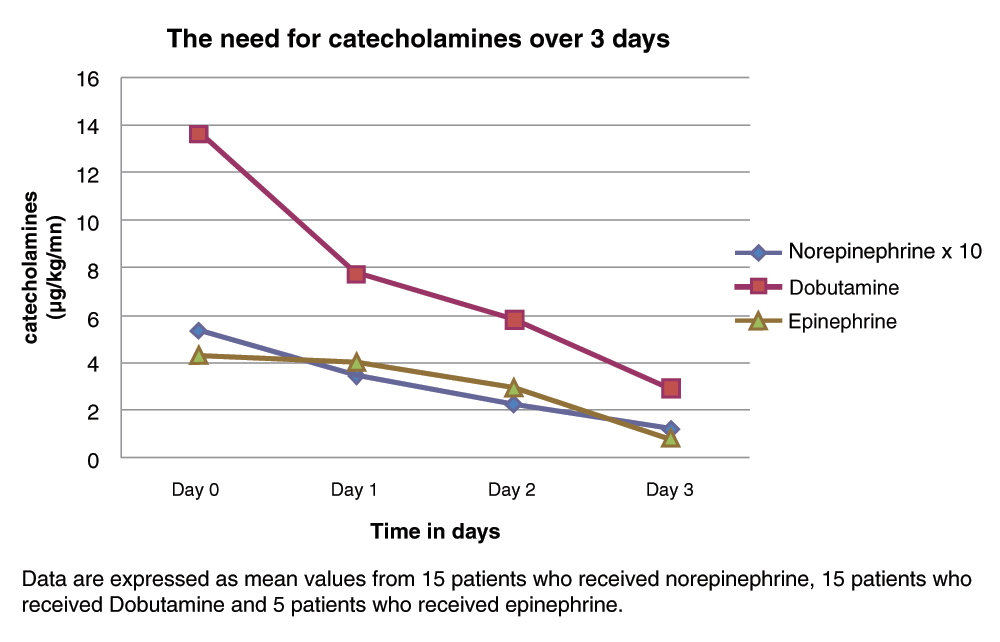
There was a significant decrease in lactate from 3.77 ± 2.93 to 1.60 ± 1.32 mmol/L after 72 h. Levosimendan significantly reduced systemic vascular resistance (1834 ± 308 to 1321 ± 304 dyne/s/cm2, p<0.0001) and pulmonary vascular resistances (289 ± 64 to 204 ± 78 dyne/s/cm2, p=0.001). Administration of levosimendan also reduced the need for catecholamines (Figure 2), thus leading to a gradual reduction in the need for catecholamines in six patients.
Administration of levosimendan improved SvO2 to 70 ± 5% after 24 h of infusion. The evolution of the left-ventricle ejection fraction showed bottom-up kinetics as a function of time, which ranged from 26 ± 5% at inclusion to 54 ± 5% by day 15.
Levosimendan is a calcium sensitizer. Its main mechanism of action is to increase the binding affinity of troponin C to Ca2+ and to stabilize its conformation. It also directly increases cardiac contractility without raising intracellular concentrations of calcium or AMPc. This makes levosimendan one of the best inotropic agents and causes least arrhythmia. In addition to its positive inotropic affects, levosimendan can caused vasodilatation of many vascular areas by opening ATP-dependent potassium channels. This increased coronary blood flow without increasing consumption of myocardial oxygen, thus explains the good tolerance of levosimendan in cases of coronary syndrome and also its effects as an anti-ischemic and a cardio-protector.
Many clinical studies have been conducted to assess the effects of levosimendan on hemodynamic parameters. The first studies focused on patients with decompensated heart failure. The main aim of these authors was to compare levosimendan with dobutamine (i.e., the main type of catecholamine used during low cardiac output). Three main studies have been published: the LIDO, SURVIVE, and CASINO studies.
In the LIDO study, 203 patients were hospitalized for severe cardiac failure and received either levosimendan (a loading dose of 24 µg/kg/min infused for 10 min; and then continuous infusion for 24 h at a dose of 0.1 µg/kg/min, n=103) or dobutamine (a dose of 5 µg/kg/min was given without a loading dose, n=100). Levosimendan increased the cardiac index by ≥30% and decreased pulmonary capillary wedge pressure by ≥25% in 29 patients compared to only 15 patients receiving dobutamine (hazard ratio=1.9, CI= of 95%, p=0.022)11,12. A decline in the need for catecholamines and the resumption of beta blockers occurred more frequently in patients that received levosimendan14. Mortality rate was also lower in patients that received levosimendan compared to dobutamine after 1 month (7.8% vs. 17%, p=0.045) and at 6 months (26% vs. 38%, p=0.029)9,11–13.
The SURVIVE study was a multicenter study that included 1327 randomized patients with severe cardiac failure and who had a left-ventricle ejection fraction of <30%. This study chose mortality as the main criterion for judgment and assessed the effectiveness of levosimendan compared to dobutamine11. When the analyses focused on early mortality rates during the first 5 days, levosimendan was more effective than dobutamine (4.4% vs. 6%), indicating that levosimendan could be an alternative to dobutamine for patients with acute cardiac insufficiency. However, this favorable trend was not found in the longer term11. During the 180 days of follow-up, there were 173 deaths (26%) in the group receiving levosimendan (n=664) compared to 185 (28%) in the group receiving dobutamine (n=663)15. However, the main criterion of mortality at 6 months may not be appropriate to judge the effectiveness of this treatment when its main benefits were observed at an early stage16.
The CASINO study was a randomized, controlled, double-blind study that included 299 patients with decompensated congestive heart failure. The study compared levosimendan therapy with dobutamine, and with a placebo. The study showed positive results for its first assessment criterion: i.e., mortality rate at 6 months. The authors reported that improved survival was associated with the use of levosimendan16. In the light of these results, levosimendan represents a significant advance in the treatment of cardiac insufficiency. These studies have helped to expand levosimendan’s indications and have also assessed its contribution to treat CS.
Many authors have evaluated the hemodynamic effects of levosimendan in the course of CS. Delle et al. concluded that administration of levosimendan to 10 patients with CS led to a significant increase in the cardiac index and significantly decreased vascular resistance17.
Russ et al. obtained similar results when they prescribed levosimendan to 56 patients with CS. They reported a significant increase in the cardiac index (from 2.1 ± 0.56 to 3.0 ± 1.11 l/min/m2 at 24 h, p<0.01) associated with a significant decrease in systemic vascular resistance (from 1208 ± 333 to 858 ± 299 dynes/s/mm-4 at 24 h, p<0.01), whereas mean arterial pressure and pulmonary wedge pressure were slightly but non-significantly decreased. An improved SOFA score was found after 72 h of levosimendan treatment, suggesting improved organic function18.
Labriola et al. also reported the same variations in hemodynamic parameters as described by Delle et al. and Russ et al., which were distinguished by the development of a significant increase in the left-ventricle ejection fraction19.
According to the study of Berry et al., a continuous decrease in noradrenalin, dobutamine, and milrinone was associated with improved hemodynamic parameters in 93 patients with CS who received a continuous infusion of levosimendan for 26 h at a dose of 0.096 µg/kg/min20.
Labbene et al. analyzed the hemodynamic effects of levosimendan in 16 patients hospitalized with CS and who were refractory to catecholamine21. The evolution of hemodynamic parameters after infusion of levosimendan was marked by a significant increase in the cardiac index (from 2.01 ± 0.4l to 3.4 ± 0.65 l/min/m2 at 48 h) of SvO2 (from 57% ± 5% to 70% ± 3%) and the left-ventricular ejection fraction (from 26 ± 6% to 52 ± 5%). There was also a significant decrease in pulmonary wedge pressure from 28 ± 5 to 17 ± 5 mmHg, and of pulmonary arterial systolic pressure, of diastolic arterial systolic pressure, of average arterial systolic pressure of systemic vascular resistance (from 1833 ± 308 to 1216 ± 304 dyne/s/cm2 at 48 h), and of pulmonary vascular resistances (from 289 ± 64 at T0 to 179 ± 44 dyne/s/cm2 at 4 h)21. Our results agree with those from the literature and show the effectiveness of levosimendan to treat CS when it is refractory to catecholamines. At the recommended dose, levosimendan improves hemodynamic parameters by increasing the cardiac index and SvO2. It is responsible for the drop in systemic vascular resistance and pulmonary vascular resistances, and reduces the need for catecholamines. Other studies have also confirmed the efficacy of levosimendan to stabilize hemodynamic parameters in patients with CS22–25.
Our results show that levosimendan was efficacious at treating CS, and that it has several advantages compared to other isotropic agents. However, it is expensive (compared to dobutamine) and the absence of a net profit on long-term survival represents a limit to its wide-spread use in pathologies with a poor prognosis, such as CS. Further randomized studies that include a placebo and a larger patient population with CS are needed to precisely determine the indications for levosimendan and to assess its cost in relation to patient survival.
F1000Research: Dataset 1. Data used for retrospective analysis of the effects of levosimendan for cardiogenic shock, 10.5256/f1000research.5820.d4017226
IL and MF conceived the study. IL and FJ designed the experiments. KEA, CR and IL carried out the research. KEA and FJ prepared the first draft of the manuscript. IL, FJ and KEA contributed to the experimental design and preparation of the manuscript. All authors were involved in the revision of the draft manuscript and have agreed to the final content.
Supported by a grant from the Research Laboratory LR12DN01 and The Military Hospital of Tunis.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors thank Newmed Publishing Services for language editing and submission support, provided on a pro bono basis.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Peer review at F1000Research is author-driven. Currently no reviewers are being invited.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)